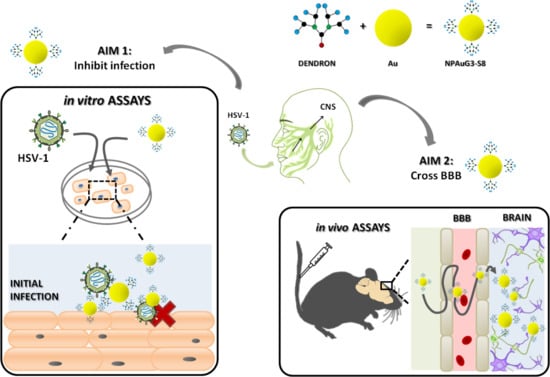
Gold Nanoparticles Crossing Blood-Brain Barrier Prevent HSV-1 Infection and Reduce Herpes Associated Amyloid-βsecretion
Abstract
1. Introduction
2. Materials and Method
2.1. Cell Lines, Culture Conditions and Virus
2.2. Nanoparticles
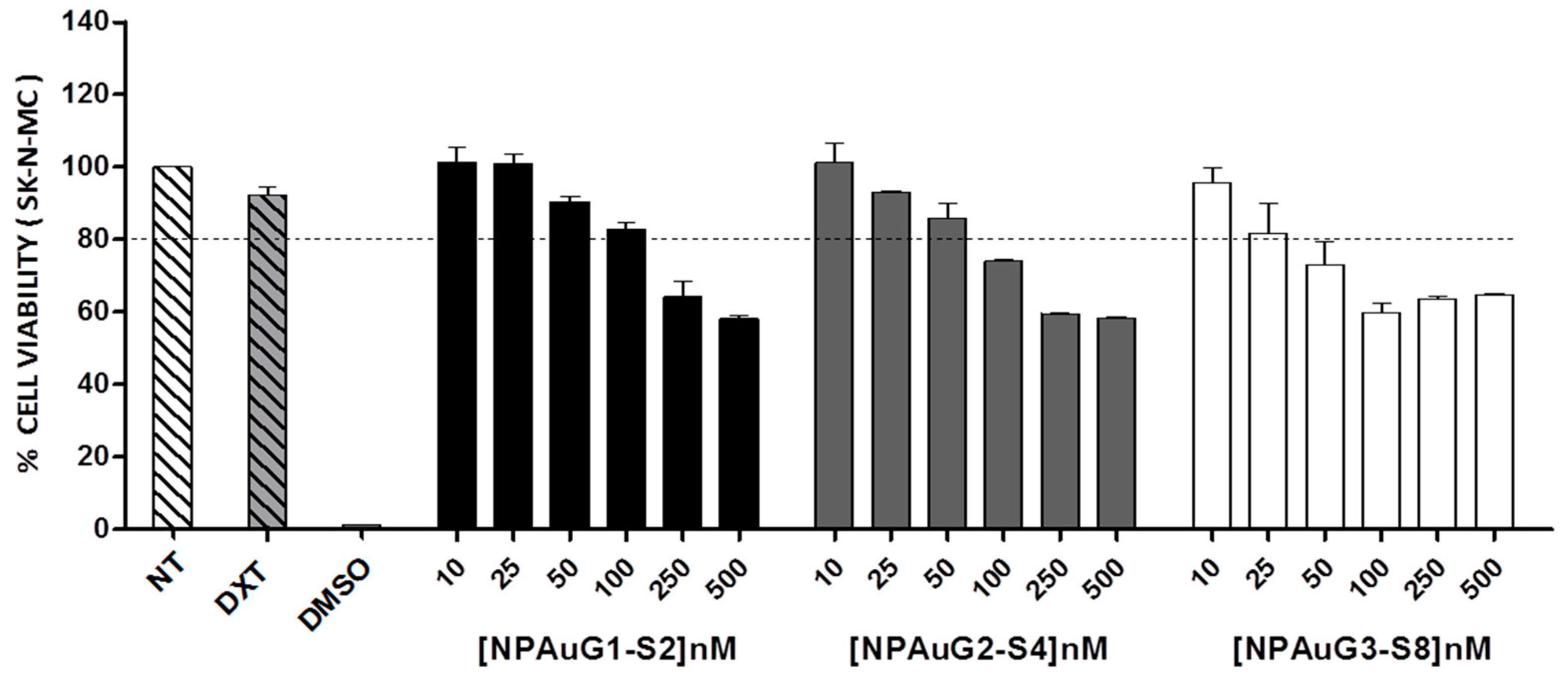
2.3. Cell Viability Assay
2.4. HSV-1 Infection
2.5. Cellular Protection against HSV-1 Infection
2.6. Interactions Nanoparticles-HSV-1
2.7. β-Secretase Activity
2.8. Measurement of Secreted Aβ
2.9. Histological Studies in CD1 Mice
2.10. Permeability of Blood-Brain Barrier to NPAuG3-S8 in CD1 Mice
2.11. Statistical Analysis
3. Results
3.1. Cytotoxicity of Nanoparticles
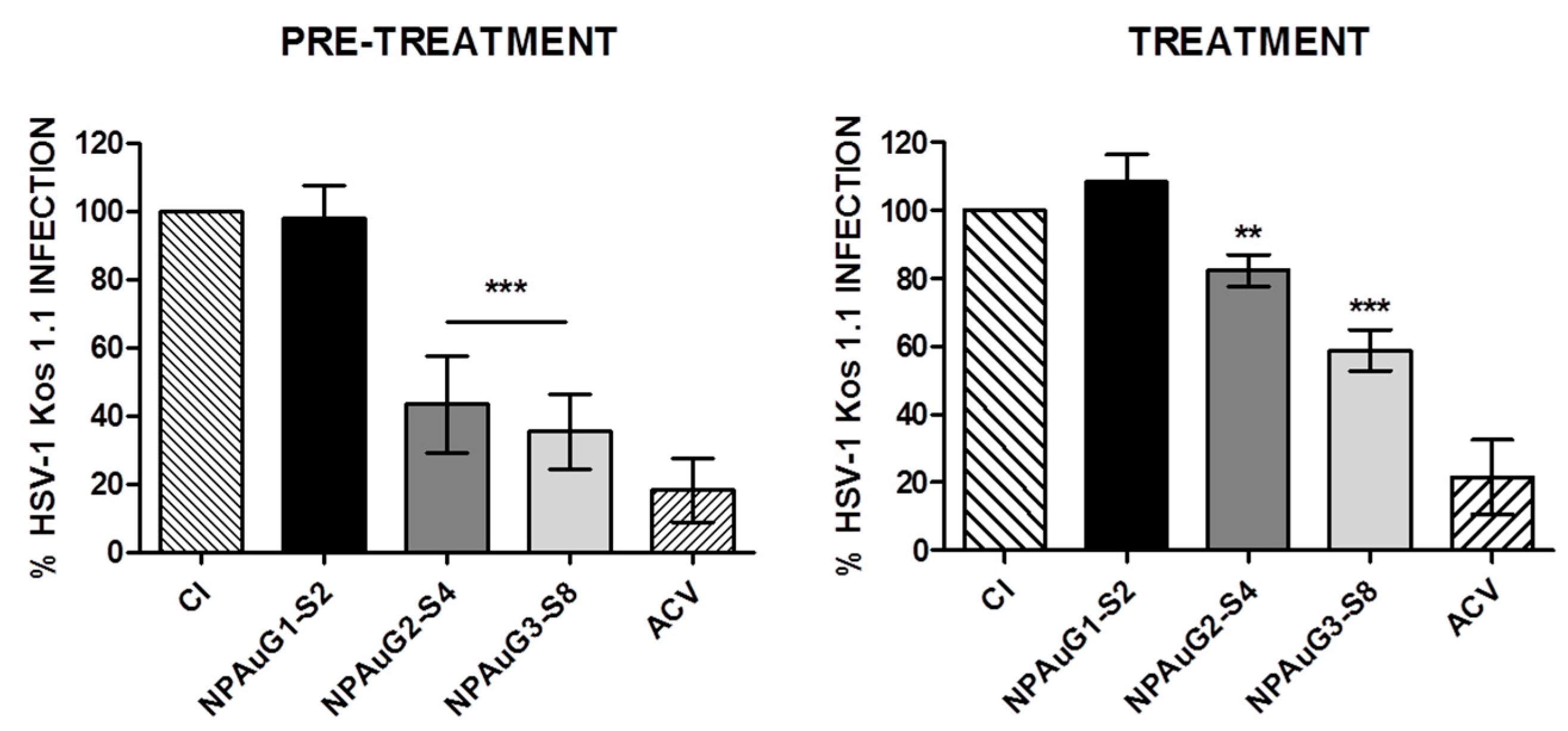
3.2. Anti-HSV-1 Activity of Nanoparticles
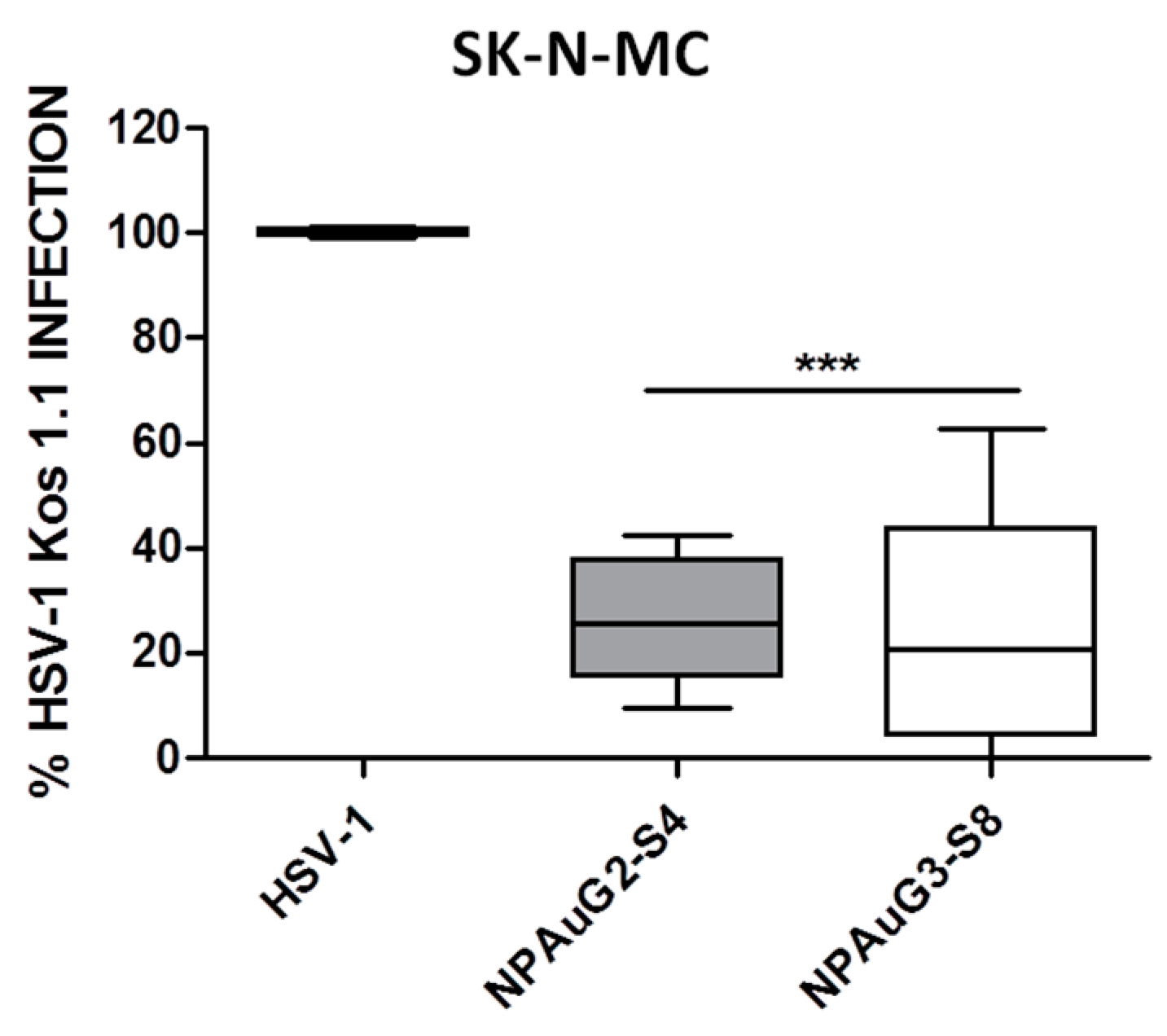
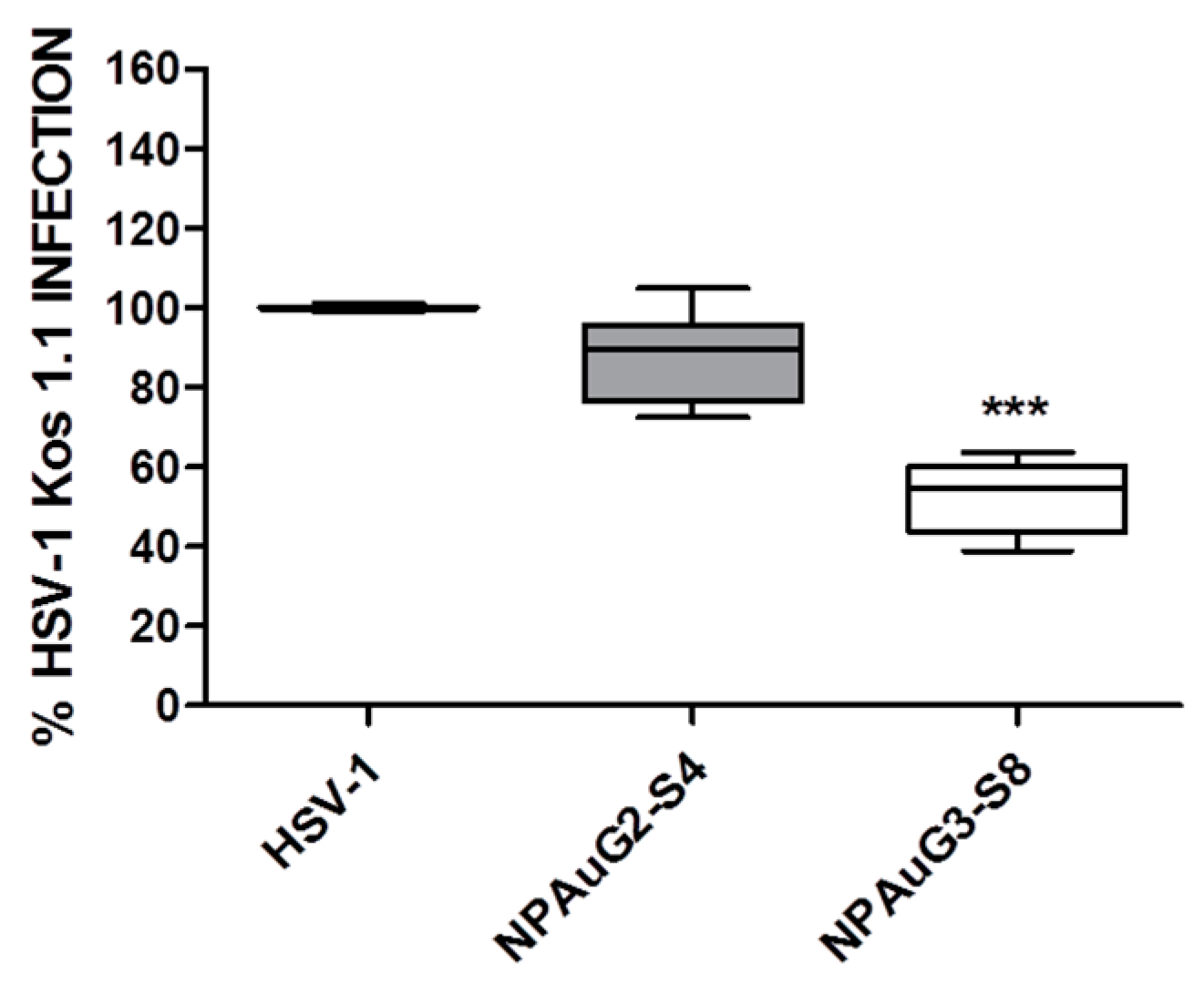
3.3. Gold Nanoparticles Inhibit HSV-1 Infection through Cellular Protection
3.4. Gold Nanoparticles Inhibit HSV-1 Infection through Viral Action
3.5. Nanoparticles Revert the β-Secretase Activity Increase Induced by HSV-1 to Basal Levels
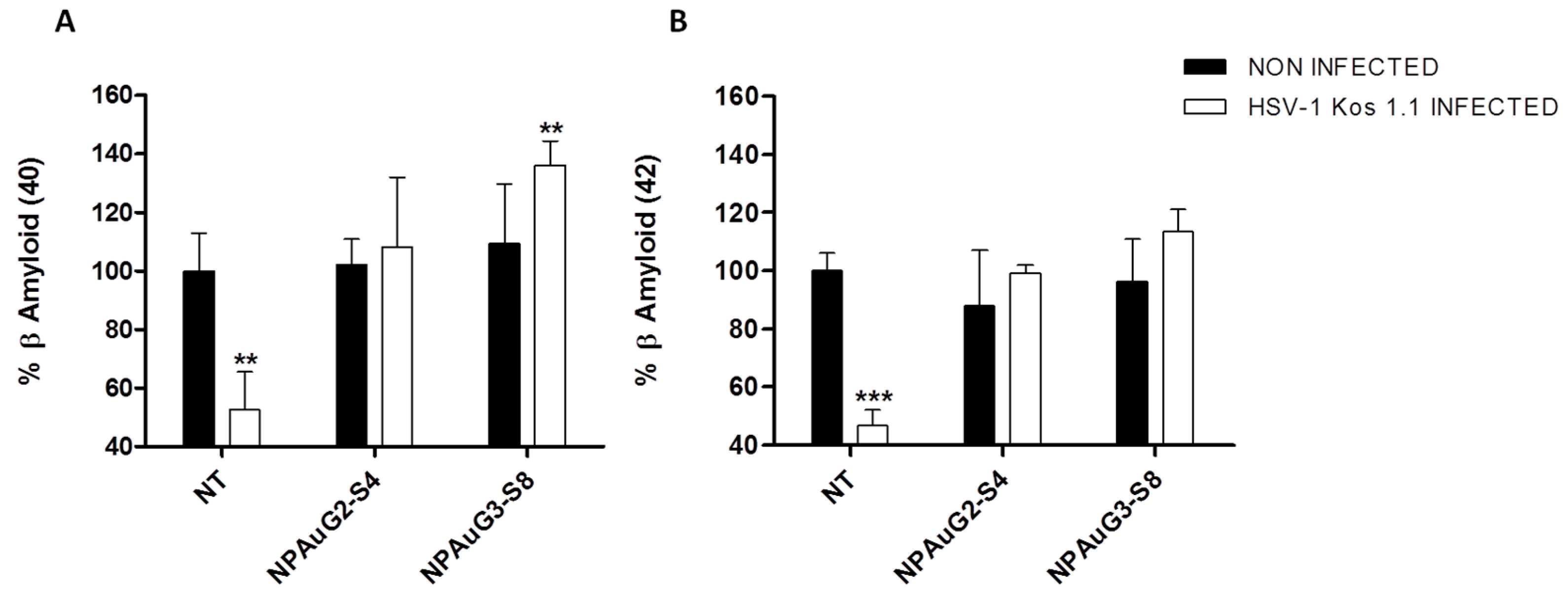
3.6. Effect of Nanoparticles on Secreted Aβ
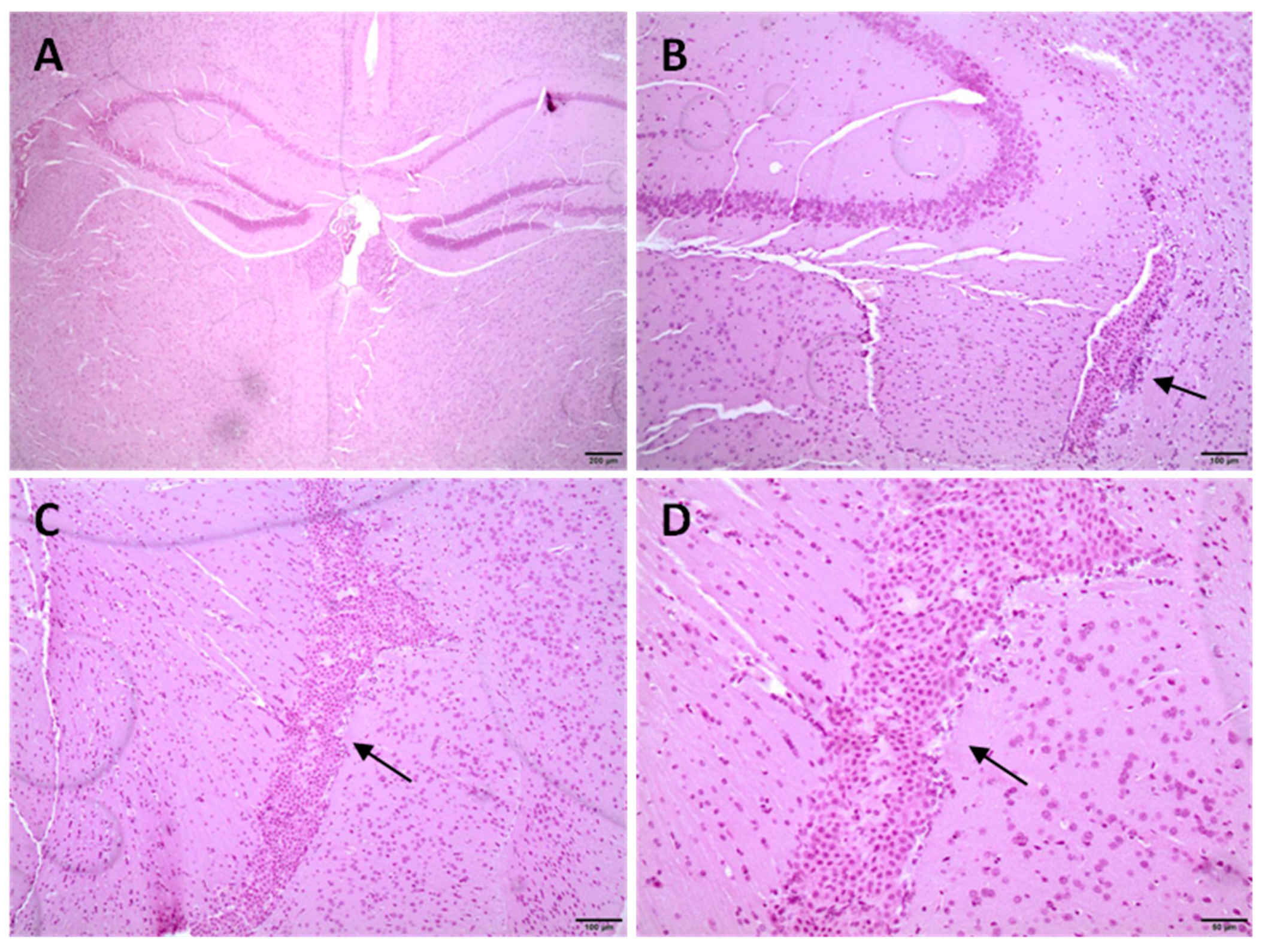
3.7. Histological Studies in CD1 Mice
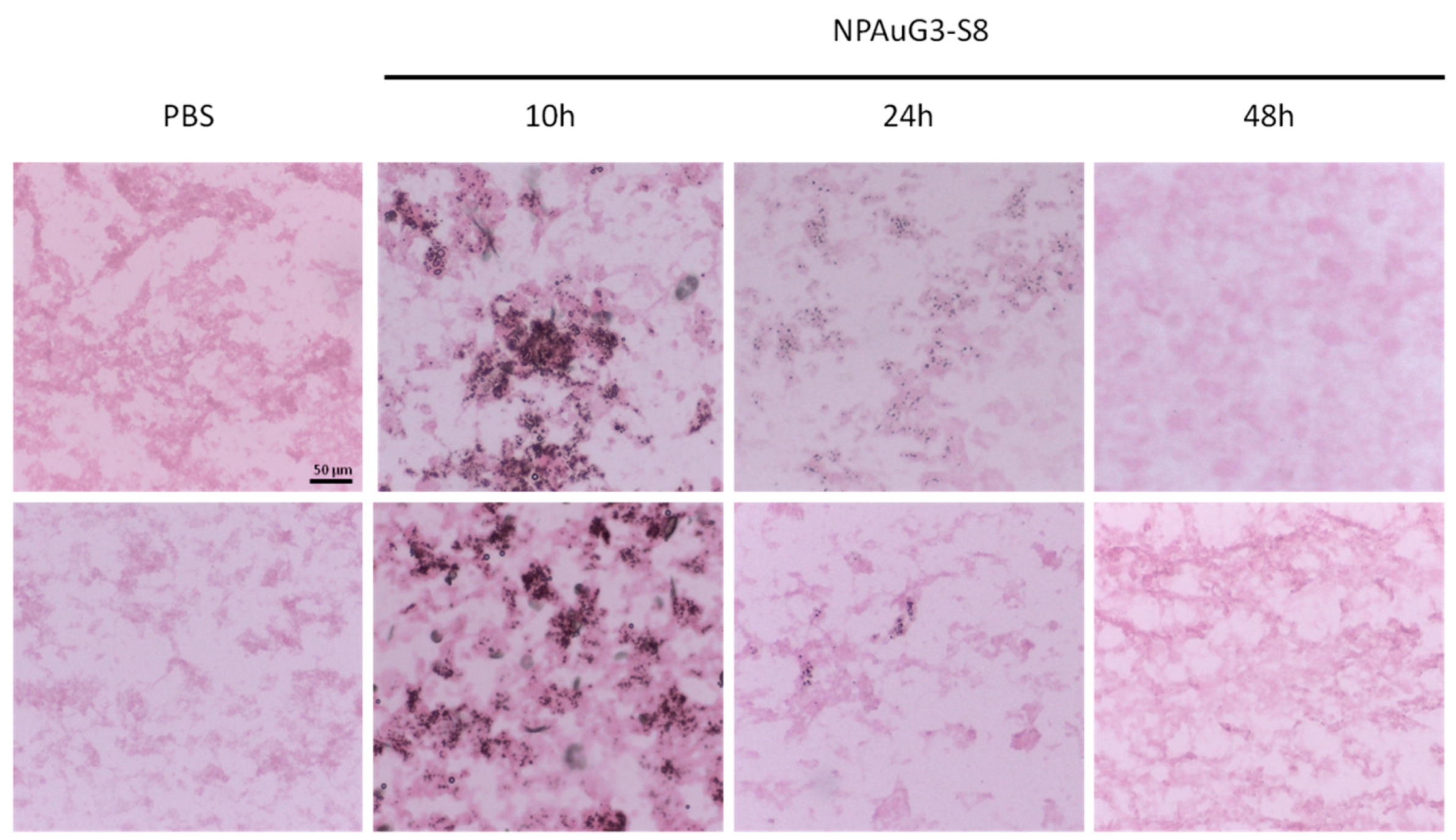
3.8. Capability of NPAuG3-S8 Crossing the Blood-Brain Barrier (BBB)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kennedy, P.G.; Rovnak, J.; Badani, H.; Cohrs, R.J. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J. Gen. Virol. 2015, 96, 1581–1602. [Google Scholar] [CrossRef]
- Nicoll, M.P.; Proenca, J.T.; Efstathiou, S. The molecular basis of herpes simplex virus latency. FEMS Microbiol. Rev. 2012, 36, 684–705. [Google Scholar] [CrossRef]
