Risk and Protective Environmental Factors Associated with Autism Spectrum Disorder: Evidence-Based Principles and Recommendations
Abstract
:1. Introduction
2. Conception Period
3. Prenatal Period
4. Perinatal/Early Postnatal Period
5. Gene–Environment Interactions and Epigenetics
6. Protective Factors
7. Early Intervention Strategies
8. Clinical Recommendations
9. Future Perspectives
Author Contributions
Conflicts of Interest
References
- Parellada, M.; Penzol, M.J.; Pina, L.; Moreno, C.; Gonzalez-Vioque, E.; Zalsman, G.; Arango, C. The neurobiology of autism spectrum disorders. Eur. Psychiatry 2014, 29, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zachor, D.A.; Curatolo, P.; Participants of Italian-Israeli Consensus Conference. Recommendations for early diagnosis and intervention in autism spectrum disorders: An Italian-Israeli consensus conference. Eur. J. Paediatr. Neurol. 2014, 18, 107–118. [Google Scholar] [CrossRef]
- Reichow, B.; Hume, K.; Barton, E.E.; Boyd, B.A. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database Syst. Rev. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Benvenuto, A.; Battan, B.; Porfirio, M.C.; Curatolo, P. Pharmacotherapy of autism spectrum disorders. Brain Dev. 2013, 35, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Idring, S.; Magnusson, C.; Lundberg, M.; Ek, M.; Rai, D.; Svensson, A.C.; Dalman, C.; Karlsson, H.; Lee, B.K. Parental age and the risk of autism spectrum disorders: Findings from a Swedish population-based cohort. Int. J. Epidemiol. 2014, 43, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.J.; Hannan, A.J.; Craig, J.M. The Role of Epigenetic Change in Autism Spectrum Disorders. Front. Neurol. 2015, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Hultman, C.; Larsson, H.; Reichenberg, A. The Heritability of Autism Spectrum Disorder. JAMA 2017, 318, 1182–1184. [Google Scholar] [CrossRef]
- Ronald, A.; Hoekstra, R.A. Autism spectrum disorders and autistic traits: A decade of new twin studies. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011, 156B, 255–274. [Google Scholar] [CrossRef]
- Girirajan, S.; Dennis, M.Y.; Baker, C.; Malig, M.; Coe, B.P.; Campbell, C.D.; Mark, K.; Vu, T.H.; Alkan, C.; Cheng, Z.; et al. Refinement and discovery of new hotspots of copy-number variation associated with autism spectrum disorder. Am. J. Hum. Genet. 2013, 92, 221–237. [Google Scholar] [CrossRef]
- Sanders, S.J.; He, X.; Willsey, A.J.; Ercan-Sencicek, A.G.; Samocha, K.E.; Cicek, A.E.; Murtha, M.T.; Bal, V.H.; Bishop, S.L.; Dong, S.; et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron 2015, 87, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Ziats, M.N.; Rennert, O.M. The Evolving Diagnostic and Genetic Landscapes of Autism Spectrum Disorder. Front. Genet. 2016, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Kushima, I.; Aleksic, B.; Nakatochi, M.; Shimamura, T.; Okada, T.; Uno, Y.; Morikawa, M.; Ishizuka, K.; Shiino, T.; Kimura, H.; et al. Comparative Analyses of Copy-Number Variation in Autism Spectrum Disorder and Schizophrenia Reveal Etiological Overlap and Biological Insights. Cell. Rep. 2018, 24, 2838–2856. [Google Scholar] [CrossRef] [PubMed]
- Bergbaum, A.; Ogilvie, C.M. Autism and chromosome abnormalities-A review. Clin. Anat. 2016, 29, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.J. Next-Generation Sequencing in Autism Spectrum Disorder. Cold Spring Harb. Perspect. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Benvenuto, A.; Moavero, R.; Alessandrelli, R.; Manzi, B.; Curatolo, P. Syndromic autism: Causes and pathogenetic pathways. World J. Pediatr. 2009, 5, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Saskin, A.; Fulginiti, V.; Birch, A.H.; Trakadis, Y. Prevalence of four Mendelian disorders associated with autism in 2392 affected families. J. Hum. Genet. 2017, 62, 657–659. [Google Scholar] [CrossRef]
- Emberti Gialloreti, L.; Curatolo, P. Autism Spectrum Disorder: Why Do We Know So Little? Front. Neurol. 2018, 9, 670. [Google Scholar] [CrossRef]
- Lyall, K.; Munger, K.L.; O’Reilly, E.J.; Santangelo, S.L.; Ascherio, A. Maternal dietary fat intake in association with autism spectrum disorders. Am. J. Epidemiol. 2013, 178, 209–220. [Google Scholar] [CrossRef]
- Lyall, K.; Croen, L.A.; Sjodin, A.; Yoshida, C.K.; Zerbo, O.; Kharrazi, M.; Windham, G.C. Polychlorinated Biphenyl and Organochlorine Pesticide Concentrations in Maternal Mid-Pregnancy Serum Samples: Association with Autism Spectrum Disorder and Intellectual Disability. Environ. Health Perspect. 2017, 125, 474–480. [Google Scholar] [CrossRef]
- Janecka, M.; Mill, J.; Basson, M.A.; Goriely, A.; Spiers, H.; Reichenberg, A.; Schalkwyk, L.; Fernandes, C. Advanced paternal age effects in neurodevelopmental disorders-review of potential underlying mechanisms. Transl. Psychiatry 2017, 7, e1019. [Google Scholar] [CrossRef] [PubMed]
- Merikangas, A.K.; Calkins, M.E.; Bilker, W.B.; Moore, T.M.; Gur, R.C.; Gur, R.E. Parental Age and Offspring Psychopathology in the Philadelphia Neurodevelopmental Cohort. J. Am. Acad. Child. Adolesc. Psychiatry 2017, 56, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.S.; Maenner, M.J.; Newschaffer, C.J.; Lee, L.C.; Cunniff, C.M.; Daniels, J.L.; Kirby, R.S.; Leavitt, L.; Miller, L.; Zahorodny, W.; et al. Advanced parental age and the risk of autism spectrum disorder. Am. J. Epidemiol. 2008, 168, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Ben Itzchak, E.; Lahat, E.; Zachor, D.A. Advanced parental ages and low birth weight in autism spectrum disorders-rates and effect on functioning. Res. Dev. Disabil. 2011, 32, 1776–1781. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Kern, J.K.; Sykes, L.K.; Geier, M.R. Examining genotypic variation in autism spectrum disorder and its relationship to parental age and phenotype. Appl. Clin. Genet. 2016, 9, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Sandin, S.; Schendel, D.; Magnusson, P.; Hultman, C.; Suren, P.; Susser, E.; Gronborg, T.; Gissler, M.; Gunnes, N.; Gross, R.; et al. Autism risk associated with parental age and with increasing difference in age between the parents. Mol. Psychiatry 2016, 21, 693–700. [Google Scholar] [CrossRef]
