Oral Appliance Therapy for Obstructive Sleep Apnoea: State of the Art
Abstract
1. Introduction
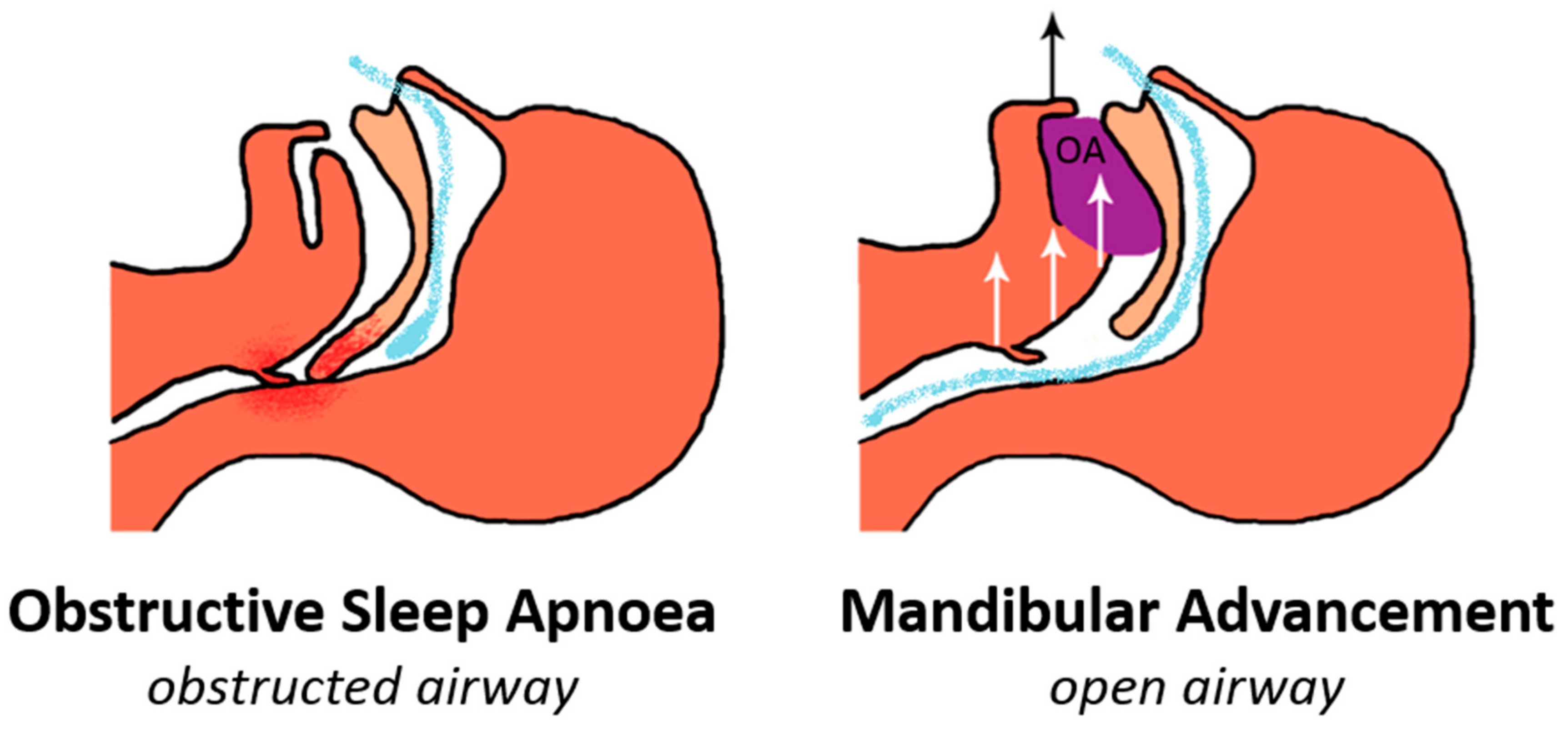
2. Mechanism of Action
3. Efficacy of Oral Appliance Therapy
4. Factors Related to Oral Appliance Efficacy and Prediction Methods
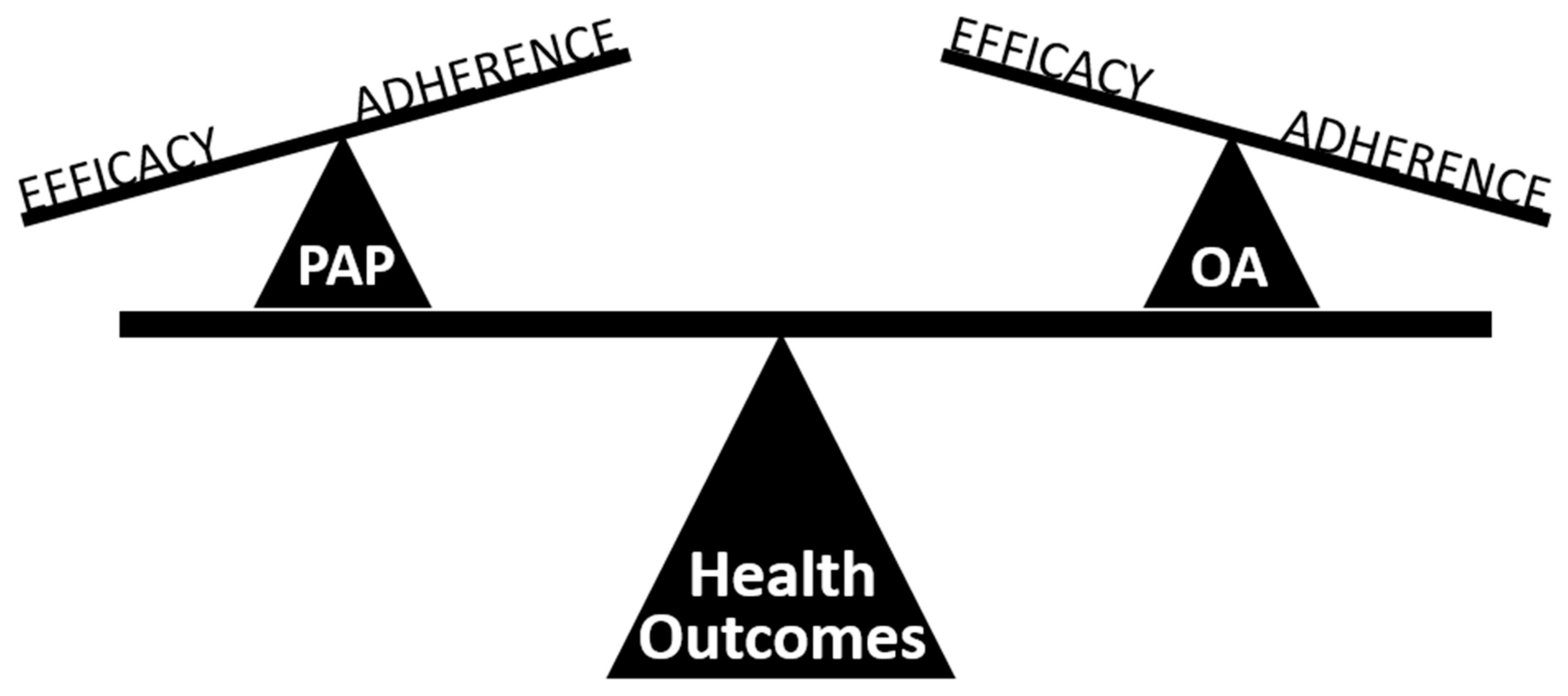
5. Treatment Effectiveness of Oral Appliance Therapy
6. Cardiovascular Health
7. Devices and Customisation
8. Long-term Side Effects
9. Objective Compliance
10. Combination Therapy Strategies
11. Models of Care
12. Toward a Precision Medicine Approach
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Marshall, N.S.; Wong, K.K.; Phillips, C.L.; Liu, P.Y.; Knuiman, M.W.; Grunstein, R.R. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J. Clin. Sleep Med. 2009, 5, 15–20. [Google Scholar] [PubMed]
- Sassani, A.; Findley, L.J.; Kryger, M.; Goldlust, E.; George, C.; Davidson, T.M. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep 2004, 27, 453–458. [Google Scholar] [CrossRef]
- Fu, Y.; Xia, Y.; Yi, H.; Xu, H.; Guan, J.; Yin, S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath 2017, 21, 181–189. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pepin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Bratton, D.J.; Gaisl, T.; Wons, A.M.; Kohler, M. CPAP vs Mandibular Advancement Devices and Blood Pressure in Patients With Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. JAMA 2015, 314, 2280–2293. [Google Scholar] [CrossRef]
- Weaver, T.E.; Maislin, G.; Dinges, D.F.; Bloxham, T.; George, C.F.; Greenberg, H.; Kader, G.; Mahowald, M.; Younger, J.; Pack, A.I. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007, 30, 711–719. [Google Scholar] [CrossRef]
- Ramar, K.; Dort, L.C.; Katz, S.G.; Lettieri, C.J.; Harrod, C.G.; Thomas, S.M.; Chervin, R.D. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J. Clin. Sleep Med. 2015, 11, 773–827. [Google Scholar] [CrossRef]
- Chang, E.T.; Fernandez-Salvador, C.; Giambo, J.; Nesbitt, B.; Liu, S.Y.; Capasso, R.; Kushida, C.A.; Camacho, M. Tongue retaining devices for obstructive sleep apnea: A systematic review and meta-analysis. Am. J. Otolaryngol. 2017, 38, 272–278. [Google Scholar] [CrossRef]
- Mehta, A.; Qian, J.; Petocz, P.; Darendeliler, M.A.; Cistulli, P.A. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2001, 163, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; Lee, R.W.; Srinivasan, V.K.; Darendeliler, M.A.; Grunstein, R.R.; Cistulli, P.A. Nasopharyngoscopic evaluation of oral appliance therapy for obstructive sleep apnoea. Eur. Respir. J. 2010, 35, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; Sutherland, K.; Schwab, R.J.; Zeng, B.; Petocz, P.; Lee, R.W.; Darendeliler, M.A.; Cistulli, P.A. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax 2010, 65, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.C.; Cheng, S.; McKenzie, D.K.; Butler, J.E.; Gandevia, S.C.; Bilston, L.E. Tongue and lateral upper airway movement with mandibular advancement. Sleep 2013, 36, 397–404. [Google Scholar] [CrossRef]
- Bamagoos, A.A.; Cistulli, P.A.; Sutherland, K.; Ngiam, J.; Burke, P.G.R.; Bilston, L.E.; Butler, J.E.; Eckert, D.J. Dose-dependent effects of mandibular advancement on upper airway collapsibility and muscle function in obstructive sleep apnea. Sleep 2019, 42. [Google Scholar] [CrossRef]
- Marques, M.; Genta, P.R.; Azarbarzin, A.; Taranto-Montemurro, L.; Messineo, L.; Hess, L.B.; Demko, G.; White, D.P.; Sands, S.A.; Wellman, A. Structure and severity of pharyngeal obstruction determine oral appliance efficacy in sleep apnoea. J. Physiol. 2019. [Google Scholar] [CrossRef]
- Kato, J.; Isono, S.; Tanaka, A.; Watanabe, T.; Araki, D.; Tanzawa, H.; Nishino, T. Dose-dependent effects of mandibular advancement on pharyngeal mechanics and nocturnal oxygenation in patients with sleep-disordered breathing. Chest 2000, 117, 1065–1072. [Google Scholar] [CrossRef]