- Wagner, E.K.; Bloom, D.C. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 1997, 10, 419–443. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.L.; McArthur, J.C.; Venkatesan, A.; Nath, A. Atypical manifestations and poor outcome of herpes simplex encephalitis in the immunocompromised. Neurology 2012, 79, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, M.P.; Hann, W.; Shivkumar, M.; Harman, L.E.; Connor, V.; Coleman, H.M.; Proenca, J.T.; Efstathiou, S. The HSV-1 Latency-Associated Transcript Functions to Repress Latent Phase Lytic Gene Expression and Suppress Virus Reactivation from Latently Infected Neurons. PLoS Pathog. 2016, 12, e1005539. [Google Scholar] [CrossRef] [PubMed]
- Roizman, B.; Zhou, G. The 3 facets of regulation of herpes simplex virus gene expression: A critical inquiry. Virology 2015, 479, 562–567. [Google Scholar] [CrossRef]
- Zhou, L.; Miranda-Saksena, M.; Saksena, N.K. Viruses and neurodegeneration. Virol. J. 2013, 10, 172. [Google Scholar] [CrossRef]
- Harris, S.A.; Harris, E.A. Molecular Mechanisms for Herpes Simplex Virus Type 1 Pathogenesis in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 48. [Google Scholar] [CrossRef]
- McNamara, J.; Murray, T.A. Connections Between Herpes Simplex Virus Type 1 and Alzheimer’s Disease Pathogenesis. Curr. Alzheimer Res. 2016, 13, 996–1005. [Google Scholar] [CrossRef]
- Itzhaki, R.F.; Lathe, R.; Balin, B.J.; Ball, M.J.; Bearer, E.L.; Braak, H.; Bullido, M.J.; Carter, C.; Clerici, M.; Cosby, S.L.; et al. Microbes and Alzheimer’s Disease. J. Alzheimers Dis. 2016, 51, 979–984. [Google Scholar] [CrossRef]
- Itzhaki, R.F.; Lathe, R. Herpes Viruses and Senile Dementia: First Population Evidence for a Causal Link. J. Alzheimers Dis. 2018, 64, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Readhead, B.; Haure-Mirande, J.V.; Funk, C.C.; Richards, M.A.; Shannon, P.; Haroutunian, V.; Sano, M.; Liang, W.S.; Beckmann, N.D.; Price, N.D.; et