- Flatscher-Bader, T.; Foldi, C.J.; Chong, S.; Whitelaw, E.; Moser, R.J.; Burne, T.H.; Eyles, D.W.; McGrath, J.J. Increased de novo copy number variants in the offspring of older males. Transl. Psychiatry 2011, 1, e34. [Google Scholar] [CrossRef]
- Kong, A.; Frigge, M.L.; Masson, G.; Besenbacher, S.; Sulem, P.; Magnusson, G.; Gudjonsson, S.A.; Sigurdsson, A.; Jonasdottir, A.; Jonasdottir, A.; et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012, 488, 471–475. [Google Scholar] [CrossRef]
- European IVF-monitoring Consortium (EIM); European Society of Human Reproduction and Embryology (ESHRE); Calhaz-Jorge, C.; De Geyter, C.; Kupka, M.S.; de Mouzon, J.; Erb, K.; Mocanu, E.; Motrenko, T.; et al. Assisted reproductive technology in Europe, 2013: Results generated from European registers by ESHRE. Hum. Reprod. 2017, 32, 1957–1973. [Google Scholar] [CrossRef]
- Liu, L.; Gao, J.; He, X.; Cai, Y.; Wang, L.; Fan, X. Association between assisted reproductive technology and the risk of autism spectrum disorders in the offspring: A meta-analysis. Sci. Rep. 2017, 7, 46207. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Kurinczuk, J.J.; Bower, C.; Webb, S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N. Engl. J. Med. 2002, 346, 725–730. [Google Scholar] [CrossRef]
- Gosden, R.; Trasler, J.; Lucifero, D.; Faddy, M. Rare congenital disorders, imprinted genes, and assisted reproductive technology. Lancet 2003, 361, 1975–1977. [Google Scholar] [CrossRef]
- Davies, M.J.; Moore, V.M.; Willson, K.J.; Van Essen, P.; Priest, K.; Scott, H.; Haan, E.A.; Chan, A. Reproductive technologies and the risk of birth defects. N. Engl. J. Med. 2012, 366, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Lidegaard, O.; Pinborg, A.; Andersen, A.N. Imprinting disorders after assisted reproductive technologies. Curr. Opin. Obstet. Gynecol. 2006, 18, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, B.; Baron-Cohen, S.; Ashwin, E.; Knickmeyer, R.; Taylor, K.; Hackett, G. Fetal testosterone and autistic traits. Br. J. Psychol. 2009, 100, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lung, F.W.; Shu, B.C.; Chiang, T.L.; Lin, S.J. Twin-singleton influence on infant development: A national birth cohort study. Child. Care Health Dev. 2009, 35, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Zachor, D.A.; Ben Itzchak, E. Assisted reproductive technology and risk for autism spectrum disorder. Res. Dev. Disabil. 2011, 32, 2950–2956. [Google Scholar] [CrossRef] [PubMed]
- Kalkbrenner, A.E.; Schmidt, R.J.; Penlesky, A.C. Environmental chemical exposures and autism spectrum disorders: A review of the epidemiological evidence. Curr. Probl. Pediatr. Adolesc. Health Care 2014, 44, 277–318. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Genuis, S.J.; Frye, R.E. Environmental toxicants and autism spectrum disorders: A systematic review. Transl. Psychiatry 2014, 4, e360. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, P.; Walker, C.K.; Bremer, A.A.; Baker, A.S.; Ozonoff, S.; Hansen, R.L.; Hertz-Picciotto, I. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 2012, 129, e1121–e1128. [Google Scholar] [CrossRef] [PubMed]
- Getz, K.D.; Anderka, M.T.; Werler, M.M.; Jick, S.S. Maternal Pre-pregnancy Body Mass Index and Autism Spectrum Disorder among Offspring: A Population-Based Case-Control Study. Paediatr. Perinat. Epidemiol. 2016, 30, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.H.; Thomsen, P.H.; Nohr, E.A.; Lemcke, S. Maternal body mass index before pregnancy as a risk factor for ADHD and autism in children. Eur. Child. Adolesc Psychiatry 2018, 27, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bugatto, F.; Fernandez-Deudero, A.; Bailen, A.; Fernandez-Macias, R.; Hervias-Vivancos, B.; Bartha, J.L. Second-trimester amniotic fluid proinflammatory cytokine levels in normal and overweight women. Obstet. Gynecol. 2010, 115, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Nutrition and the developing brain: Nutrient priorities and measurement. Am. J. Clin. Nutr. 2007, 85, 614S620S. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Kogan, V.; Shelton, J.F.; Delwiche, L.; Hansen, R.L.; Ozonoff, S.; Ma, C.C.; McCanlies, E.C.; Bennett, D.H.; Hertz-Picciotto, I.; et al. Combined Prenatal Pesticide Exposure and Folic Acid Intake in Relation to Autism Spectrum Disorder. Environ. Health Perspect. 2017, 125, 097007. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.M.; Panser, L.A.; Katusic, S.K. Is excess folic acid supplementation a risk factor for autism? Med. Hypotheses 2011, 77, 15–17. [Google Scholar] [CrossRef]
- Wiens, D.; DeSoto, M.C. Is High Folic Acid Intake a Risk Factor for Autism?–A Review. Brain Sci. 2017, 7. [Google Scholar] [CrossRef]
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2018, 32, 100–111. [Google Scholar] [CrossRef]
- Powers, H.J. Folic acid under scrutiny. Br. J. Nutr. 2007, 98, 665–666. [Google Scholar] [CrossRef]
