Patient-Derived Colorectal Cancer Organoids Upregulate Revival Stem Cell Marker Genes following Chemotherapeutic Treatment
Abstract
1. Introduction
2. Experimental Section
2.1. Ethics and Consent
2.2. Patient Data
2.3. Establishing Colorectal Cancer Organoids
2.4. Organoid Drug Sensitivity Testing
2.5. Histological Sections
2.6. Immunohistochemistry
2.7. Quantitative RT–PCR Analysis
2.8. Survival Analysis
3. Results
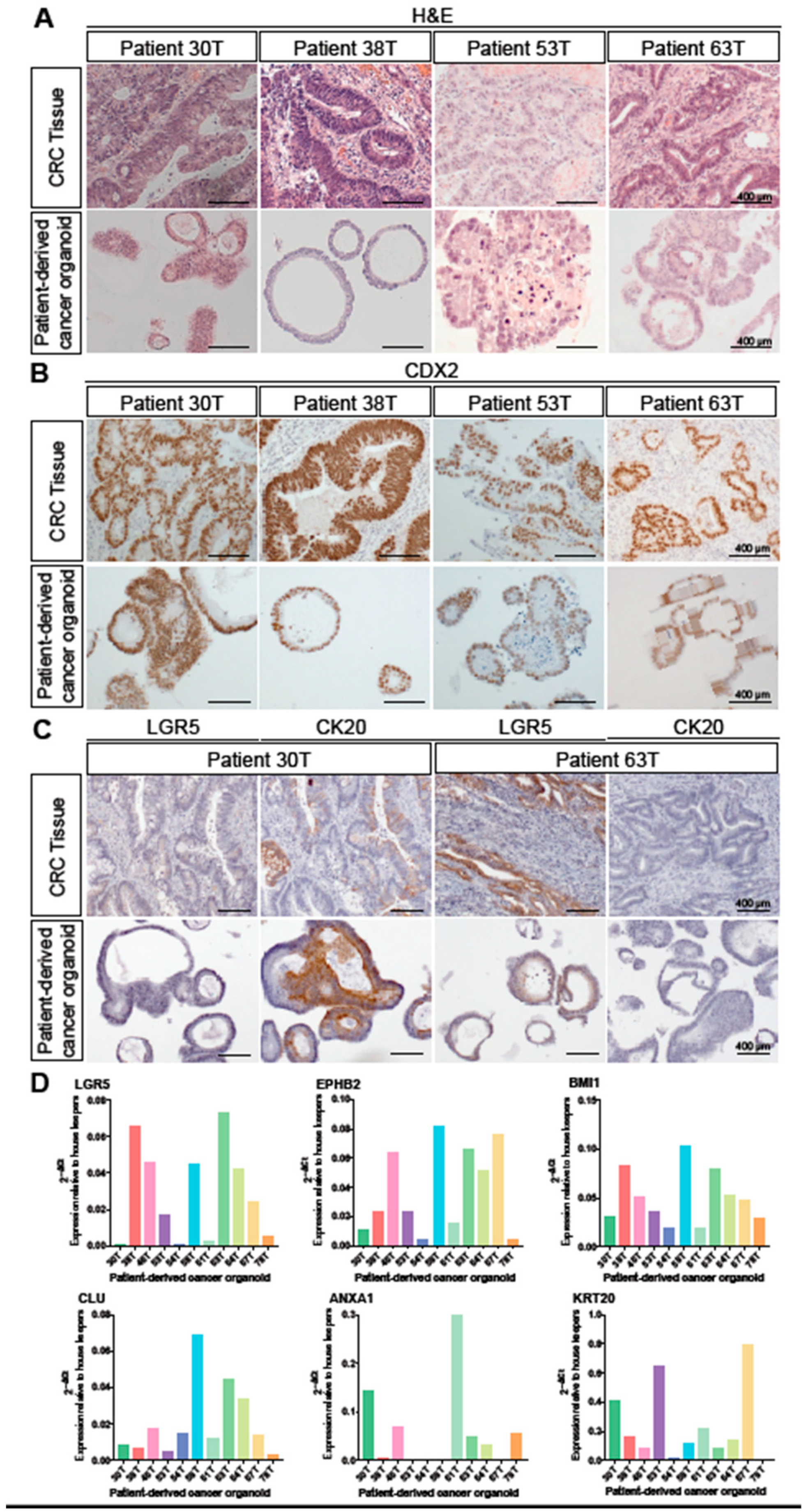
3.1. Patient-Derived Colorectal Cancer Organoids Recapitulate the Histopathological Characteristics of Their Primary Tumours and Display Inter-Tumoural Heterogeneity in Stem Cell Signatures
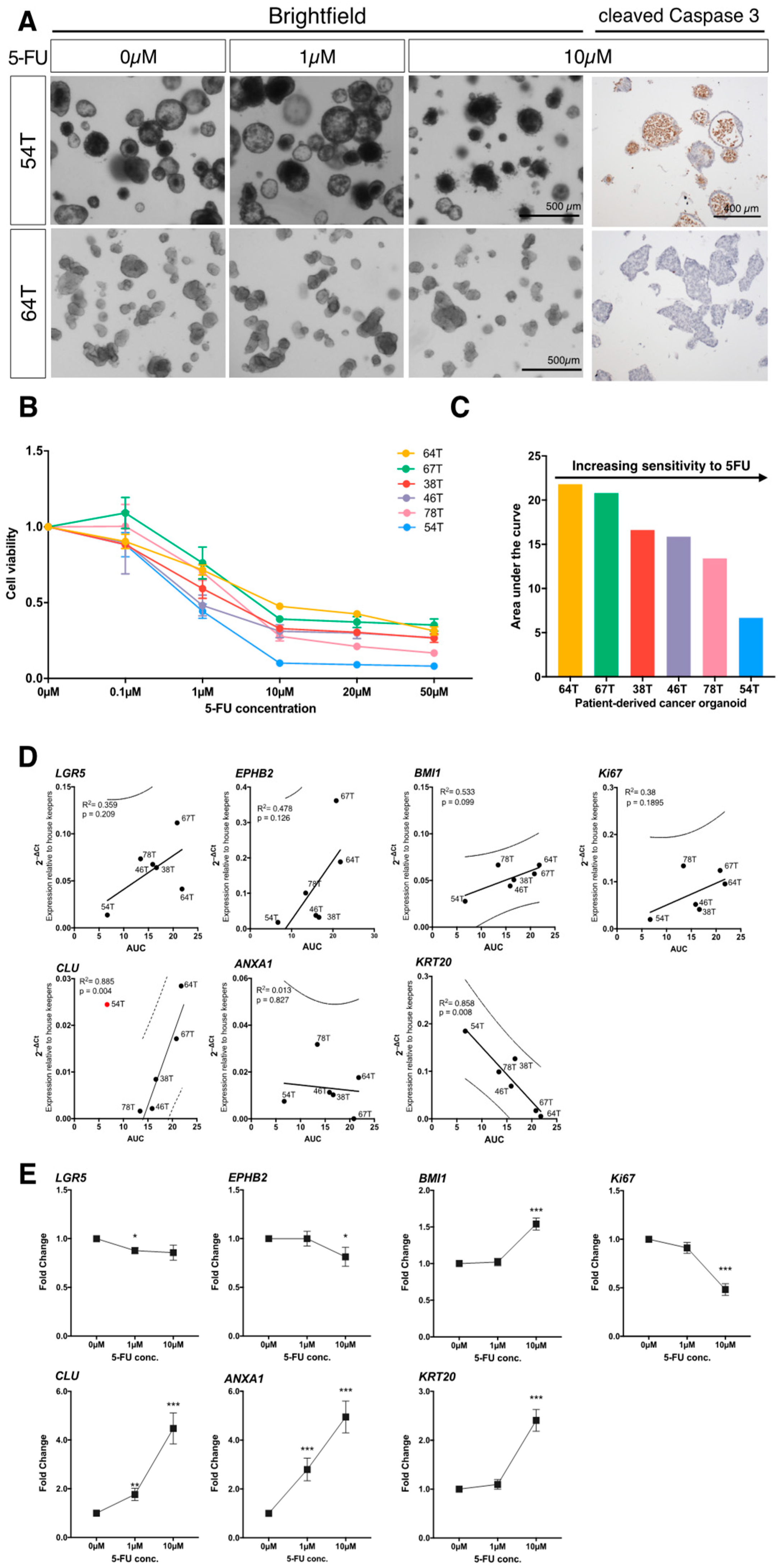
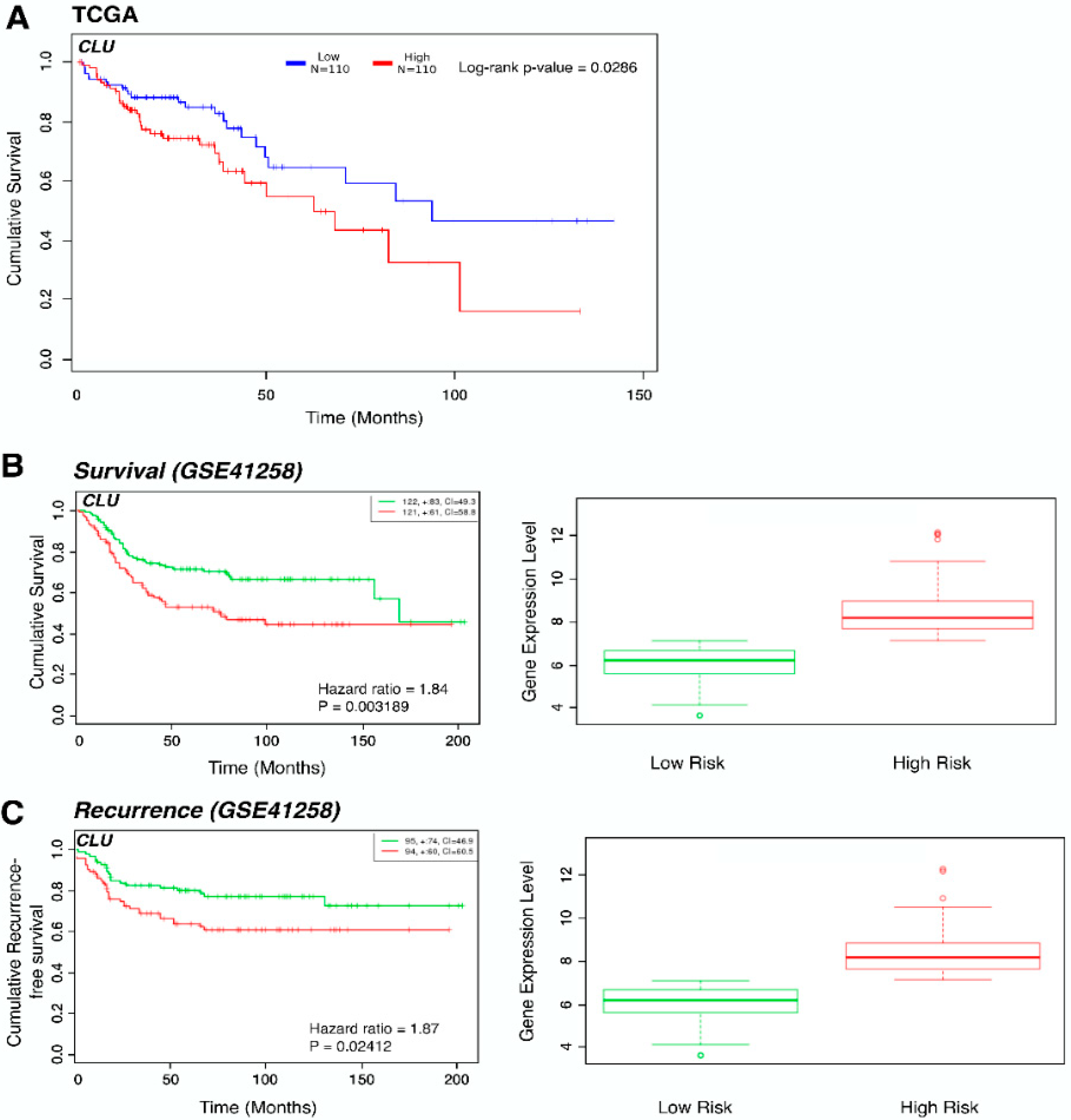
3.2. Elevated Expression of a Subset of Stem Cell Markers Correlates with Resistance to Chemotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Are, C. Current Status and Future Directions in Colorectal Cancer. Indian J. Surg Oncol. 2017, 8, 455–456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lintoiu-Ursut, B.; Tulin, A.; Constantinoiu, S. Recurrence after hepatic resection in colorectal cancer liver metastasis—Review article. J. Med. Life 2015, 8, 12–14. [Google Scholar] [PubMed]
- Regnard, J.-F.; Grunenwald, D.; Spaggiari, L.; Girard, P.; Elias, D.; Ducreux, M.; Baldeyrou, P.; Levasseur, P. Surgical treatment of hepatic and pulmonary metastases from colorectal cancers. Ann. Thorac. Surg. 1998, 66, 214–218. [Google Scholar] [CrossRef]
- Grothey, A.; Sargent, D.; Goldberg, R.M.; Schmoll, H.J. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J. Clin. Oncol. 2004, 22, 1209–1214. [Google Scholar] [CrossRef]
- Tournigand, C.; Andre, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J. Clin. Oncol. 2004, 22, 229–237. [Google Scholar] [CrossRef]