al. Multiscale Analysis of Independent Alzheimer’s Cohorts Finds Disruption of Molecular, Genetic, and Clinical Networks by Human Herpesvirus. Neuron 2018, 99, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Eimer, W.A.; Kumar, D.K.V.; Shanmugam, N.K.N.; Rodriguez, A.S.; Mitchell, T.; Washicosky, K.J.; Gyorgy, B.; Breakefield, X.O.; Tanzi, R.E.; Moir, R.D. Alzheimer’s Disease-Associated beta-Amyloid Is Rapidly Seeded by Herpesviridae to Protect against Brain Infection. Neuron 2018, 99, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Gatz, M.; Reynolds, C.A.; Fratiglioni, L.; Johansson, B.; Mortimer, J.A.; Berg, S.; Fiske, A.; Pedersen, N.L. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 2006, 63, 168–174. [Google Scholar] [CrossRef]
- Mancuso, R.; Baglio, F.; Agostini, S.; Cabinio, M.; Lagana, M.M.; Hernis, A.; Margaritella, N.; Guerini, F.R.; Zanzottera, M.; Nemni, R.; et al. Relationship between herpes simplex virus-1-specific antibody titers and cortical brain damage in Alzheimer’s disease and amnestic mild cognitive impairment. Front. Aging Neurosci. 2014, 6, 285. [Google Scholar] [CrossRef]
- Lovheim, H.; Gilthorpe, J.; Adolfsson, R.; Nilsson, L.G.; Elgh, F. Reactivated herpes simplex infection increases the risk of Alzheimer’s disease. Alzheimers Dement. 2015, 11, 593–599. [Google Scholar] [CrossRef]
- Tanzi, R.E. The genetics of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006296. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- Andrew, R.J.; Kellett, K.A.; Thinakaran, G.; Hooper, N.M. A Greek Tragedy: The Growing Complexity of Alzheimer Amyloid Precursor Protein Proteolysis. J. Biol. Chem. 2016, 291, 19235–19244. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Berrocal, R.; Rao, K.S.; Durant, A.A. Polyphenols as therapeutic molecules in Alzheimer’s disease through modulating amyloid pathways. Mol. Neurobiol. 2015, 51, 466–479. [Google Scholar] [CrossRef]
