The Effect of a Transdermal Scopolamine Patch on Postoperative Nausea and Vomiting after Retromastoid Craniectomy with Microvascular Decompression: A Preliminary Single Center, Double-Blind, Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
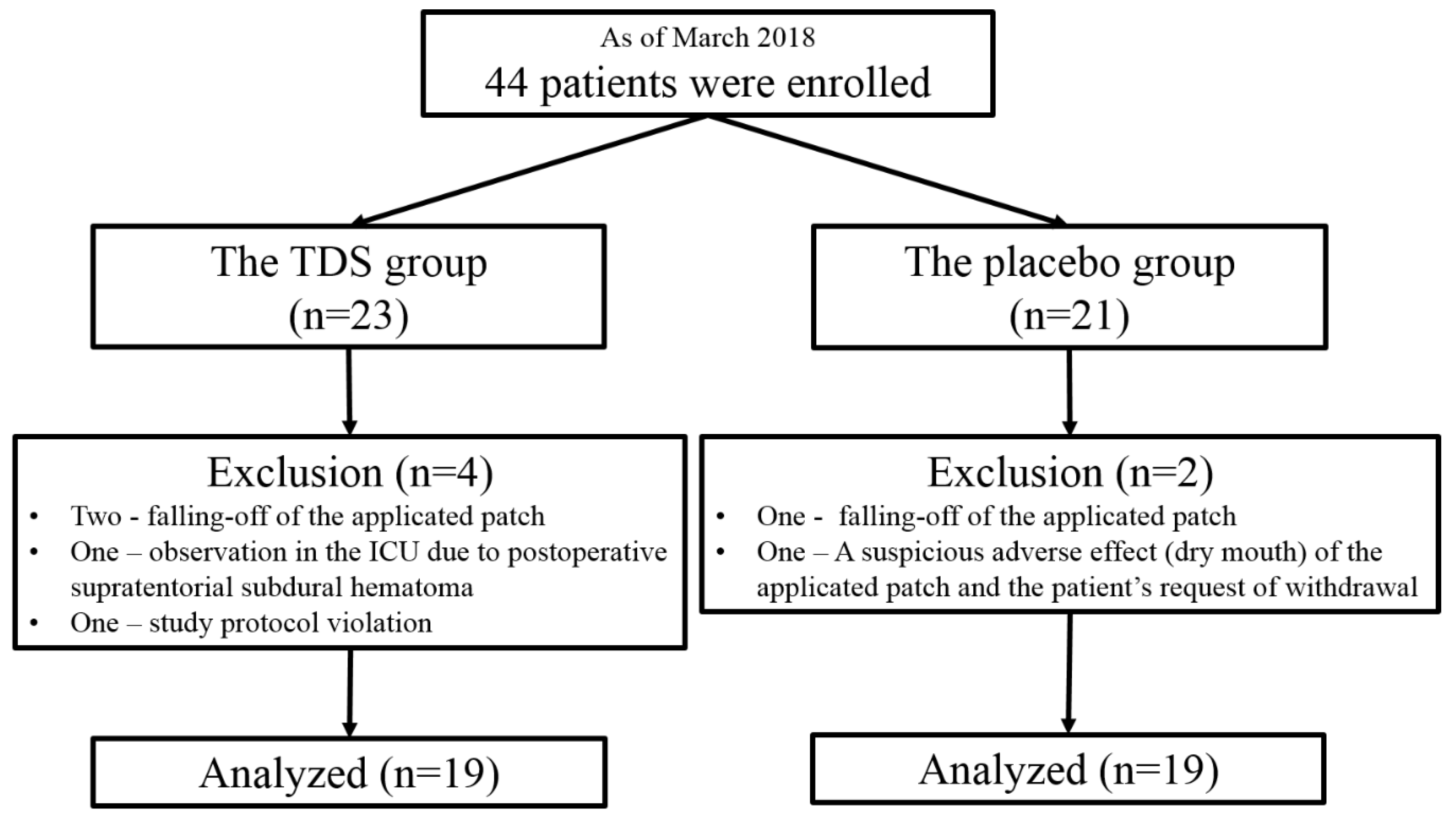
2.1. Patients
2.2. Anesthesia
2.3. Surgery of RMC-MVD
2.4. Outcome Measurements and Statistical Analyses
3. Results
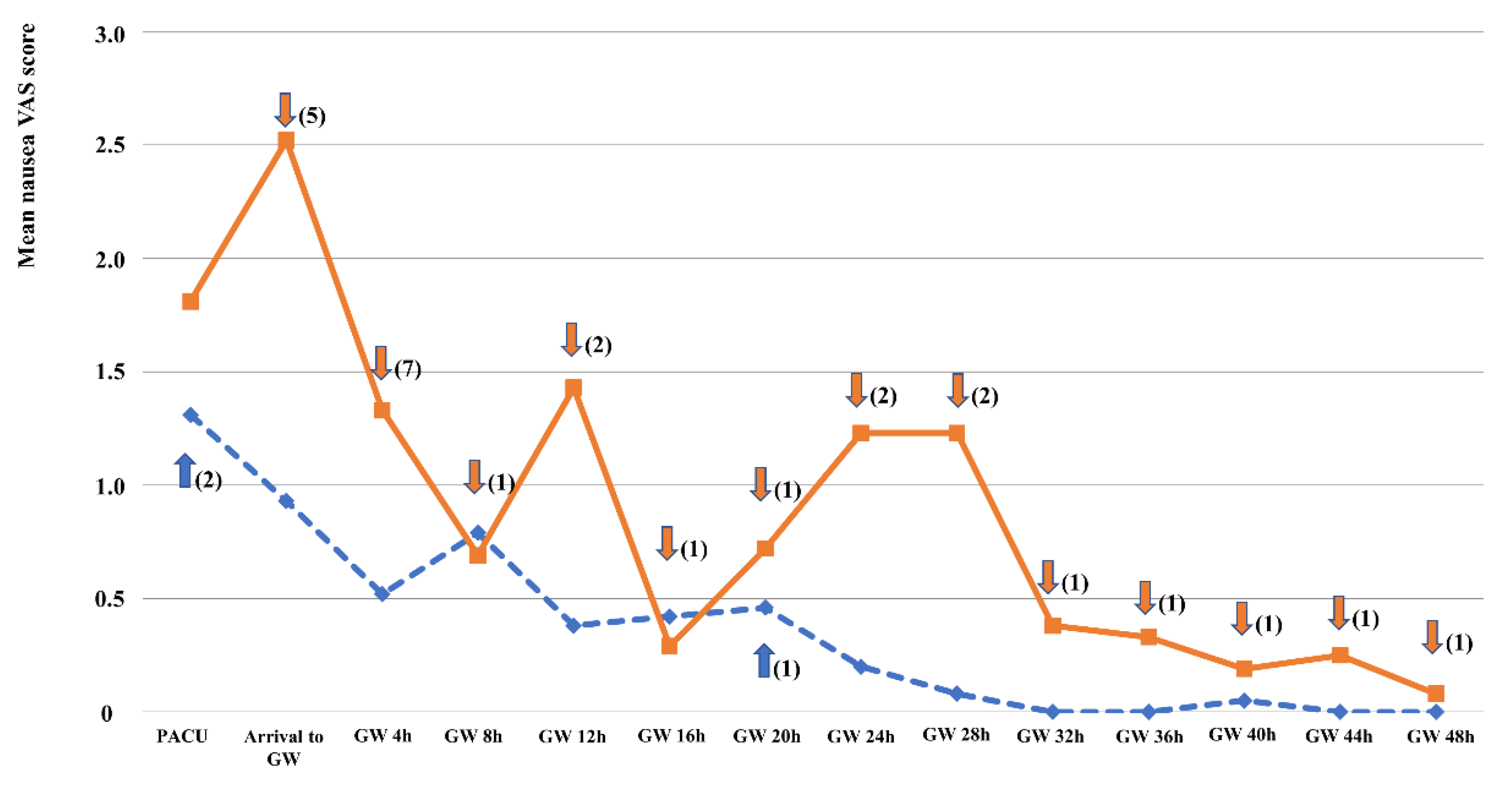
3.1. The Incidence and Severity of PONV
3.2. The Use of Rescue Antiemetics and Side Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergese, S.D.; Antor, M.A.; Uribe, A.A.; Yildiz, V.; Werner, J. Triple Therapy with Scopolamine, Ondansetron, and Dexamethasone for Prevention of Postoperative Nausea and Vomiting in Moderate to High-Risk Patients Undergoing Craniotomy Under General Anesthesia: A Pilot Study. Front. Med. 2015, 2, 40. [Google Scholar] [CrossRef] [PubMed]
- Kovac, A.L. Prevention and treatment of postoperative nausea and vomiting. Drugs 2000, 59, 213–243. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, J.E.; Lim, Y.J.; Hong, D.M.; Park, H.P.; Han, J.I. A prospective, randomized, double-blind, and multicenter trial of prophylactic effects of ramosetronon postoperative nausea and vomiting (PONV) after craniotomy: Comparison with ondansetron. BMC Anesthesiol. 2014, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, S.M.; Newburn-Cook, C.V. The efficacy of 5-HT3 receptor antagonists for the prevention of postoperative nausea and vomiting after craniotomy: A meta-analysis. J. Neurosurg. Anesthesiol. 2007, 19, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.V., Jr.; Philip, B.K.; Leslie, J.B.; Taylor, R., Jr.; Raffa, R.B. Perspectives on transdermal scopolamine for the treatment of postoperative nausea and vomiting. J. Clin. Anesth. 