- Girotto, F.; Scott, L.; Avchalumov, Y.; Harris, J.; Iannattone, S.; Drummond-Main, C.; Tobias, R.; Bello-Espinosa, L.; Rho, J.M.; Davidsen, J.; et al. High dose folic acid supplementation of rats alters synaptic transmission and seizure susceptibility in offspring. Sci. Rep. 2013, 3, 1465. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Chadman, K.K.; Kuizon, S.; Buenaventura, D.; Stapley, N.W.; Ruocco, F.; Begum, U.; Guariglia, S.R.; Brown, W.T.; Junaid, M.A. Increasing maternal or post-weaning folic acid alters gene expression and moderately changes behavior in the offspring. PLoS One 2014, 9, e101674. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Tancredi, D.J.; Krakowiak, P.; Hansen, R.L.; Ozonoff, S. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am. J. Epidemiol. 2014, 180, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Gidaya, N.B.; Lee, B.K.; Burstyn, I.; Yudell, M.; Mortensen, E.L.; Newschaffer, C.J. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. J. Autism Dev. Disord. 2014, 44, 2558–2567. [Google Scholar] [CrossRef]
- Andalib, S.; Emamhadi, M.R.; Yousefzadeh-Chabok, S.; Shakouri, S.K.; Hoilund-Carlsen, P.F.; Vafaee, M.S.; Michel, T.M. Maternal SSRI exposure increases the risk of autistic offspring: A meta-analysis and systematic review. Eur. Psychiatry 2017, 45, 161–166. [Google Scholar] [CrossRef]
- Brown, H.K.; Hussain-Shamsy, N.; Lunsky, Y.; Dennis, C.E.; Vigod, S.N. The Association between Antenatal Exposure to Selective Serotonin Reuptake Inhibitors and Autism: A Systematic Review and Meta-Analysis. J. Clin. Psychiatry 2017, 78, e48–e58. [Google Scholar] [CrossRef] [PubMed]
- Mezzacappa, A.; Lasica, P.A.; Gianfagna, F.; Cazas, O.; Hardy, P.; Falissard, B.; Sutter-Dallay, A.L.; Gressier, F. Risk for Autism Spectrum Disorders According to Period of Prenatal Antidepressant Exposure: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017, 171, 555–563. [Google Scholar] [CrossRef]
- Rai, D.; Lee, B.K.; Dalman, C.; Golding, J.; Lewis, G.; Magnusson, C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: Population based case-control study. BMJ 2013, 346, 2059. [Google Scholar] [CrossRef]
- Malm, H.; Brown, A.S.; Gissler, M.; Gyllenberg, D.; Hinkka-Yli-Salomaki, S.; McKeague, I.W.; Weissman, M.; Wickramaratne, P.; Artama, M.; Gingrich, J.A.; et al. Gestational Exposure to Selective Serotonin Reuptake Inhibitors and Offspring Psychiatric Disorders: A National Register-Based Study. J. Am. Acad. Child. Adolesc. Psychiatry 2016, 55, 359–366. [Google Scholar] [CrossRef]
- Hviid, A.; Melbye, M.; Pasternak, B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N. Engl. J. Med. 2013, 369, 2406–2415. [Google Scholar] [CrossRef]
- Sørensen, M.J.; Gronborg, T.K.; Christensen, J.; Parner, E.T.; Vestergaard, M.; Schendel, D.; Pedersen, L.H. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin. Epidemiol. 2013, 5, 449–459. [Google Scholar] [PubMed]
- Jung, C.R.; Lin, Y.T.; Hwang, B.F. Air pollution and newly diagnostic autism spectrum disorders: A population-based cohort study in Taiwan. PLoS ONE 2013, 8, e75510. [Google Scholar] [CrossRef]
- Kim, D.; Volk, H.; Girirajan, S.; Pendergrass, S.; Hall, M.A.; Verma, S.S.; Schmidt, R.J.; Hansen, R.L.; Ghosh, D.; Ludena-Rodriguez, Y.; et al. The joint effect of air pollution exposure and copy number variation on risk for autism. Autism Res. 2017, 10, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, A.J.; Volk, H.E.; Tancredi, D.J.; McConnell, R.; Lurmann, F.W.; Hansen, R.L.; Schmidt, R.J. Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder. Autism Res. 2018, 11, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Gaita, L.; Manzi, B.; Sacco, R.; Lintas, C.; Altieri, L.; Lombardi, F.; Pawlowski, T.L.; Redman, M.; Craig, D.W.; Huentelman, M.J.; et al. Decreased serum arylesterase activity in autism spectrum disorders. Psychiatry Res. 2010, 180, 105–113. [Google Scholar] [CrossRef]
- Thummler, S.; Dor, E.; David, R.; Leali, G.; Battista, M.; David, A.; Askenazy, F.; Verstuyft, C. Pharmacoresistant Severe Mental Health Disorders in Children and Adolescents: Functional Abnormalities of Cytochrome P450 2D6. Front. Psychiatry 2018, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Weisskopf, M.G.; Kioumourtzoglou, M.A.; Roberts, A.L. Air Pollution and Autism Spectrum Disorders: Causal or Confounded? Curr. Environ. Health Rep. 2015, 2, 430–439. [Google Scholar] [CrossRef]
- Lam, J.; Sutton, P.; Kalkbrenner, A.; Windham, G.; Halladay, A.; Koustas, E.; Lawler, C.; Davidson, L.; Daniels, N.; Newschaffer, C.; et al. A Systematic Review and Meta-Analysis of Multiple Airborne Pollutants and Autism Spectrum Disorder. PLoS ONE 2016, 11, e0161851. [Google Scholar] [CrossRef]
- Volk, H.E.; Hertz-Picciotto, I.; Delwiche, L.; Lurmann, F.; McConnell, R. Residential proximity to freeways and autism in the CHARGE study. Environ. Health Perspect. 2011, 119, 873–877. [Google Scholar] [CrossRef]
- Becerra, T.A.; Wilhelm, M.; Olsen, J.; Cockburn, M.; Ritz, B. Ambient air pollution and autism in Los Angeles county, California. Environ. Health Perspect. 2013, 121, 380–386. [Google Scholar] [CrossRef]
- Gong, T.; Dalman, C.; Wicks, S.; Dal, H.; Magnusson, C.; Lundholm, C.; Almqvist, C.; Pershagen, G. Perinatal Exposure to Traffic-Related Air Pollution and Autism Spectrum Disorders. Environ. Health Perspect. 2017, 125, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Guxens, M.; Ghassabian, A.; Gong, T.; Garcia-Esteban, R.; Porta, D.; Giorgis-Allemand, L.; Almqvist, C.; Aranbarri, A.; Beelen, R.; Badaloni, C.; et al. Air Pollution Exposure during Pregnancy and Childhood Autistic Traits in Four European Population-Based Cohort Studies: The ESCAPE Project. Environ. Health Perspect. 2016, 124, 133–140. [Google Scholar] [CrossRef]
- Raz, R.; Levine, H.; Pinto, O.; Broday, D.M.; Yuval; Weisskopf, M.G. Traffic-Related Air Pollution and Autism Spectrum Disorder: A Population-Based Nested Case-Control Study in Israel. Am. J. Epidemiol. 2018, 187, 717–725. [Google Scholar] [CrossRef]
- Pagalan, L.; Bickford, C.; Weikum, W.; Lanphear, B.; Brauer, M.; Lanphear, N.; Hanley, G.E.; Oberlander, T.F.; Winters, M. Association of Prenatal Exposure to Air Pollution with Autism Spectrum Disorder. JAMA Pediatr. 2018. [Google Scholar] [CrossRef]