- Marlow, L.; Cain, M.; Pappolla, M.A.; Sambamurti, K. Beta-secretase processing of the Alzheimer’s amyloid protein precursor (APP). J. Mol. Neurosci. 2003, 20, 233–239. [Google Scholar] [CrossRef]
- Santana, S.; Bullido, M.J.; Recuero, M.; Valdivieso, F.; Aldudo, J. Herpes simplex virus type I induces an incomplete autophagic response in human neuroblastoma cells. J. Alzheimers Dis. 2012, 30, 815–831. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, M.M.; Otte-Holler, I.; Fransen, J.A.; de Waal, R.M. Accumulation of the amyloid-beta precursor protein in multivesicular body-like organelles. J. Histochem. Cytochem. 2002, 50, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Santana, S.; Recuero, M.; Bullido, M.J.; Valdivieso, F.; Aldudo, J. Herpes simplex virus type I induces the accumulation of intracellular beta-amyloid in autophagic compartments and the inhibition of the non-amyloidogenic pathway in human neuroblastoma cells. Neurobiol. Aging 2012, 33, 430-e19. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, R.; Puma, D.D.L.; Ripoli, C.; Marcocci, M.E.; de Chiara, G.; Garaci, E.; Palamara, A.T.; Grassi, C. Herpes Simplex Virus type-1 infection induces synaptic dysfunction in cultured cortical neurons via GSK-3 activation and intraneuronal amyloid-beta protein accumulation. Sci. Rep. 2015, 5, 15444. [Google Scholar] [CrossRef]
- Alvarez, G.; Aldudo, J.; Alonso, M.; Santana, S.; Valdivieso, F. Herpes simplex virus type 1 induces nuclear accumulation of hyperphosphorylated tau in neuronal cells. J. Neurosci. Res. 2012, 90, 1020–1029. [Google Scholar] [CrossRef]