2012, 24, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Diemunsch, P.; Habib, A.S.; Kovac, A.; Kranke, P.; Meyer, T.A. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth. Analg. 2014, 118, 85–113. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sai, S.; Adachi, T. Is microvascular decompression surgery a high risk for postoperative nausea and vomiting in patients undergoing craniotomy? J. Anesth. 2013, 27, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Barker, F.G., 2nd; Jannetta, P.J.; Bissonette, D.J.; Larkins, M.V.; Jho, H.D. The long-term outcome of microvascular decompression for trigeminal neuralgia. N. Engl. J. Med. 1996, 334, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Miller, V.M. Safety and effectiveness of microvascular decompression for treatment of hemifacial spasm: A systematic review. Br. J. Neurosurg. 2012, 26, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Quinlan, J.J. Assessing risk factors for postoperative nausea and vomiting: A retrospective study in patients undergoing retromastoid craniectomy with microvascular decompression of cranial nerves. J. Neurosurg. Anesthesiol. 2006, 18, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Antor, M.A.; Uribe, A.A.; Erminy-Falcon, N.; Werner, J.G.; Candiotti, K.A.; Pergolizzi, J.V. The effect of transdermal scopolamine for the prevention of postoperative nausea and vomiting. Front. Pharmacol. 2014, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Han, J.H.; Kim, C.Y.; Oh, C.W. Closed-suction drainage and cerebrospinal fluid leakage following microvascular decompression: A retrospective comparison study. J. Korean Neurosurg. Soc. 2013, 54, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Korttila, K.; Abdalla, M.; Kerger, H.; Turan, A.; Vedder, I. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N. Engl. J. Med. 2004, 350, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Kapur, P.A. The big “little problem”. Anesth. Analg. 1991, 73, 243–245. [Google Scholar] [PubMed]