- Roberts, E.M.; English, P.B.; Grether, J.K.; Windham, G.C.; Somberg, L.; Wolff, C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ. Health Perspect. 2007, 115, 1482–1489. [Google Scholar] [CrossRef]
- Roberts, E.M.; English, P.B. Bayesian modeling of time-dependent vulnerability to environmental hazards: An example using autism and pesticide data. Stat. Med. 2013, 32, 2308–2319. [Google Scholar] [CrossRef] [PubMed]
- Cheslack-Postava, K.; Rantakokko, P.V.; Hinkka-Yli-Salomaki, S.; Surcel, H.M.; McKeague, I.W.; Kiviranta, H.A.; Sourander, A.; Brown, A.S. Maternal serum persistent organic pollutants in the Finnish Prenatal Study of Autism: A pilot study. Neurotoxicol. Teratol. 2013, 38, 1–5. [Google Scholar] [CrossRef]
- Braun, J.M.; Kalkbrenner, A.E.; Just, A.C.; Yolton, K.; Calafat, A.M.; Sjodin, A.; Hauser, R.; Webster, G.M.; Chen, A.; Lanphear, B.P. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: The HOME study. Environ. Health Perspect. 2014, 122, 513–520. [Google Scholar] [CrossRef]
- Eskenazi, B.; Marks, A.R.; Bradman, A.; Harley, K.; Barr, D.B.; Johnson, C.; Morga, N.; Jewell, N.P. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ. Health Perspect. 2007, 115, 792–798. [Google Scholar] [CrossRef]
- Shelton, J.F.; Geraghty, E.M.; Tancredi, D.J.; Delwiche, L.D.; Schmidt, R.J.; Ritz, B.; Hansen, R.L.; Hertz-Picciotto, I. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: The CHARGE study. Environ. Health Perspect. 2014, 122, 1103–1109. [Google Scholar] [CrossRef]
- Bjorling-Poulsen, M.; Andersen, H.R.; Grandjean, P. Potential developmental neurotoxicity of pesticides used in Europe. Environ. Health 2008, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Martinez, M.A.; Dai, M.; Chen, D.; Ares, I.; Romero, A.; Castellano, V.; Martinez, M.; Rodriguez, J.L.; Martinez-Larranaga, M.R.; et al. Permethrin-induced oxidative stress and toxicity and metabolism. A review. Environ. Res. 2016, 149, 86–104. [Google Scholar] [CrossRef]
- Hicks, S.D.; Wang, M.; Fry, K.; Doraiswamy, V.; Wohlford, E.M. Neurodevelopmental Delay Diagnosis Rates Are Increased in a Region with Aerial Pesticide Application. Front. Pediatr. 2017, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Catalani, S.; Franco, A.; Brighenti, M.; Apostoli, P. Lack of correlation between metallic elements analyzed in hair by ICP-MS and autism. J. Autism Dev. Disord. 2012, 42, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Yoshimasu, K.; Kiyohara, C.; Takemura, S.; Nakai, K. A meta-analysis of the evidence on the impact of prenatal and early infancy exposures to mercury on autism and attention deficit/hyperactivity disorder in the childhood. Neurotoxicology 2014, 44, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Roullet, F.I.; Lai, J.K.; Foster, J.A. In utero exposure to valproic acid and autism-a current review of clinical and animal studies. Neurotoxicol. Teratol. 2013, 36, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Rios, P.; Cogo, E.; Straus, S.E.; Finkelstein, Y.; Kealey, R.; Reynen, E.; Soobiah, C.; Thavorn, K.; Hutton, B.; et al. Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: A systematic review and network meta-analysis. BMJ Open 2017, 7, e017248. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. Antidepressant Exposure during Pregnancy and Risk of Autism in the Offspring, 1: Meta-Review of Meta-Analyses. J. Clin. Psychiatry 2017, 78, e1047–e1051. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, Y.C.; Keskin-Arslan, E.; Acar, S.; Sozmen, K. Maternal SSRI discontinuation, use, psychiatric disorder and the risk of autism in children: A meta-analysis of cohort studies. Br. J. Clin. Pharmacol. 2017, 83, 2798–2806. [Google Scholar] [CrossRef] [PubMed]
- Oberlander, T.F.; Zwaigenbaum, L. Disentangling Maternal Depression and Antidepressant Use during Pregnancy as Risks for Autism in Children. JAMA 2017, 317, 1533–1534. [Google Scholar] [CrossRef] [PubMed]
- Atladottir, H.O.; Henriksen, T.B.; Schendel, D.E.; Parner, E.T. Autism after infection, febrile episodes, and antibiotic use during pregnancy: An exploratory study. Pediatrics 2012, 130, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Mian, F.M.; Stanisz, A.M.; Bindels, L.B.; Cambier, E.; Ben-Amram, H.; Koren, O.; Forsythe, P.; Bienenstock, J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 2017, 8, 15062. [Google Scholar] [CrossRef] [PubMed]
- Aronson, M.; Hagberg, B.; Gillberg, C. Attention deficits and autistic spectrum problems in children exposed to alcohol during gestation: A follow-up study. Dev. Med. Child. Neurol. 1997, 39, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Eliasen, M.; Tolstrup, J.S.; Nybo Andersen, A.M.; Gronbaek, M.; Olsen, J.; Strandberg-Larsen, K. Prenatal alcohol exposure and autistic spectrum disorders--a population-based prospective study of 80,552 children and their mothers. Int. J. Epidemiol. 2010, 39, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.; McCarthy, F.P.; Ryan, R.M.; Khashan, A.S. Maternal Alcohol Consumption during Pregnancy and the Risk of Autism Spectrum Disorders in Offspring: A Retrospective Analysis of the Millennium Cohort Study. J. Autism Dev. Disord. 2018, 48, 3773–3782. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Weiss, B.; Janson, S.; Sundell, J.; Bornehag, C.G. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6-8 years of age. Neurotoxicology 2009, 30, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.N.; Lee, B.K.; Lee, N.L.; Yang, Y.; Burstyn, I. Maternal Smoking and Autism Spectrum Disorder: A Meta-analysis. J. Autism Dev. Disord. 2015, 45, 1689–1698. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Y.; Gong, X.; Wang, G. A Meta-Analysis of Maternal Smoking during Pregnancy and Autism Spectrum Disorder Risk in Offspring. Int. J. Environ. Res. Public Health 2015, 26, 10418–10431. [Google Scholar] [CrossRef]