- Bamagoos, A.A.; Eckert, D.J.; Sutherland, K.; Ngiam, J.; Cistulli, P.A. Dose-dependent effects of mandibular advancement on optimal positive airway pressure requirements in obstructive sleep apnoea. Sleep Breath 2019. [Google Scholar] [CrossRef]
- Vroegop, A.V.; Vanderveken, O.M.; Dieltjens, M.; Wouters, K.; Saldien, V.; Braem, M.J.; Van de Heyning, P.H. Sleep endoscopy with simulation bite for prediction of oral appliance treatment outcome. J. Sleep Res. 2013, 22, 348–355. [Google Scholar] [CrossRef]
- Johal, A.; Gill, G.; Ferman, A.; McLaughlin, K. The effect of mandibular advancement appliances on awake upper airway and masticatory muscle activity in patients with obstructive sleep apnoea. Clin. Physiol. Funct. Imaging 2007, 27, 47–53. [Google Scholar] [CrossRef]
- Tsuiki, S.; Ono, T.; Kuroda, T. Mandibular Advancement Modulates Respiratory-Related Genioglossus Electromyographic Activity. Sleep Breath 2000, 4, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.R.; Tsuiki, S.; Hattori, Y.; Takei, Y.; Inoue, Y.; Lowe, A.A. Dose-dependent effects of mandibular protrusion on genioglossus activity in sleep apnoea. Eur. Respir. J. 2011, 37, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.; Takaya, H.; Qian, J.; Petocz, P.; Ng, A.T.; Cistulli, P.A. Oral Appliance Treatment Response and Polysomnographic Phenotypes of Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Sharples, L.D.; Clutterbuck-James, A.L.; Glover, M.J.; Bennett, M.S.; Chadwick, R.; Pittman, M.A.; Quinnell, T.G. Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea. Sleep Med. Rev. 2016, 27, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Okuno, K.; Pliska, B.T.; Hamoda, M.; Lowe, A.A.; Almeida, F.R. Prediction of oral appliance treatment outcomes in obstructive sleep apnea: A systematic review. Sleep Med. Rev. 2016, 30, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Marklund, M.; Stenlund, H.; Franklin, K.A. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: Tolerability and predictors of treatment success. Chest 2004, 125, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Vecchierini, M.F.; Attali, V.; Collet, J.M.; d’Ortho, M.P.; Goutorbe, F.; Kerbrat, J.B.; Leger, D.; Lavergne, F.; Monaca, C.; Monteyrol, P.J.; et al. Sex differences in mandibular repositioning device therapy effectiveness in patients with obstructive sleep apnea syndrome. Sleep Breath 2019, 23, 837–848. [Google Scholar] [CrossRef]
- Remmers, J.; Charkhandeh, S.; Grosse, J.; Topor, Z.; Brant, R.; Santosham, P.; Bruehlmann, S. Remotely controlled mandibular protrusion during sleep predicts therapeutic success with oral appliances in patients with obstructive sleep apnea. Sleep 2013, 36, 1517–1525. [Google Scholar] [CrossRef]
- Denolf, P.L.; Vanderveken, O.M.; Marklund, M.E.; Braem, M.J. The status of cephalometry in the prediction of non-CPAP treatment outcome in obstructive sleep apnea patients. Sleep Med. Rev. 2016, 27, 56–73. [Google Scholar] [CrossRef]
- Alessandri-Bonetti, G.; Ippolito, D.R.; Bartolucci, M.L.; D’Anto, V.; Incerti-Parenti, S. Cephalometric predictors of treatment outcome with mandibular advancement devices in adult patients with obstructive sleep apnea: A systematic review. Korean J. Orthod. 2015, 45, 308–321. [Google Scholar] [CrossRef]
- Tsuiki, S.; Ito, E.; Isono, S.; Ryan, C.F.; Komada, Y.; Matsuura, M.; Inoue, Y. Oropharyngeal crowding and obesity as predictors of oral appliance treatment response to moderate obstructive sleep apnea. Chest 2013, 144, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.T.; Darendeliler, M.A.; Petocz, P.; Cistulli, P.A. Cephalometry and prediction of oral appliance treatment outcome. Sleep Breath 2012, 16, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Storesund, A.; Johansson, A.; Bjorvatn, B.; Lehmann, S. Oral appliance treatment outcome can be predicted by continuous positive airway pressure in moderate to severe obstructive sleep apnea. Sleep Breath 2018, 22, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Dort, L.C.; Savard, N.; Dort, E.; Dort, J. Does CPAP pressure predict treatment outcome with oral appliances? J. Dent. Sleep Med. 2016, 3, 113–117. [Google Scholar] [CrossRef]