- Lin, W.R.; Wozniak, M.A.; Cooper, R.J.; Wilcock, G.K.; Itzhaki, R.F. Herpesviruses in brain and Alzheimer’s disease. J. Pathol. 2002, 197, 395–402. [Google Scholar] [CrossRef]
- Coen, D.M.; Schaffer, P.A. Antiherpesvirus drugs: A promising spectrum of new drugs and drug targets. Nat. Rev. Drug Discov. 2003, 2, 278–288. [Google Scholar] [CrossRef]
- Superti, F.; Ammendolia, M.G.; Marchetti, M. New advances in anti-HSV chemotherapy. Curr. Med. Chem. 2008, 15, 900–911. [Google Scholar] [CrossRef]
- Burrel, S.; Boutolleau, D.; Azar, G.; Doan, S.; Deback, C.; Cochereau, I.; Agut, H.; Gabison, E.E. Phenotypic and genotypic characterization of acyclovir-resistant corneal HSV-1 isolates from immunocompetent patients with recurrent herpetic keratitis. J. Clin. Virol. 2013, 58, 321–324. [Google Scholar] [CrossRef]
- van Velzen, M.; van Loenen, F.B.; Meesters, R.J.; de Graaf, M.; Remeijer, L.; Luider, T.M.; Osterhaus, A.D.; Verjans, G.M. Latent acyclovir-resistant herpes simplex virus type 1 in trigeminal ganglia of immunocompetent individuals. J. Infect. Dis. 2012, 205, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Ellis, M.N.; Hackman, R.C.; Shepp, D.H.; Meyers, J.D. Acyclovir-resistant herpes simplex virus causing pneumonia after marrow transplantation. J. Infect. Dis. 1990, 162, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Briz, V.; Sepulveda-Crespo, D.; Diniz, A.R.; Borrego, P.; Rodes, B.; de la Mata, F.J.; Gomez, R.; Taveira, N.; Munoz-Fernandez, M.A. Development of water-soluble polyanionic carbosilane dendrimers as novel and highly potent topical anti-HIV-2 microbicides. Nanoscale 2015, 7, 14669–14683. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltran, C.; Cena-Diez, R.; Sepulveda-Crespo, D.; de la Mata, J.; Gomez, R.; Leal, M.; Munoz-Fernandez, M.A.; Jimenez, J.L. Carbosilane dendrons with fatty acids at the core as a new potential microbicide against HSV-2/HIV-1 co-infection. Nanoscale 2017, 9, 17263–17273. [Google Scholar] [CrossRef] [PubMed]