- Peretti, S.; Mariano, M.; Mazzocchetti, C.; Mazza, M.; Pino, M.C.; Verrotti Di Pianella, A.; Valenti, M. Diet: The keystone of autism spectrum disorder? Nutr. Neurosci. 2018, 1–15. [Google Scholar] [CrossRef]
- Suren, P.; Roth, C.; Bresnahan, M.; Haugen, M.; Hornig, M.; Hirtz, D.; Lie, K.K.; Lipkin, W.I.; Magnus, P.; Reichborn-Kjennerud, T.; et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 2013, 309, 570–577. [Google Scholar] [CrossRef]
- Vinkhuyzen, A.A.E.; Eyles, D.W.; Burne, T.H.J.; Blanken, L.M.E.; Kruithof, C.J.; Verhulst, F.; White, T.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational vitamin D deficiency and autism spectrum disorder. BJPsych Open 2017, 3, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.G.; Monte, W.C. Dietary methanol and autism. Med. Hypotheses 2015, 85, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.L.; Nousen, E.K.; Chamlou, K.A. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol. Behav. 2014, 123, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Parboosing, R.; Bao, Y.; Shen, L.; Schaefer, C.A.; Brown, A.S. Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry 2013, 70, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Zerbo, O.; Iosif, A.M.; Walker, C.; Ozonoff, S.; Hansen, R.L.; Hertz-Picciotto, I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J. Autism Dev. Disord. 2013, 43, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Brucato, M.; Ladd-Acosta, C.; Li, M.; Caruso, D.; Hong, X.; Kaczaniuk, J.; Stuart, E.A.; Fallin, M.D.; Wang, X. Prenatal exposure to fever is associated with autism spectrum disorder in the boston birth cohort. Autism Res. 2017, 10, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Bresnahan, M.A.; Che, X.; Schultz, A.F.; Ukaigwe, J.E.; Eddy, M.L.; Hirtz, D.; Gunnes, N.; Lie, K.K.; Magnus, P.; et al. Prenatal fever and autism risk. Mol. Psychiatry 2018, 23, 759–766. [Google Scholar] [CrossRef]
- Knuesel, I.; Chicha, L.; Britschgi, M.; Schobel, S.A.; Bodmer, M.; Hellings, J.A.; Toovey, S.; Prinssen, E.P. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014, 10, 643–660. [Google Scholar] [CrossRef]
- Parker-Athill, E.C.; Tan, J. Maternal immune activation and autism spectrum disorder: Interleukin-6 signaling as a key meachanistic pathway. Neurosignals 2010, 18, 113–128. [Google Scholar] [CrossRef]
- Jones, K.L.; Croen, L.A.; Yoshida, C.K.; Heuer, L.; Hansen, R.; Zerbo, O.; DeLorenze, G.N.; Kharrazi, M.; Yolken, R.; Ashwood, P.; et al. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol. Psychiatry 2017, 22, 273–279. [Google Scholar] [CrossRef]
- Jones, K.L.; Van de Water, J. Maternal autoantibody related autism: mechanisms and pathways. Mol. Psychiat. 2018. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, A.; Van de Water, J. The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology 2017, 42, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, P.; Walker, C.K.; Tancredi, D.; Hertz-Picciotto, I.; Van de Water, J. Autism-specific maternal anti-fetal brain autoantibodies are associated with metabolic conditions. Autism Res. 2017, 10, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Spiegelman, D.; Buka, S.L. Prenatal risk factors for autism: Comprehensive meta-analysis. Br. J. Psychiatry 2009, 195, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Pauls, D.L.; Spiegelman, D.; Ascherio, A.; Santangelo, S.L. Pregnancy complications and obstetric suboptimality in association with autism spectrum disorders in children of the Nurses’ Health Study II. Autism Res. 2012, 5, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.H.; Wang, X.; Martinez, M.P.; Walthall, J.C.; Curry, E.S.; Page, K.; Buchanan, T.A.; Coleman, K.J.; Getahun, D. Association of maternal diabetes with autism in offspring. JAMA 2015, 313, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Ratzon, N.; Greenbaum, C.; Wolf, A.; Dulitzky, M. School-age children born to diabetic mothers and to mothers with gestational diabetes exhibit a high rate of inattention and fine and gross motor impairment. J. Pediatr. Endocrinol. Metab. 2001, 14, 681–689. [Google Scholar] [CrossRef]
- Banik, A.; Kandilya, D.; Ramya, S.; Stunkel, W.; Chong, Y.S.; Dheen, S.T. Maternal Factors that Induce Epigenetic Changes Contribute to Neurological Disorders in Offspring. Genes (Basel) 2017, 8. [Google Scholar] [CrossRef]
- Braam, W.; Ehrhart, F.; Maas, A.; Smits, M.G.; Curfs, L. Low maternal melatonin level increases autism spectrum disorder risk in children. Res. Dev. Disabil. 2018, 82, 79–89. [Google Scholar] [CrossRef]
- Jin, Y.; Choi, J.; Won, J.; Hong, Y. The Relationship between Autism Spectrum Disorder and Melatonin during Fetal Development. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Curran, E.A.; O’Neill, S.M.; Cryan, J.F.; Kenny, L.C.; Dinan, T.G.; Khashan, A.S.; Kearney, P.M. Research review: Birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. J. Child. Psychol. Psychiatry 2015, 56, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Dodds, L.; Fell, D.B.; Shea, S.; Armson, B.A.; Allen, A.C.; Bryson, S. The role of prenatal, obstetric and neonatal factors in the development of autism. J. Autism Dev. Disord. 2011, 41, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Emberti Gialloreti, L.; Benvenuto, A.; Benassi, F.; Curatolo, P. Are caesarean sections, induced labor and oxytocin regulation linked to Autism Spectrum Disorders? Med. Hypotheses 2014, 82, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Harony, H.; Wagner, S. The contribution of oxytocin and vasopressin to mammalian social behavior: Potential role in autism spectrum disorder. Neurosignals 2010, 18, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Olza Fernandez, I.; Marin Gabriel, M.A.; Lopez Sanchez, F.; Malalana Martinez, A.M. Oxytocin and autism: A hypothesis to research. Can perinatal oxitocinergic manipulation facilitate autism? Rev. Psiquiatr. Salud Ment. 2011, 4, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Geng, H.; Liu, W.; Zhang, G. Prenatal, perinatal, and postnatal factors associated with autism: A meta-analysis. Medicine (Baltimore) 2017, 96, e6696. [Google Scholar] [CrossRef]