- Sutherland, K.; Phillips, C.L.; Davies, A.; Srinivasan, V.K.; Dalci, O.; Yee, B.J.; Darendeliler, M.A.; Grunstein, R.R.; Cistulli, P.A. CPAP pressure for prediction of oral appliance treatment response in obstructive sleep apnea. J. Clin. Sleep Med. 2014, 10, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Ng, A.T.; Darendeliler, M.A.; Petocz, P.; Cistulli, P.A. Use of flow-volume curves to predict oral appliance treatment outcome in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2007, 175, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; Lee, R.W.; Srinivasan, V.K.; Darendeliler, M.A.; Cistulli, P.A. Use of flow-volume curves to predict oral appliance treatment outcome in obstructive sleep apnea: A prospective validation study. Sleep Breath 2011, 15, 157–162. [Google Scholar] [CrossRef]
- Okuno, K.; Sasao, Y.; Nohara, K.; Sakai, T.; Pliska, B.T.; Lowe, A.A.; Ryan, C.F.; Almeida, F.R. Endoscopy evaluation to predict oral appliance outcomes in obstructive sleep apnoea. Eur. Respir. J. 2016, 47, 1410–1419. [Google Scholar] [CrossRef]
- Ng, A.T.; Qian, J.; Cistulli, P.A. Oropharyngeal collapse predicts treatment response with oral appliance therapy in obstructive sleep apnea. Sleep 2006, 29, 666–671. [Google Scholar]
- Sutherland, K.; Ngiam, J.; Cistulli, P.A. Performance of Remotely Controlled Mandibular Protrusion Sleep Studies for Prediction of Oral Appliance Treatment Response. J. Clin. Sleep Med. 2017, 13, 411–417. [Google Scholar] [CrossRef][Green Version]
- Tsai, W.H.; Vazquez, J.C.; Oshima, T.; Dort, L.; Roycroft, B.; Lowe, A.A.; Hajduk, E.; Remmers, J.E. Remotely controlled mandibular positioner predicts efficacy of oral appliances in sleep apnea. Am. J. Respir. Crit. Care Med. 2004, 170, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.A.; Andara, C.; Landry, S.; Sands, S.A.; Joosten, S.A.; Owens, R.L.; White, D.P.; Hamilton, G.S.; Wellman, A. Upper-Airway Collapsibility and Loop Gain Predict the Response to Oral Appliance Therapy in Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2016, 194, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.; Almeida, F.R.; de Chazal, P.; Cistulli, P.A. Prediction in obstructive sleep apnoea: Diagnosis, comorbidity risk, and treatment outcomes. Expert Rev. Respir. Med. 2018, 12, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Tsuiki, S.; Isono, S.; Ishikawa, T.; Yamashiro, Y.; Tatsumi, K.; Nishino, T. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology 2008, 108, 1009–1015. [Google Scholar] [CrossRef]
- Mostafiz, W.; Dalci, O.; Sutherland, K.; Malhotra, A.; Srinivasan, V.; Darendeliler, M.A.; Cistulli, P.A. Influence of oral and craniofacial dimensions on mandibular advancement splint treatment outcome in patients with obstructive sleep apnea. Chest 2011, 139, 1331–1339. [Google Scholar] [CrossRef]
- Sutherland, K.; Chan, A.S.; Cistulli, P.A. Three-dimensional assessment of anatomical balance and oral appliance treatment outcome in obstructive sleep apnoea. Sleep Breath 2016, 20, 903–910. [Google Scholar] [CrossRef]
- Landry, S.A.; Joosten, S.A.; Eckert, D.J.; Jordan, A.S.; Sands, S.A.; White, D.P.; Malhotra, A.; Wellman, A.; Hamilton, G.S.; Edwards, B.A. Therapeutic CPAP Level Predicts Upper Airway Collapsibility in Patients With Obstructive Sleep Apnea. Sleep 2017, 40. [Google Scholar] [CrossRef]
- Tsuiki, S.; Kobayashi, M.; Namba, K.; Oka, Y.; Komada, Y.; Kagimura, T.; Inoue, Y. Optimal positive airway pressure predicts oral appliance response to sleep apnoea. Eur. Respir. J. 2010, 35, 1098–1105. [Google Scholar] [CrossRef]