- Macario, A.; Weinger, M.; Carney, S.; Kim, A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth. Analg. 1999, 89, 652–658. [Google Scholar] [PubMed]
| TDS Group (n = 19) N (%) / Mean ± SD | Placebo Group (n = 19) N (%) / Mean ± SD | p Value | ||
|---|---|---|---|---|
| Sex | Male | 9 (47.4) | 3 (15.8) | 0.079 § |
| Female | 10 (52.6) | 16 (84.2) | ||
| Age (year) | 46.63 ± 11.02 | 53.63 ± 8.780 | 0.037 | |
| HDCN syndrome * | HFS ¶ | 16 (84.2) | 16 (84.2) | 1.00 § |
| TN ¶¶ | 3 (15.8) | 3 (15.8) | ||
| Symptom duration (mo) | 38.26 ± 32.62 | 52.66 ± 37.68 | 0.216 § | |
| History of previous surgery | Yes | 13 (68.4) | 12 (63.2) | 1.00 § |
| No | 6 (31.6) | 7 (36.8) | ||
| History of PONV † | Yes | 3 (15.8) | 0 | 0.220 § |
| No | 10 (52.6) | 12 (63.2) | ||
| Smoking | Yes | 3 (15.8) | 2 (10.5) | 1.00 § |
| No | 16 (84.2) | 17 (89.5) | ||
| History of Motion sickness | Yes | 9 (47.4) | 7 (36.8) | 0.743 § |
| No | 10 (52.6) | 12 (63.2) | ||
| GI disturbance | Yes | 4 (21.1) | 3 (15.8) | 1.00 § |
| No | 15 (78.9) | 16 (84.2) | ||
| ASA ‡ physical status | I | 14 (73.7) | 12 (63.2) | 0.728 § |
| II | 5 (26.3) | 7 (36.8) | ||
| Operation time (min) | 90.37 ± 23.52 | 83.16 ± 14.83 | 0.266 | |
| Anesthetic agents | Propofol (mL) | 87.06 ± 23.85 | 74.72 ± 18.11 | 0.093 |
| Fentanyl (µg) | 1214.71 ± 441.50 | 1133.33 ± 280.76 | 0.517 | |
| Postoperative opioids | Yes | 10 (52.6) | 16 (84.2) | 0.079 § |
| No | 9 (47.4) | 3 (15.8) | ||
| Mean Opioid MME | 2.75 ± 0.79 | 2.66 ± 0.63 | 0.740 | |
| TDS Group (n = 19) Mean ± SD | Placebo Group (n = 19) Mean ± SD | p Value | ||
|---|---|---|---|---|