- Limperopoulos, C.; Bassan, H.; Sullivan, N.R.; Soul, J.S.; Robertson, R.L., Jr.; Moore, M.; Ringer, S.A.; Volpe, J.J.; Du Plessis, A.J. Positive screening for autism in ex-preterm infants: Prevalence and risk factors. Pediatrics 2008, 121, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, J.; Yang, B. Targeting gut microbiome: A novel and potential therapy for autism. Life Sci. 2018, 194, 111–119. [Google Scholar] [CrossRef]
- Grimaldi, R.; Cela, D.; Swann, J.R.; Vulevic, J.; Gibson, G.R.; Tzortzis, G.; Costabile, A. In vitro fermentation of B-GOS: Impact on faecal bacterial populations and metabolic activity in autistic and non-autistic children. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef]
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejia, J.L.; Hansen, L.H.; Leigh Gibson, E.; Nielsen, D.S.; Costabile, A. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome 2018, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Weber-Stadlbauer, U. Epigenetic and transgenerational mechanisms in infection-mediated neurodevelopmental disorders. Transl. Psychiatry 2017, 7, e1113. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Yim, Y.S.; Ha, S.; Atarashi, K.; Tan, T.G.; Longman, R.S.; Honda, K.; Littman, D.R.; Choi, G.B.; et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 2017, 549, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.M.; Schmidt, R.J.; Tancredi, D.; Barkoski, J.; Ozonoff, S.; Bennett, D.H.; Hertz-Picciotto, I. Prenatal exposure to phthalates and autism spectrum disorder in the MARBLES study. Environ. Health 2018, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Edlow, A.G.; Vora, N.L.; Hui, L.; Wick, H.C.; Cowan, J.M.; Bianchi, D.W. Maternal obesity affects fetal neurodevelopmental and metabolic gene expression: A pilot study. PLoS One 2014, 9, e88661. [Google Scholar] [CrossRef]
- Anwar, A.; Abruzzo, P.M.; Pasha, S.; Rajpoot, K.; Bolotta, A.; Ghezzo, A.; Marini, M.; Posar, A.; Visconti, P.; Thornalley, P.J.; et al. Advanced glycation endproducts, dityrosine and arginine transporter dysfunction in autism–a source of biomarkers for clinical diagnosis. Mol. Autism 2018, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Xu, L.L.; Shao, L.; Xia, R.M.; Yu, Z.H.; Ling, Z.X.; Yang, F.; Deng, M.; Ruan, B. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain Behav. Immun. 2016, 58, 165–172. [Google Scholar] [CrossRef]
- Mazina, V.; Gerdts, J.; Trinh, S.; Ankenman, K.; Ward, T.; Dennis, M.Y.; Girirajan, S.; Eichler, E.E.; Bernier, R. Epigenetics of autism-related impairment: Copy number variation and maternal infection. J. Dev. Behav. Pediatr. 2015, 36, 61–67. [Google Scholar] [CrossRef]
- Kinney, D.K.; Barch, D.H.; Chayka, B.; Napoleon, S.; Munir, K.M. Environmental risk factors for autism: Do they help cause de novo genetic mutations that contribute to the disorder? Med. Hypotheses 2010, 74, 102–106. [Google Scholar] [CrossRef]
- Rangasamy, S.; D’Mello, S.R.; Narayanan, V. Epigenetics, autism spectrum, and neurodevelopmental disorders. Neurotherapeutics 2013, 10, 742–756. [Google Scholar] [CrossRef]
- Waye, M.M.Y.; Cheng, H.Y. Genetics and epigenetics of autism: A Review. Psychiatry Clin. Neurosci. 2018, 72, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Szatmari, P. Risk and resilience in autism spectrum disorder: A missed translational opportunity? Dev. Med. Child. Neurol. 2018, 60, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Tancredi, D.J.; Ozonoff, S.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Schmidt, L.C.; Tassone, F.; Hertz-Picciotto, I. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am. J. Clin. Nutr. 2012, 96, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, G.; Henley, K.; Green, J. Autism: Will vitamin D supplementation during pregnancy and early childhood reduce the recurrence rate of autism in newborn siblings? Med. Hypotheses 2016, 88, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.T.; Chen, Y.W.; Stubbs, B.; Carvalho, A.F.; Whiteley, P.; Tang, C.H.; Yang, W.C.; Chen, T.Y.; Li, D.J.; Chu, C.S.; et al. Maternal breastfeeding and autism spectrum disorder in children: A systematic review and meta-analysis. Nutr. Neurosci. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bar, S.; Milanaik, R.; Adesman, A. Long-term neurodevelopmental benefits of breastfeeding. Curr. Opin. Pediatr. 2016, 28, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Boucher, O.; Julvez, J.; Guxens, M.; Arranz, E.; Ibarluzea, J.; Sanchez de Miguel, M.; Fernandez-Somoano, A.; Tardon, A.; Rebagliato, M.; Garcia-Esteban, R.; et al. Association between breastfeeding duration and cognitive development, autistic traits and ADHD symptoms: A multicenter study in Spain. Pediatr. Res. 2017, 81, 434–442. [Google Scholar] [CrossRef]
- Morgese, M.G.; Trabace, L. Maternal Malnutrition in the Etiopathogenesis of Psychiatric Diseases: Role of Polyunsaturated Fatty Acids. Brain Sci. 2016, 6. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Schmidt, L.C.; Tancredi, D.J.; Tassone, F.; Hertz-Picciotto, I. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology 2011, 22, 476–485. [Google Scholar] [CrossRef]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.G.; Frye, R.E.; James, S.J. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl. Psychiatry 2012, 2, e134. [Google Scholar] [CrossRef]