- Bosshard, V.; Masse, J.F.; Series, F. Prediction of oral appliance efficiency in patients with apnoea using phrenic nerve stimulation while awake. Thorax 2011, 66, 220–225. [Google Scholar] [CrossRef][Green Version]
- Sutherland, K.; Chan, A.S.L.; Ngiam, J.; Darendeliler, M.A.; Cistulli, P.A. Qualitative assessment of awake nasopharyngoscopy for prediction of oral appliance treatment response in obstructive sleep apnoea. Sleep Breath 2018, 22, 1029–1036. [Google Scholar] [CrossRef]
- Eckert, D.J. Phenotypic approaches to obstructive sleep apnoea—New pathways for targeted therapy. Sleep Med. Rev. 2018, 37, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.; Chan, A.S.L.; Ngiam, J.; Dalci, O.; Darendeliler, M.A.; Cistulli, P.A. Awake Multimodal Phenotyping for Prediction of Oral Appliance Treatment Outcome. J. Clin. Sleep Med. 2018, 14, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Vanderveken, O.M.; Devolder, A.; Marklund, M.; Boudewyns, A.N.; Braem, M.J.; Okkerse, W.; Verbraecken, J.A.; Franklin, K.A.; De Backer, W.A.; Van de Heyning, P.H. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am. J. Respir. Crit. Care Med. 2008, 178, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Johal, A.; Haria, P.; Manek, S.; Joury, E.; Riha, R. Ready-Made Versus Custom-Made Mandibular Repositioning Devices in Sleep Apnea: A Randomized Clinical Trial. J. Clin. Sleep Med. 2017, 13, 175–182. [Google Scholar] [CrossRef]
- Dort, L.C.; Hadjuk, E.; Remmers, J.E. Mandibular advancement and obstructive sleep apnoea: A method for determining effective mandibular protrusion. Eur. Respir. J. 2006, 27, 1003–1009. [Google Scholar] [CrossRef]
- Petelle, B.; Vincent, G.; Gagnadoux, F.; Rakotonanahary, D.; Meyer, B.; Fleury, B. One-night mandibular advancement titration for obstructive sleep apnea syndrome: A pilot study. Am. J. Respir. Crit. Care Med. 2002, 165, 1150–1153. [Google Scholar] [CrossRef]
- Kastoer, C.; Dieltjens, M.; Oorts, E.; Hamans, E.; Braem, M.J.; Van de Heyning, P.H.; Vanderveken, O.M. The Use of Remotely Controlled Mandibular Positioner as a Predictive Screening Tool for Mandibular Advancement Device Therapy in Patients with Obstructive Sleep Apnea through Single-Night Progressive Titration of the Mandible: A Systematic Review. J. Clin. Sleep Med. 2016, 12, 1411–1421. [Google Scholar] [CrossRef][Green Version]
- Remmers, J.E.; Topor, Z.; Grosse, J.; Vranjes, N.; Mosca, E.V.; Brant, R.; Bruehlmann, S.; Charkhandeh, S.; Zareian Jahromi, S.A. A Feedback-Controlled Mandibular Positioner Identifies Individuals With Sleep Apnea Who Will Respond to Oral Appliance Therapy. J. Clin. Sleep Med. 2017, 13, 871–880. [Google Scholar] [CrossRef]
- Alshaer, H.; Ryan, C.; Fernie, G.R.; Bradley, T.D. Reproducibility and predictors of the apnea hypopnea index across multiple nights. Sleep Sci. 2018, 11, 28–33. [Google Scholar] [CrossRef]
- Younes, M.; Raneri, J.; Hanly, P. Staging Sleep in Polysomnograms: Analysis of Inter-Scorer Variability. J. Clin. Sleep Med. 2016, 12, 885–894. [Google Scholar] [CrossRef]
- Vuorjoki-Ranta, T.R.; Aarab, G.; Lobbezoo, F.; Tuomilehto, H.; Ahlberg, J. Weight gain may affect mandibular advancement device therapy in patients with obstructive sleep apnea: A retrospective study. Sleep Breath 2019, 23, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Marklund, M. Long-term efficacy of an oral appliance in early treated patients with obstructive sleep apnea. Sleep Breath 2016, 20, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.C.; Sutherland, K.; Cistulli, P.A.; Pack, A.I. P4 medicine approach to obstructive sleep apnoea. Respirology 2017, 22, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Muriel, A.; Peker, Y. CPAP Adherence for Prevention of Major Adverse Cerebrovascular and Cardiovascular Events in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.P.; Edwards, B.A.; Gautam, S.P.; Montesi, S.B.; Duran-Cantolla, J.; Aizpuru, F.; Barbe, F.; Sanchez-de-la-Torre, M.; Malhotra, A. Blood pressure improvement with continuous positive airway pressure is independent of obstructive sleep apnea severity. J. Clin. Sleep Med. 2014, 10, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Lv, Y.; Li, K.; Ma, L.; Du, G.; Xiang, Y.; Li, X. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: A systematic review and meta-analysis of six randomized controlled trials. J. Bras. Pneumol. 2017, 43, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.L.; Grunstein, R.R.; Darendeliler, M.A.; Mihailidou, A.S.; Srinivasan, V.K.; Yee, B.J.; Marks, G.B.; Cistulli, P.A. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2013, 187, 879–887. [Google Scholar] [CrossRef]
- Sutherland, K.; Phillips, C.L.; Cistulli, P.A. Efficacy versus Effectiveness in the treatment of Obstructive Sleep Apnea: CPAP and oral appliances. J. Dent. Sleep Med. 2015, 2, 175–181. [Google Scholar] [CrossRef]
- Vanderveken, O.M.; Dieltjens, M.; Wouters, K.; De Backer, W.A.; Van de Heyning, P.H.; Braem, M.J. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax 2013, 68, 91–96. [Google Scholar] [CrossRef]
- Ravesloot, M.J.; de Vries, N. Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited. Sleep 2011, 34, 105–110. [Google Scholar] [CrossRef]
- Ravesloot, M.J.; de Vries, N.; Stuck, B.A. Treatment adherence should be taken into account when reporting treatment outcomes in obstructive sleep apnea. Laryngoscope 2014, 124, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.B.; Upender, R.; Walters, A.S.; Goodpaster, R.L.; Stanley, J.J.; Wang, L.; Chandrasekhar, R. Effective Apnea-Hypopnea Index (“Effective AHI”): A New Measure of Effectiveness for Positive Airway Pressure Therapy. Sleep 2016, 39, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Hla, K.M.; Young, T.; Hagen, E.W.; Stein, J.H.; Finn, L.A.; Nieto, F.J.; Peppard, P.E. Coronary heart disease incidence in sleep disordered breathing: The Wisconsin Sleep Cohort Study. Sleep 2015, 38, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.J.; Young, T.B.; Lind, B.K.; Shahar, E.; Samet, J.M.; Redline, S.; D’Agostino, R.B.; Newman, A.B.; Lebowitz, M.D.; Pickering, T.G. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000, 283, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Redline, S.; Yenokyan, G.; Gottlieb, D.J.; Shahar, E.; O’Connor, G.T.; Resnick, H.E.; Diener-West, M.; Sanders, M.H.; Wolf, P.A.; Geraghty, E.M.; et al. Obstructive sleep apnea-hypopnea and incident stroke: The sleep heart health study. Am. J. Respir. Crit. Care Med. 2010, 182, 269–277. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- De Vries, G.E.; Wijkstra, P.J.; Houwerzijl, E.J.; Kerstjens, H.A.M.; Hoekema, A. Cardiovascular effects of oral appliance therapy in obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2018, 40, 55–68. [Google Scholar] [CrossRef]
- Anandam, A.; Patil, M.; Akinnusi, M.; Jaoude, P.; El-Solh, A.A. Cardiovascular mortality in obstructive sleep apnoea treated with continuous positive airway pressure or oral appliance: An observational study. Respirology 2013, 18, 1184–1190. [Google Scholar] [CrossRef]
- Gagnadoux, F.; Pepin, J.L.; Vielle, B.; Bironneau, V.; Chouet-Girard, F.; Launois, S.; Meslier, N.; Meurice, J.C.; Nguyen, X.L.; Paris, A.; et al. Impact of Mandibular Advancement Therapy on Endothelial Function in Severe Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2017, 195, 1244–1252. [Google Scholar] [CrossRef]
- Gagnadoux, F.; Nguyen, X.L.; Le Vaillant, M.; Priou, P.; Meslier, N.; Eberlein, A.; Kun-Darbois, J.D.; Chaufton, C.; Villiers, B.; Levy, M.; et al. Comparison of titrable thermoplastic versus custom-made mandibular advancement device for the treatment of obstructive sleep apnoea. Respir. Med. 2017, 131, 35–42. [Google Scholar] [CrossRef]