- Cena-Diez, R.; Vacas-Cordoba, E.; Garcia-Broncano, P.; de la Mata, F.J.; Gomez, R.; Maly, M.; Munoz-Fernandez, M.A. Prevention of vaginal and rectal herpes simplex virus type 2 transmission in mice: Mechanism of antiviral action. Int. J. Nanomed. 2016, 11, 2147–2162. [Google Scholar]
- Garcia-Broncano, P.; Cena-Diez, R.; de la Mata, F.J.; Gomez, R.; Resino, S.; Munoz-Fernandez, M.A. Efficacy of carbosilane dendrimers with an antiretroviral combination against HIV-1 in the presence of semen-derived enhancer of viral infection. Eur. J. Pharmacol. 2017, 811, 155–163. [Google Scholar] [CrossRef]
- Cena-Diez, R.; Garcia-Broncano, P.; de la Mata, F.J.; Gomez, R.; Munoz-Fernandez, M.A. Efficacy of HIV antiviral polyanionic carbosilane dendrimer G2-S16 in the presence of semen. Int. J. Nanomed. 2016, 11, 2443–2450. [Google Scholar]
- Vacas-Cordoba, E.; Maly, M.; de la Mata, F.J.; Gomez, R.; Pion, M.; Munoz-Fernandez, M.A. Antiviral mechanism of polyanionic carbosilane dendrimers against HIV-1. Int. J. Nanomed. 2016, 11, 1281–1294. [Google Scholar]
- Bermejo, J.F.; Ortega, P.; Chonco, L.; Eritja, R.; Samaniego, R.; Mullner, M.; de Jesus, E.; de la Mata, F.J.; Flores, J.C.; Gomez, R.; et al. Water-soluble carbosilane dendrimers: Synthesis biocompatibility and complexation with oligonucleotides; evaluation for medical applications. Chemistry 2007, 13, 483–495. [Google Scholar] [CrossRef]
- Sepulveda-Crespo, D.; Sanchez-Rodriguez, J.; Serramia, M.J.; Gomez, R.; de la Mata, F.J.; Jimenez, J.L.; Munoz-Fernandez, M.A. Triple combination of carbosilane dendrimers, tenofovir and maraviroc as potential microbicide to prevent HIV-1 sexual transmission. Nanomedicine 2015, 10, 899–914. [Google Scholar] [CrossRef]