- Ghezzo, A.; Visconti, P.; Abruzzo, P.M.; Bolotta, A.; Ferreri, C.; Gobbi, G.; Malisardi, G.; Manfredini, S.; Marini, M.; Nanetti, L.; et al. Oxidative Stress and Erythrocyte Membrane Alterations in Children with Autism: Correlation with Clinical Features. PLoS ONE 2013, 8, e66418. [Google Scholar] [CrossRef] [PubMed]
- Bolotta, A.; Visconti, P.; Fedrizzi, G.; Ghezzo, A.; Marini, M.; Manunta, P.; Messaggio, E.; Posar, A.; Vignini, A.; Abruzzo, P.M. Na(+), K(+)-ATPase activity in children with autism spectrum disorder: Searching for the reason(s) of its decrease in blood cells. Autism Res. 2018, 11, 1388–1403. [Google Scholar] [CrossRef] [PubMed]
- Madore, C.; Leyrolle, Q.; Lacabanne, C.; Benmamar-Badel, A.; Joffre, C.; Nadjar, A.; Laye, S. Neuroinflammation in Autism: Plausible Role of Maternal Inflammation, Dietary Omega 3, and Microbiota. Neural Plast. 2016, 2016, 3597209. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.R.; Yang, H.; Serena, G.; Sturgeon, C.; Ma, B.; Careaga, M.; Hughes, H.K.; Angkustsiri, K.; Rose, M.; Hertz-Picciotto, I.; et al. Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brain Behav. Immun. 2018, 70, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Lussu, M.; Noto, A.; Masili, A.; Rinaldi, A.C.; Dessi, A.; De Angelis, M.; De Giacomo, A.; Fanos, V.; Atzori, L.; Francavilla, R. The urinary (1) H-NMR metabolomics profile of an italian autistic children population and their unaffected siblings. Autism Res. 2017, 10, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Babenko, O.; Kovalchuk, I.; Metz, G.A. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci. Biobehav. Rev. 2015, 48, 70–91. [Google Scholar] [CrossRef]
- Curatolo, P.; Nabbout, R.; Lagae, L.; Aronica, E.; Ferreira, J.C.; Feucht, M.; Hertzberg, C.; Jansen, A.C.; Jansen, F.; Kotulska, K.; et al. Management of epilepsy associated with tuberous sclerosis complex: Updated clinical recommendations. Eur. J. Paediatr. Neurol. 2018, 22, 738–748. [Google Scholar] [CrossRef]
- Peralta-Carcelen, M.; Schwartz, J.; Carcelen, A.C. Behavioral and Socioemotional Development in Preterm Children. Clin. Perinatol. 2018, 45, 529–546. [Google Scholar] [CrossRef]
- McDonald, N.M.; Varcin, K.J.; Bhatt, R.; Wu, J.Y.; Sahin, M.; Nelson, C.A., 3rd; Jeste, S.S. Early autism symptoms in infants with tuberous sclerosis complex. Autism Res. 2017. [Google Scholar] [CrossRef]
- Zwaigenbaum, L. Congenital anomalies and etiological diversity in autism. Dev. Med. Child. Neurol. 2015, 57, 10–11. [Google Scholar] [CrossRef]
- Bonati, M.T.; Russo, S.; Finelli, P.; Valsecchi, M.R.; Cogliati, F.; Cavalleri, F.; Roberts, W.; Elia, M.; Larizza, L. Evaluation of autism traits in Angelman syndrome: A resource to unfold autism genes. Neurogenetics 2007, 8, 169–178. [Google Scholar] [CrossRef] [PubMed]
- McCary, L.M.; Roberts, J.E. Early identification of autism in fragile X syndrome: A review. J. Intellect. Disabil. Res. 2013, 57, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.J.H.; Dawson, G.; Kelly, J.; Estes, A.; Jane Webb, S. Parent-delivered early intervention in infants at risk for ASD: Effects on electrophysiological and habituation measures of social attention. Autism Res. 2017, 10, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Ou, J.J.; Li, Y.M.; Xiang, D.X. Dietary Supplement for Core Symptoms of Autism Spectrum Disorder: Where Are We Now and Where Should We Go? Front. Psychiatry 2017, 8, 155. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; Geis, E.; Gehn, E.; Fimbres, V.; Pollard, E.L.; Mitchell, J.; Ingram, J.; Hellmers, R.; Laake, D.; et al. Comprehensive Nutritional and Dietary Intervention for Autism Spectrum Disorder-A Randomized, Controlled 12-Month Trial. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Newman, N.C.; Ryan, P.; Lemasters, G.; Levin, L.; Bernstein, D.; Hershey, G.K.; Lockey, J.E.; Villareal, M.; Reponen, T.; Grinshpun, S.; et al. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ. Health Perspect. 2013, 121, 731–736. [Google Scholar] [CrossRef] [PubMed]
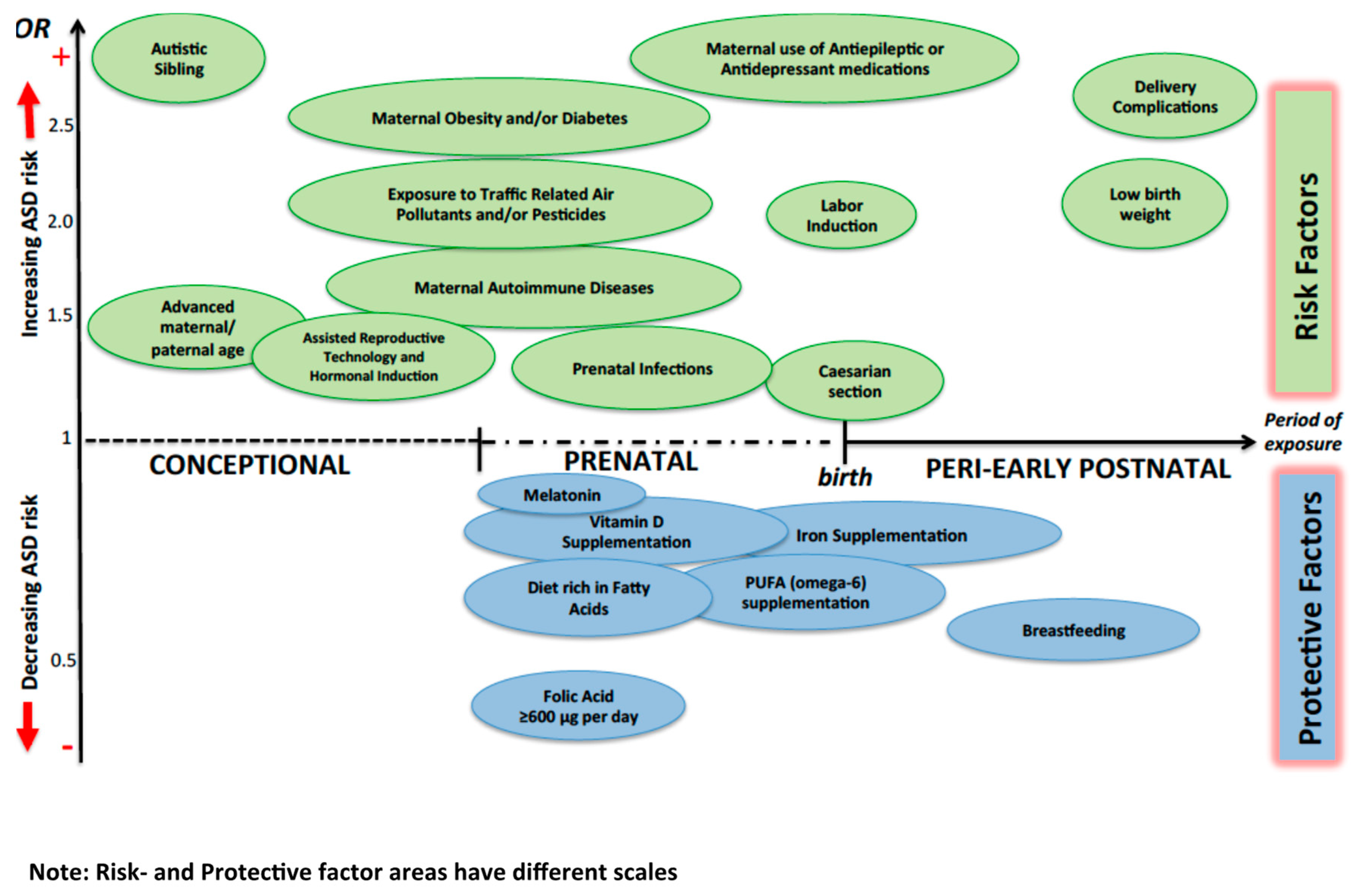
| Risk Factor | Hypothesized Period of Action | Selected Studies |
|---|---|---|
| Advanced parental age | Conception | Durkin et al., 2008 [23]; Ben Itzchak et al., 2011 [24]; Geier et al., 2016 [25]; Sandin et al., 2016 [27]; Modabbernia et al., 2017 [26] |
| Use of hormonal induction; Assisted Reproductive Technologies (ART) | Conception | Auyeung et al., 2009 [36]; Zachor & Ben Itzchak., 2011 [38]; Liu et al., 2017 [31] |
| Environmental chemicals and toxicants: air pollution pesticides phthalates | Conception, prenatal | Volk et al., 2011 [69]; Becerra et al., 2013 [70]; Rossignol et al., 2014 [40]; Weisskopf et al., 2015 [67]; Gong et al., 2017 [71]; Raz et al., 2018 [73]; Eskenazi et al., 2007 [79]; Cheslack-Postava, 2013 [77]; Shin et al., 2018 [134]; Braun et al., 2014 [78] |
| Nutritional factors: maternal obesity or undernutrition folates vitamin D deficiency iron deficiency | Conception; prenatal; early postnatal | Georgieff et al., 2007 [45]; Krakowiak et al., 2012 [41]; Getz et al., 2016 [42]; Andersen et al., 2018 [43]; Schmidt et al., 2011, 2012 and 2017 [46,135,136]; Vinkhuyzen et al., 2017 [101]; Schmidt et al., 2014 [53] |
| Medications: valproate other AEDs SSRIs antibiotics antibiotic | Prenatal | Roullet, et al., 2013 [86]; Veroniki et al., 2017 [87]; Mezzacappa et al., 2017 [57]; Atladottir, 2012 [91] |
| Infections; Fever; Maternal Immune Activation (MIA) | Prenatal | Zerbo et al., 2013 [105]; Jiang et al., 2016 [137]; Brucato et al., 2017 [106]; Zerbo et al., 2013 [105]; Parker-Athill et al., 2010 [109]; Jones et al., 2017 [110] |
| Maternal individual factors and diseases: gestational diabetes; maternal melatonin levels; depression (?) | Prenatal | Gardener at al., 2009 [114]; Lyall et al., 2012 [115]; Jin et al., 2018 [120] |