- Johal, A.; Agha, B. Ready-made versus custom-made mandibular advancement appliances in obstructive sleep apnea: A systematic review and meta-analysis. J. Sleep Res. 2018, 27, e12660. [Google Scholar] [CrossRef] [PubMed]
- Aarab, G.; Lobbezoo, F.; Hamburger, H.L.; Naeije, M. Effects of an oral appliance with different mandibular protrusion positions at a constant vertical dimension on obstructive sleep apnea. Clin. Oral Investig. 2010, 14, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Dieltjens, M.; Vanderveken, O.M.; Heyning, P.H.; Braem, M.J. Current opinions and clinical practice in the titration of oral appliances in the treatment of sleep-disordered breathing. Sleep Med. Rev. 2012, 16, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.E.; Attarian, H.P.; Harrison, M.C.; Blank, J.E.; Takacs, C.M.; Smith, D.L.; Gozal, D. High-Resolution Pulse Oximetry and Titration of a Mandibular Advancement Device for Obstructive Sleep Apnea. Front. Neurol. 2019, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Okuno, K.; Ikai, K.; Matsumura-Ai, E.; Araie, T. Titration technique using endoscopy for an oral appliance treatment of obstructive sleep apnea. J. Prosthet. Dent. 2018, 119, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Kastoer, C.; Dieltjens, M.; Op de Beeck, S.; Braem, M.J.; Van de Heyning, P.H.; Vanderveken, O.M. Remotely Controlled Mandibular Positioning During Drug-Induced Sleep Endoscopy Toward Mandibular Advancement Device Therapy: Feasibility and Protocol. J. Clin. Sleep Med. 2018, 14, 1409–1413. [Google Scholar] [CrossRef]
- Nikolopoulou, M.; Naeije, M.; Aarab, G.; Hamburger, H.L.; Visscher, C.M.; Lobbezoo, F. The effect of raising the bite without mandibular protrusion on obstructive sleep apnoea. J. Oral Rehabil. 2011, 38, 643–647. [Google Scholar] [CrossRef]
- Vroegop, A.V.; Vanderveken, O.M.; Van de Heyning, P.H.; Braem, M.J. Effects of vertical opening on pharyngeal dimensions in patients with obstructive sleep apnoea. Sleep Med. 2012, 13, 314–316. [Google Scholar] [CrossRef]
- Pitsis, A.J.; Darendeliler, M.A.; Gotsopoulos, H.; Petocz, P.; Cistulli, P.A. Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2002, 166, 860–864. [Google Scholar] [CrossRef]
- Norrhem, N.; Marklund, M. An oral appliance with or without elastic bands to control mouth opening during sleep-a randomized pilot study. Sleep Breath 2016, 20, 929–938. [Google Scholar] [CrossRef]
- Milano, F.; Mutinelli, S.; Sutherland, K.; Milioli, G.; Scaramuzzino, G.; Cortesi, A.B.; Siciliani, G.; Lombardo, L.; Cistulli, P.A. Influence of vertical mouth opening on oral appliance treatment outcome in positional obstructive sleep apnea: A pilot study. J. Dent. Sleep Med. 2018, 5, 17–23. [Google Scholar] [CrossRef]
- Vecchierini, M.F.; Attali, V.; Collet, J.M.; d’Ortho, M.P.; El Chater, P.; Kerbrat, J.B.; Leger, D.; Monaca, C.; Monteyrol, P.J.; Morin, L.; et al. A custom-made mandibular repositioning device for obstructive sleep apnoea-hypopnoea syndrome: The ORCADES study. Sleep Med. 2016, 19, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Schell, T.G. Avoiding and Managing Oral Appliance Therapy Side Effects. Sleep Med. Clin. 2018, 13, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Marklund, M. Subjective versus objective dental side effects from oral sleep apnea appliances. Sleep Breath 2019. [Google Scholar] [CrossRef]
- Hamoda, M.M.; Almeida, F.R.; Pliska, B.T. Long-term side effects of sleep apnea treatment with oral appliances: Nature, magnitude and predictors of long-term changes. Sleep Med. 2019, 56, 184–191. [Google Scholar] [CrossRef]
- Minagi, H.O.; Okuno, K.; Nohara, K.; Sakai, T. Predictors of Side Effects With Long-Term Oral Appliance Therapy for Obstructive Sleep Apnea. J. Clin. Sleep Med. 2018, 14, 119–125. [Google Scholar] [CrossRef]