- Grossman, J.G.; Nywening, T.M.; Belt, B.A.; Panni, R.Z.; Krasnick, B.A.; DeNardo, D.G.; Hawkins, W.G.; Goedegebuure, S.P.; Linehan, D.C.; Fields, R.C. Recruitment of CCR2(+) tumor associated macrophage to sites of liver metastasis confers a poor prognosis in human colorectal cancer. Oncoimmunology 2018, 7, e1470729. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, 106–110. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Touil, Y.; Igoudjil, W.; Corvaisier, M.; Dessein, A.F.; Vandomme, J.; Monte, D.; Stechly, L.; Skrypek, N.; Langlois, C.; Grard, G.; et al. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin. Cancer Res. 2014, 20, 837–846. [Google Scholar] [CrossRef] [PubMed]
- de Sousa e Melo, F.; Kurtova, A.V.; Harnoss, J.M.; Kljavin, N.; Hoeck, J.D.; Hung, J.; Anderson, J.E.; Storm, E.E.; Modrusan, Z.; Koeppen, H.; et al. A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature 2017, 543, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, M.; Ohta, Y.; Nishikori, S.; Matano, M.; Takano, A.; Fujii, M.; Date, S.; Sugimoto, S.; Kanai, T.; Sato, T. Visualization and targeting of LGR5(+) human colon cancer stem cells. Nature 2017, 545, 187–192. [Google Scholar] [CrossRef]
- Jardé, T.; Kass, L.; Staples, M.; Lescesen, H.; Carne, P.; Oliva, K.; McMurrick, P.J.; Abud, H.E. ERBB3 Positively Correlates with Intestinal Stem Cell Markers but Marks a Distinct Non Proliferative Cell Population in Colorectal Cancer. PLoS ONE 2015, 10, e0138336. [Google Scholar] [CrossRef]
- Merlos-Suárez, A.; Barriga, F.M.; Jung, P.; Iglesias, M.; Céspedes, M.V.; Rossell, D.; Sevillano, M.; Hernando-Momblona, X.; da Silva-Diz, V.; Muñoz, P.; et al. The Intestinal Stem Cell Signature Identifies Colorectal Cancer Stem Cells and Predicts Disease Relapse. Cell Stem Cell 2011, 8, 511–524. [Google Scholar] [CrossRef]