| PACU* | Nausea VAS | 1.31 ± 2.22 | 1.81 ± 2.16 | 0.487 |
| Vomiting | 0 | 0.05 ± 0.23 | 0.331 | |
| Arrival to general wards | Nausea VAS | 0.93 ± 1.71 | 2.52 ± 2.85 | 0.046 |
| Vomiting | 0 | 0.05 ± 0.23 | 0.331 | |
| GW 4 h† | Nausea VAS | 0.52 ± 1.00 | 1.33 ± 2.12 | 0.114 |
| Vomiting | 0 | 0.11 ± 0.32 | 0.163 | |
| GW 8 h | Nausea VAS | 0.79 ± 1.39 | 0.69 ± 1.16 | 0.821 |
| Vomiting | 0.05 ± 0.23 | 0.16 ± 0.37 | 0.305 | |
| GW 12 h | Nausea VAS | 0.38 ± 0.81 | 1.43 ± 3.02 | 0.162 |
| Vomiting | 0 | 0.11 ± 0.46 | 0.331 | |
| GW 16 h | Nausea VAS | 0.42 ± 0.89 | 0.29 ± 0.52 | 0.596 |
| Vomiting | 0 | 0 | - | |
| GW 20 h | Nausea VAS | 0.46 ± 1.18 | 0.72 ± 1.70 | 0.590 |
| Vomiting | 0.05 + 0.23 | 0 | 0.331 | |
| GW 24 h | Nausea VAS | 0.20 ± 0.39 | 1.23 ± 2.18 | 0.057 |
| Vomiting | 0 | 0.05 ± 0.23 | 0.331 | |
| GW 28 h | Nausea VAS | 0.08 ± 0.25 | 1.23 ± 2.32 | 0.051 |
| Vomiting | 0 | 0 | - | |
| GW 32 h | Nausea VAS | 0 | 0.38 ± 0.76 | 0.050 |
| Vomiting | 0 | 0 | - | |
| GW 36 h | Nausea VAS | 0 | 0.33 ± 0.97 | 0.163 |
| Vomiting | 0 | 0.06 ± 0.24 | 0.331 | |
| GW 40 h | Nausea VAS | 0.05 ± 0.15 | 0.19 ± 0.52 | 0.261 |
| Vomiting | 0 | 0 | - | |
| GW 44 h | Nausea VAS | 0 | 0.25 ± 0.58 | 0.104 |
| Vomiting | 0 | 0 | - | |
| GW 48 h | Nausea VAS | 0 | 0.08 ± 0.19 | 0.165 |
| Vomiting | 0 | 0 | - | |
| TDS Group (n = 19) N (%) / Mean ± SD | Placebo Group (n = 19) N (%) / Mean ± SD | p Value | |||
|---|---|---|---|---|---|
| Within GW 4 h* | Use of rescue antiemetics | Yes | 1 (5.3) | 8 (42.1) | 0.019 |
| No | 18 (94.7) | 11 (57.9) | |||
| Total No. of use of rescue antiemetics | 0.11 ± 0.46 | 0.63 ± 0.83 | 0.022 | ||
| Within GW 24 h | Use of rescue antiemetics | Yes | 2 (10.5) | 9 (47.4) | 0.029 |
| No | 17 (89.5) | 10 (52.6) | |||
| Total No. of use of rescue antiemetics | 0.16 ± 0.50 | 1.00 ± 1.49 | 0.029 | ||