- Pliska, B.T.; Nam, H.; Chen, H.; Lowe, A.A.; Almeida, F.R. Obstructive sleep apnea and mandibular advancement splints: Occlusal effects and progression of changes associated with a decade of treatment. J. Clin. Sleep Med. 2014, 10, 1285–1291. [Google Scholar] [CrossRef]
- Marklund, M. Predictors of long-term orthodontic side effects from mandibular advancement devices in patients with snoring and obstructive sleep apnea. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 214–221. [Google Scholar] [CrossRef]
- Norrhem, N.; Nemeczek, H.; Marklund, M. Changes in lower incisor irregularity during treatment with oral sleep apnea appliances. Sleep Breath 2017, 21, 607–613. [Google Scholar] [CrossRef]
- Kirshenblatt, S.; Chen, H.; Dieltjens, M.; Pliska, B.T.; Almeida, F.R. Accuracy of thermosensitive microsensors intended to monitor patient use of removeable oral appliances. J. Can. Dent. Assoc. 2018, 84, i2. [Google Scholar]
- Dieltjens, M.; Braem, M.J.; Vroegop, A.; Wouters, K.; Verbraecken, J.A.; De Backer, W.A.; Van de Heyning, P.H.; Vanderveken, O.M. Objectively measured vs self-reported compliance during oral appliance therapy for sleep-disordered breathing. Chest 2013, 144, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Mullane, S.; Loke, W. Influence of short-term side effects on oral sleep appliance compliance among CPAP-intolerant patients: An objective monitoring of compliance. J. Oral Rehabil. 2019, 46, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Dieltjens, M.; Verbruggen, A.E.; Braem, M.J.; Wouters, K.; Verbraecken, J.A.; De Backer, W.A.; Hamans, E.; Van de Heyning, P.H.; Vanderveken, O.M. Determinants of Objective Compliance During Oral Appliance Therapy in Patients With Sleep-Disordered Breathing: A Prospective Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.; Kairaitis, K.; Yee, B.J.; Cistulli, P.A. From CPAP to tailored therapy for obstructive sleep Apnoea. Multidiscip. Respir. Med. 2018, 13, 44. [Google Scholar] [CrossRef]
- De Vries, G.E.; Doff, M.H.; Hoekema, A.; Kerstjens, H.A.; Wijkstra, P.J. Continuous positive airway pressure and oral appliance hybrid therapy in obstructive sleep apnea: Patient comfort, compliance and preference: A pilot study. J. Dent. Sleep Med. 2016, 3, 5–10. [Google Scholar] [CrossRef]
- El-Solh, A.A.; Moitheennazima, B.; Akinnusi, M.E.; Churder, P.M.; Lafornara, A.M. Combined oral appliance and positive airway pressure therapy for obstructive sleep apnea: A pilot study. Sleep Breath 2011, 15, 203–208. [Google Scholar] [CrossRef]
- Liu, H.W.; Chen, Y.J.; Lai, Y.C.; Huang, C.Y.; Huang, Y.L.; Lin, M.T.; Han, S.Y.; Chen, C.L.; Yu, C.J.; Lee, P.L. Combining MAD and CPAP as an effective strategy for treating patients with severe sleep apnea intolerant to high-pressure PAP and unresponsive to MAD. PLoS ONE 2017, 12, e0187032. [Google Scholar] [CrossRef]
- Almeida, F.R.; Mulgrew, A.; Ayas, N.; Tsuda, H.; Lowe, A.A.; Fox, N.; Harrison, S.; Fleetham, J.A. Mandibular advancement splint as short-term alternative treatment in patients with obstructive sleep apnea already effectively treated with continuous positive airway pressure. J. Clin. Sleep Med. 2013, 9, 319–324. [Google Scholar] [CrossRef]
- Dieltjens, M.; Vroegop, A.V.; Verbruggen, A.E.; Wouters, K.; Willemen, M.; De Backer, W.A.; Verbraecken, J.A.; Van de Heyning, P.H.; Braem, M.J.; de Vries, N.; et al. A promising concept of combination therapy for positional obstructive sleep apnea. Sleep Breath 2015, 19, 637–644. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, H.F.; Xie, Y. Efficacy of uvulopalatopharyngoplasty combined with oral appliance in treatment of obstructive sleep apnea-hypopnea syndrome. Ir. J. Med. Sci. 2015, 184, 329–334. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Gurubhagavatula, I.; Teff, K.; Rader, D.J.; Wadden, T.A.; Townsend, R.; Foster, G.D.; Maislin, G.; Saif, H.; Broderick, P.; et al. CPAP, weight loss, or both for obstructive sleep apnea. N. Engl. J. Med. 2014, 370, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Lai, V.; Tong, B.K.; Tran, C.; Ricciardiello, A.; Donegan, M.; Murray, N.P.; Carberry, J.C.; Eckert, D.J. Combination therapy with mandibular advancement and expiratory positive airway pressure valves reduces obstructive sleep apnea severity. Sleep 2019, 42. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, J.; Yaggi, H.K. Patient-centered care in obstructive sleep apnea: A vision for the future. Sleep Med. Rev. 2018, 37, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Cistulli, P.A.; Sutherland, K. Phenotyping obstructive sleep apnoea-Bringing precision to oral appliance therapy. J. Oral Rehabil. 2019. [Google Scholar] [CrossRef] [PubMed]
| Treatment Response Definitions | All OSA | OSA Severity | |||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||
| ‘Complete response’ | Treatment AHI < 5/h | 36.5% | 52.2% | 38.3% | 23.6% |
| ‘Near-complete response’ | Treatment AHI < 10/h + ≥ 50% AHI reduction | 52.2% | 52.2% | 59.6% | 42.1% |
| ‘Partial response’ | ≥50% AHI reduction | 63.8% | 52.2% | 64.8% | 70.0% |
| Prediction Test | Range of Diagnostic Accuracy | Accuracy Classification | Applicability Concerns | Reference | |||
|---|---|---|---|---|---|---|---|
| AUC | Sensitivity | Specificity | Accuracy | ||||
| Craniofacial (cephalometry) | 0.73–0.86 | 0.96 | 0.72 | Fair–Poor | Radiation, poor prediction | [30] | |
| Clinical factors (age, BMI) and OSA severity | 0.66 | 58% | Poor | Clinically applicable, but poor prediction | [23] | ||
| Obesity and Mallampati score | 0.85 | 0.55 | Easy to perform, no prospective studies | [31] | |||
| Clinical factors and craniofacial (cephalometry) | 0.73 | 0.61–0.78 | 0.55–0.82 | 51% | Poor | Radiation, poor prediction | [32] |
| PAP optimal pressure | 0.65–0.70 | 0.86–0.87 | 0.32–0.62 | Poor | Requires available pressure value, clinically applicable but variation between studies | [33,34,35] | |
| Spirometry | 0.91 | 0.36–0.80 | 0.30–0.80 | 45–57% | Excellent–Poor | Excellent performance in derivation study, but poor on prospective validation | [36,37] |
| Drug-induced sleep endoscopy | 0.82 | 0.49 | 0.78 | 58% | Good | Costly and not widely available | [19] |
| Awake nasendoscopy | 0.74–0.87 | 0.65–0.88 | 0.68–0.80 | 80% | Good | Excellent only in a small study of Japanese patients | [38] |
| Site of pharyngeal collapse (multisensory catheter) | 0.57–0.80 | 0.73–1.0 | Good | Invasive, not clinically applicable | [39] | ||
| Remotely controlled mandibular protrusion sleep studies | 0.60–0.86 | 0.89–0.92 | 88% | Good–Excellent | Excellent if based on ODI, good if based on AHI. Potentially poor if accounting for inconclusive tests | [28,40,41] | |
| Pathophysiology (airway collapsibility and unstable ventilator control) | 0.86–0.96 | 1.0 | 0.87 | 63% | Good | Small sample, no prospective validation, not clinically applicable | [42] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutherland, K.; Cistulli, P.A. Oral Appliance Therapy for Obstructive Sleep Apnoea: State of the Art. J. Clin. Med. 2019, 8, 2121. https://doi.org/10.3390/jcm8122121
Sutherland K, Cistulli PA. Oral Appliance Therapy for Obstructive Sleep Apnoea: State of the Art. Journal of Clinical Medicine. 2019; 8(12):2121. https://doi.org/10.3390/jcm8122121
Chicago/Turabian StyleSutherland, Kate, and Peter A. Cistulli. 2019. "Oral Appliance Therapy for Obstructive Sleep Apnoea: State of the Art" Journal of Clinical Medicine 8, no. 12: 2121. https://doi.org/10.3390/jcm8122121
APA StyleSutherland, K., & Cistulli, P. A. (2019). Oral Appliance Therapy for Obstructive Sleep Apnoea: State of the Art. Journal of Clinical Medicine, 8(12), 2121. https://doi.org/10.3390/jcm8122121