- Asfaha, S.; Hayakawa, Y.; Muley, A.; Stokes, S.; Graham, T.A.; Ericksen, R.E.; Westphalen, C.B.; Von Burstin, J.; Mastracci, T.L.; Worthley, D.L.; et al. Krt19+/Lgr5− Cells Are Radioresistant Cancer-Initiating Stem Cells in the Colon and Intestine. Cell Stem Cell 2015, 16, 627–638. [Google Scholar] [CrossRef]
- Tian, H.; Biehs, B.; Warming, S.; Leong, K.G.; Rangell, L.; Klein, O.D.; de Sauvage, F.J. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 2011, 478, 255–259. [Google Scholar] [CrossRef]
- Metcalfe, C.; Kljavin, N.M.; Ybarra, R.; de Sauvage, F.J. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 2014, 14, 149–159. [Google Scholar] [CrossRef]
- Li, D.W.; Tang, H.M.; Fan, J.W.; Yan, D.W.; Zhou, C.Z.; Li, S.X.; Wang, X.L.; Peng, Z.H. Expression level of Bmi-1 oncoprotein is associated with progression and prognosis in colon cancer. J. Cancer Res. Clin. Oncol. 2010, 136, 997–1006. [Google Scholar] [CrossRef]
- Ayyaz, A.; Kumar, S.; Sangiorgi, B.; Ghoshal, B.; Gosio, J.; Ouladan, S.; Fink, M.; Barutcu, S.; Trcka, D.; Shen, J.; et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 2019, 569, 121–125. [Google Scholar] [CrossRef] [PubMed]
- McMurrick, P.J.; Oliva, K.; Carne, P.; Reid, C.; Polglase, A.; Bell, S.; Farmer, K.C.; Ranchod, P. The first 1000 patients on an internet-based colorectal neoplasia database across private and public medicine in Australia: Development of a binational model for the Colorectal Surgical Society of Australia and New Zealand. Dis. Colon Rectum 2014, 57, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Horvay, K.; Jardé, T.; Casagranda, F.; Perreau, V.M.; Haigh, K.; Nefzger, C.M.; Akhtar, R.; Gridley, T.; Berx, G.; Haigh, J.J.; et al. Snai1 regulates cell lineage allocation and stem cell maintenance in the mouse intestinal epithelium. EMBO J. 2015, 34, 1319–1335. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; Mao, W.; Wang, X.; Wang, B.E.; Pham, T.; Flygare, J.; Yu, S.F.; Yee, S.; Goldenberg, D.; Fields, C.; et al. Targeting LGR5+ cells with an antibody-drug conjugate for the treatment of colon cancer. Sci. Transl. Med. 2015, 7, 314ra186. [Google Scholar] [CrossRef] [PubMed]
- Anaya, J. OncoLnc: Linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput. Sci. 2016, 2, e67. [Google Scholar] [CrossRef]
- Aguirre-Gamboa, R.; Gomez-Rueda, H.; Martínez-Ledesma, E.; Martínez-Torteya, A.; Chacolla-Huaringa, R.; Rodriguez-Barrientos, A.; Tamez-Peña, J.G.; Treviño, V. SurvExpress: An online biomarker validation tool and database for cancer gene expression data using survival analysis. PLoS ONE 2013, 8, e74250. [Google Scholar] [CrossRef]
- Moskaluk, C.A.; Zhang, H.; Powell, S.M.; Cerilli, L.A.; Hampton, G.M.; Frierson, H.F., Jr. Cdx2 protein expression in normal and malignant human tissues: An immunohistochemical survey using tissue microarrays. Mod. Pathol. 2003, 16, 913–919. [Google Scholar] [CrossRef]
- Werling, R.W.; Yaziji, H.; Bacchi, C.E.; Gown, A.M. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: An immunohistochemical survey of 476 primary and metastatic carcinomas. Am. J. Surg. Pathol. 2003, 27, 303–310. [Google Scholar] [CrossRef]
- Redondo, M.; Rodrigo, I.; Alcaide, J.; Tellez, T.; Roldan, M.J.; Funez, R.; Diaz-Martin, A.; Rueda, A.; Jimenez, E. Clusterin expression is associated with decreased disease-free survival of patients with colorectal carcinomas. Histopathology 2010, 56, 932–936. [Google Scholar] [CrossRef]