- Sepulveda-Crespo, D.; Serramia, M.J.; Tager, A.M.; Vrbanac, V.; Gomez, R.; de la Mata, F.J.; Jimenez, J.L.; Munoz-Fernandez, M.A. Prevention vaginally of HIV-1 transmission in humanized BLT mice and mode of antiviral action of polyanionic carbosilane dendrimer G2-S16. Nanomedicine 2015, 11, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Hormozi-Nezhad, M.R.; Mahmoudi, M. Label-free detection of beta-amyloid peptides (Abeta40 and Abeta42): A colorimetric sensor array for plasma monitoring of Alzheimer’s disease. Nanoscale 2018, 10, 6361–6368. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Liu, Q.; Wang, S.; Ren, Z.; Kitazato, K.; Yang, D.; Wang, Y. HSV-1-encoded microRNA miR-H1 targets Ubr1 to promote accumulation of neurodegeneration-associated protein. Virus Genes 2018, 54, 343–350. [Google Scholar] [CrossRef] [PubMed]
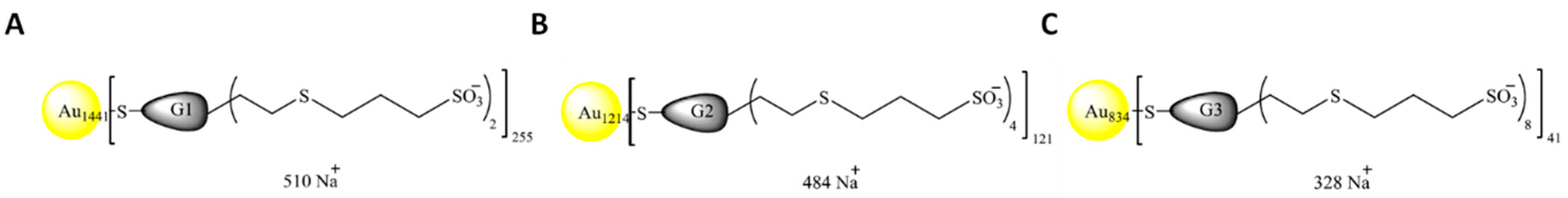

| Nomenclature | Nuclei | Generation | Chargues N | Funtional Group | Molecular Weight (g/mol) |
|---|---|---|---|---|---|
| NPAuG1-S2 | GOLD | 1 | 2 | SULFONATE | 421978 |
| NPAuG2-S4 | GOLD | 2 | 4 | SULFONATE | 374956 |
| NPAuG3-S8 | GOLD | 3 | 8 | SULFONATE | 257929 |
| PBS | 10 H | 24 H | 48 H | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mouse ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| TELENCEPHALON | ||||||||||||||||||||||||
| Microglia infiltration | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| satellitism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| vacuolization | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| necrosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| DILENCEPHAL | ||||||||||||||||||||||||
| microgliainfiltration | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| satellitism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| vacuolization | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| necrosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| MIDBRAIN | ||||||||||||||||||||||||