| Delivery method | Perinatal | Dodds et al., 2011 [122]; Emberti Gialloreti et al., 2014 [123] |
| Fetal distress | Perinatal | Modabbernia et al., 2017 [26]; Wang et al., 2017 [126] |
| Nutritional Protective Factors | Period of Exposure | Study |
|---|---|---|
| Folic acid of ≥600 μg Folic acid + MTHFR 677 C > T variant genotype | Prenatal Prenatal | Schmidt et al., 2012 [143]; Schmidt et al., 2011 [149]; Suren et al., 2013 [100] |
| Fatty acid PUFA | Prenatal | Lyall et al., 2013 [19]; Morgese et al., 2016 [148] |
| Vitamin D | Prenatal | Stubbs et al., 2016 [144] |
| Iron Iron + Breastfeeding | Prenatal; postnatal | Schmidt et al., 2014 [53] |
| Melatonin | Prenatal | Jin et al., 2018 [120] |
| Breast feeding | Postnatal | Bar et al., 2016 [146]; Boucher et al., 2017 [147]; Tseng et al., 2017 [145] |
| Clinical Recommendations | Minimizing Risk Factors | Maximizing Protective Factors | References |
|---|---|---|---|
| Periconception Period | Encouraging women weight loss in case of obesity and strict glycaemia control in case of diabetes; close monitoring and/or treatment of preconception maternal diseases and/or conditions (psychiatric conditions, vitamin D or folic acid deficiencies); close follow-up of children born after ART use using frequent developmental surveillance after birth | Monitor diet of women; encourage assumption of daily folic acid and vitamin D intake from natural sources before pregnancy; have reasonable exposure to sunlight. | Peretti et al., 2017 [99]; Schmidt et al., 2012 [143]; Andersen et al., 2018 [43]; Oberlander et al., 2017 [90]; Zachor & Ben Itzchak, 2011 [38] |
| Prenatal Period | Close monitoring and symptomatic treatment even for mothers with minor infections or inflammatory episodes; prevention of infections during pregnancy with vaccination programs; surveillance of mothers who are using long-term medications. Mothers who had already autistic children and/or with de novo or inherited ASD-associated CNVs are more susceptible to environmental insults in the subsequent pregnancy; therefore, a strict surveillance and treatment of infections or inflammatory episodes during whole pregnancy is highly recommended. | Recommend daily folic acid intake of ≥600 μg during the first month of pregnancy; recommend a constant intake of vitamin D and iron | Babenko et al., 2015 [156]; Schmidt et al., 2014 [53]; Mezzacappa et al., 2017 [57]; Veroniki et al., 2017 [87] |
| Perinatal/Early Postnatal Period | Close monitoring not only of premature newborns, but also of those with minor perinatal complications; defined medical and neuropsychological follow-up of preterm children; ASD screening in all preterm infants, as recommended by AAP, using instruments such as M-CHAT In case of syndromic ASD: early and frequent neurodevelopment assessment to promptly identify early signs suggestive of ASD (i.e. deficits in social communication behaviors in TSC, low adaptive behaviors in social area in FXS, lack of language development in Angelman syndrome, and difficulties in joint attention in preterm infants) In case of high risk for epilepsy, EEG monitoring and immediate treatment of seizures (to minimize the impact on long-term outcome) In all high-risk infants, (genetic syndromes, preterm birth, and familial history) parental education to warrant early referral and parent-mediated intervention | Whenever possible, encourage breastfeeding; monitor diet of infants and toddlers; early targeted behavioral interventions to potentiate cognitive abilities, which can act as protective factors reducing the severity of ASD symptoms | Curatolo et al., 2018 [157]; Peralta-Carcelen et al., 2018 [158]; McDonald et al., 2017 [159]; Tseng et al., 2017 [145]; Peretti et al., 2017 [99]; Zwaigenbaum et al., 2015 [160]; Bonati et al., 2007 [161]; McCary et al., [162]; Jones et al., 2017 [163] |
| As early as possible in high-risk infants and in newly diagnosed toddlers/children | Following the evaluation of biological parameters, provide appropriate nutraceutical supplementations | Li et al., 2017 [164]; Adams et al., 2018 [165] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emberti Gialloreti, L.; Mazzone, L.; Benvenuto, A.; Fasano, A.; Garcia Alcon, A.; Kraneveld, A.; Moavero, R.; Raz, R.; Riccio, M.P.; Siracusano, M.; et al. Risk and Protective Environmental Factors Associated with Autism Spectrum Disorder: Evidence-Based Principles and Recommendations. J. Clin. Med. 2019, 8, 217. https://doi.org/10.3390/jcm8020217
Emberti Gialloreti L, Mazzone L, Benvenuto A, Fasano A, Garcia Alcon A, Kraneveld A, Moavero R, Raz R, Riccio MP, Siracusano M, et al. Risk and Protective Environmental Factors Associated with Autism Spectrum Disorder: Evidence-Based Principles and Recommendations. Journal of Clinical Medicine. 2019; 8(2):217. https://doi.org/10.3390/jcm8020217
Chicago/Turabian StyleEmberti Gialloreti, Leonardo, Luigi Mazzone, Arianna Benvenuto, Alessio Fasano, Alicia Garcia Alcon, Aletta Kraneveld, Romina Moavero, Raanan Raz, Maria Pia Riccio, Martina Siracusano, and et al. 2019. "Risk and Protective Environmental Factors Associated with Autism Spectrum Disorder: Evidence-Based Principles and Recommendations" Journal of Clinical Medicine 8, no. 2: 217. https://doi.org/10.3390/jcm8020217
APA StyleEmberti Gialloreti, L., Mazzone, L., Benvenuto, A., Fasano, A., Garcia Alcon, A., Kraneveld, A., Moavero, R., Raz, R., Riccio, M. P., Siracusano, M., Zachor, D. A., Marini, M., & Curatolo, P. (2019). Risk and Protective Environmental Factors Associated with Autism Spectrum Disorder: Evidence-Based Principles and Recommendations. Journal of Clinical Medicine, 8(2), 217. https://doi.org/10.3390/jcm8020217