- Kevans, D.; Foley, J.; Tenniswood, M.; Sheahan, K.; Hyland, J.; O’Donoghue, D.; Mulcahy, H.; O’Sullivan, J. High clusterin expression correlates with a poor outcome in stage II colorectal cancers. Cancer Epidemiol. Biomark. Prev. 2009, 18, 393–399. [Google Scholar] [CrossRef][Green Version]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van Den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Weeber, F.; van de Wetering, M.; Hoogstraat, M.; Dijkstra, K.K.; Krijgsman, O.; Kuilman, T.; Gadellaa-van Hooijdonk, C.G.; van der Velden, D.L.; Peeper, D.S.; Cuppen, E.P.; et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc. Natl. Acad. Sci. USA 2015, 112, 13308–13311. [Google Scholar] [CrossRef] [PubMed]
- Van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Saad, R.S.; Ghorab, Z.; Khalifa, M.A.; Xu, M. CDX2 as a marker for intestinal differentiation: Its utility and limitations. World J. Gastrointest. Surg. 2011, 3, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Van Den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Dame, M.K.; Attili, D.; McClintock, S.D.; Dedhia, P.H.; Ouillette, P.; Hardt, O.; Chin, A.M.; Xue, X.; Laliberte, J.; Katz, E.L.; et al. Identification, isolation and characterization of human LGR5-positive colon adenoma cells. Development 2018, 145, dev153049. [Google Scholar] [CrossRef]
- Lee, S.H.; Hu, W.; Matulay, J.T.; Silva, M.V.; Owczarek, T.B.; Kim, K.; Chua, C.W.; Barlow, L.J.; Kandoth, C.; Williams, A.B.; et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 2018, 173, 515–528. [Google Scholar] [CrossRef]