| microgliainfiltration | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| satellitism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| vacuolization | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| necrosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CEREBELLUM | ||||||||||||||||||||||||
| microgliainfiltration | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| satellitism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| vacuolization | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| necrosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
I, R.-I.; MJ, S.; R, G.; FJ, D.L.M.; MJ, B.; MA, M.-F. Gold Nanoparticles Crossing Blood-Brain Barrier Prevent HSV-1 Infection and Reduce Herpes Associated Amyloid-βsecretion. J. Clin. Med. 2020, 9, 155. https://doi.org/10.3390/jcm9010155
I R-I, MJ S, R G, FJ DLM, MJ B, MA M-F. Gold Nanoparticles Crossing Blood-Brain Barrier Prevent HSV-1 Infection and Reduce Herpes Associated Amyloid-βsecretion. Journal of Clinical Medicine. 2020; 9(1):155. https://doi.org/10.3390/jcm9010155
Chicago/Turabian StyleI, Rodriguez-Izquierdo, Serramia MJ, Gomez R, De La Mata FJ, Bullido MJ, and Muñoz-Fernández MA. 2020. "Gold Nanoparticles Crossing Blood-Brain Barrier Prevent HSV-1 Infection and Reduce Herpes Associated Amyloid-βsecretion" Journal of Clinical Medicine 9, no. 1: 155. https://doi.org/10.3390/jcm9010155
APA StyleI, R.-I., MJ, S., R, G., FJ, D. L. M., MJ, B., & MA, M.-F. (2020). Gold Nanoparticles Crossing Blood-Brain Barrier Prevent HSV-1 Infection and Reduce Herpes Associated Amyloid-βsecretion. Journal of Clinical Medicine, 9(1), 155. https://doi.org/10.3390/jcm9010155