| Within GW 48 h | Use of rescue antiemetics | Yes | 2 (10.5) | 9 (47.4) | 0.029 |
| No | 17 (89.5) | 10 (52.6) | |||
| Total No. of use of rescue antiemetics | 0.16 ± 0.50 | 1.37 ± 2.19 | 0.029 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.H.; Kim, H.-M.; Lee, J.E.; Jeon, Y.-T.; Park, S.; Hwang, K.; Han, J.H. The Effect of a Transdermal Scopolamine Patch on Postoperative Nausea and Vomiting after Retromastoid Craniectomy with Microvascular Decompression: A Preliminary Single Center, Double-Blind, Randomized Controlled Trial. J. Clin. Med. 2020, 9, 156. https://doi.org/10.3390/jcm9010156
Lee HH, Kim H-M, Lee JE, Jeon Y-T, Park S, Hwang K, Han JH. The Effect of a Transdermal Scopolamine Patch on Postoperative Nausea and Vomiting after Retromastoid Craniectomy with Microvascular Decompression: A Preliminary Single Center, Double-Blind, Randomized Controlled Trial. Journal of Clinical Medicine. 2020; 9(1):156. https://doi.org/10.3390/jcm9010156
Chicago/Turabian StyleLee, Hyun Hee, Hyun-Mi Kim, Ji Eun Lee, Young-Tae Jeon, Sanghon Park, Kihwan Hwang, and Jung Ho Han. 2020. "The Effect of a Transdermal Scopolamine Patch on Postoperative Nausea and Vomiting after Retromastoid Craniectomy with Microvascular Decompression: A Preliminary Single Center, Double-Blind, Randomized Controlled Trial" Journal of Clinical Medicine 9, no. 1: 156. https://doi.org/10.3390/jcm9010156
APA StyleLee, H. H., Kim, H.-M., Lee, J. E., Jeon, Y.-T., Park, S., Hwang, K., & Han, J. H. (2020). The Effect of a Transdermal Scopolamine Patch on Postoperative Nausea and Vomiting after Retromastoid Craniectomy with Microvascular Decompression: A Preliminary Single Center, Double-Blind, Randomized Controlled Trial. Journal of Clinical Medicine, 9(1), 156. https://doi.org/10.3390/jcm9010156