- Ooft, S.N.; Weeber, F.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; van Werkhoven, E.; Schipper, L.; Hoes, L.; Vis, D.J.; van de Haar, J.; et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 2019, 11, eaay2574. [Google Scholar] [CrossRef]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386. [Google Scholar] [CrossRef]
- Tiriac, H.; Belleau, P.; Engle, D.D.; Plenker, D.; Deschênes, A.; Somerville, T.D.; Froeling, F.E.; Burkhart, R.A.; Denroche, R.E.; Jang, G.H.; et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov. 2018, 8, 1112–1129. [Google Scholar] [CrossRef] [PubMed]
- Du Puch, C.B.; Nouaille, M.; Giraud, S.; Labrunie, A.; Luce, S.; Preux, P.M.; Labrousse, F.; Gainant, A.; Tubiana-Mathieu, N.; Le Brun-Ly, V.; et al. Chemotherapy outcome predictive effectiveness by the Oncogramme: Pilot trial on stage-IV colorectal cancer. J. Transl. Med. 2016, 14, 10. [Google Scholar] [CrossRef] [PubMed]
| Antibodies/Dye | Host | Dilution | Supplier | Cat/Lot number |
|---|---|---|---|---|
| Primary | ||||
| Cleaved Caspase 3 (Asp175) | Rabbit | 1:250 | Cell Signaling | 9661S |
| Cytokeratin 20 (CK20) (SP33) | Rabbit | 1:1 | Roche Ventana | 790-4431 |
| Caudal type homeobox 2 (CDX2) | Rabbit | 1:1000 | Abcam | Ab76541 |
| Leucine rich repeat containing G protein-coupled receptor 5 (LGR5) | Rabbit | 1:200 | Genentech | n/a |
| Secondary | ||||
| Anti-rabbit horseradish peroxidase | Goat | 1:200 | Life Technologies | G21234 |
| Gene | Forward Primer Sequences | Reverse Primer Sequences | Product Length (bp) |
|---|---|---|---|
| ACTB | CTGGCACCACACCTTCTACAATG | GGTCTCAAACATGATCTGGGTC | 124 |
| ANXA1 | TTTGCAAGAAGGTAGAGATAAAGAC | GGATGACTTCACAGTTTGAACAT | 121 |
| B2M | GTGCTCGCGCTACTCTCTC | GTCAACTTCAATGTCGGAT | 142 |
| BMI1 | GGTACTTCATTGATGCCACAACC | CTGGTCTTGTGAACTTGGACATC | 104 |
| CLU | CAGGCCATGGACATCCACTT | GTCATCGTCGCCTTCTCGTA | 78 |
| EPHB2 | TTGGGCTCTCACGCTTTCTA | AGGTGAACTTCCGGTACTGG | 120 |
| Ki67 | CAGCACCTGCTTGTTTGGAAG | TAATATTGCCTCCTGCTCATGGAT | 109 |
| KRT20 | CTGAGGTTCAACTAACGGAGCTG | AACAGCGACTGGAGGTTGGCTA | 129 |
| LGR5 | CCTTCCAACCTCAGCGTCTT | AGGGATTGAAGGCTTCGCAA | 250 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engel, R.M.; Chan, W.H.; Nickless, D.; Hlavca, S.; Richards, E.; Kerr, G.; Oliva, K.; McMurrick, P.J.; Jardé, T.; Abud, H.E. Patient-Derived Colorectal Cancer Organoids Upregulate Revival Stem Cell Marker Genes following Chemotherapeutic Treatment. J. Clin. Med. 2020, 9, 128. https://doi.org/10.3390/jcm9010128
Engel RM, Chan WH, Nickless D, Hlavca S, Richards E, Kerr G, Oliva K, McMurrick PJ, Jardé T, Abud HE. Patient-Derived Colorectal Cancer Organoids Upregulate Revival Stem Cell Marker Genes following Chemotherapeutic Treatment. Journal of Clinical Medicine. 2020; 9(1):128. https://doi.org/10.3390/jcm9010128
Chicago/Turabian StyleEngel, Rebekah M., Wing Hei Chan, David Nickless, Sara Hlavca, Elizabeth Richards, Genevieve Kerr, Karen Oliva, Paul J. McMurrick, Thierry Jardé, and Helen E. Abud. 2020. "Patient-Derived Colorectal Cancer Organoids Upregulate Revival Stem Cell Marker Genes following Chemotherapeutic Treatment" Journal of Clinical Medicine 9, no. 1: 128. https://doi.org/10.3390/jcm9010128
APA StyleEngel, R. M., Chan, W. H., Nickless, D., Hlavca, S., Richards, E., Kerr, G., Oliva, K., McMurrick, P. J., Jardé, T., & Abud, H. E. (2020). Patient-Derived Colorectal Cancer Organoids Upregulate Revival Stem Cell Marker Genes following Chemotherapeutic Treatment. Journal of Clinical Medicine, 9(1), 128. https://doi.org/10.3390/jcm9010128





