Elevated Serum 25-Hydroxyvitamin D Levels Are Associated with Improved Bone Formation and Micro-Structural Measures in Elderly Hip Fracture Patients
Abstract
1. Introduction
2. Experimental Section
2.1. Bone and Serum Specimens
2.2. Serum Analyses
2.3. Bone Biopsy Prepareation
2.4. Bone Biopsy Micro-Computed Tomography
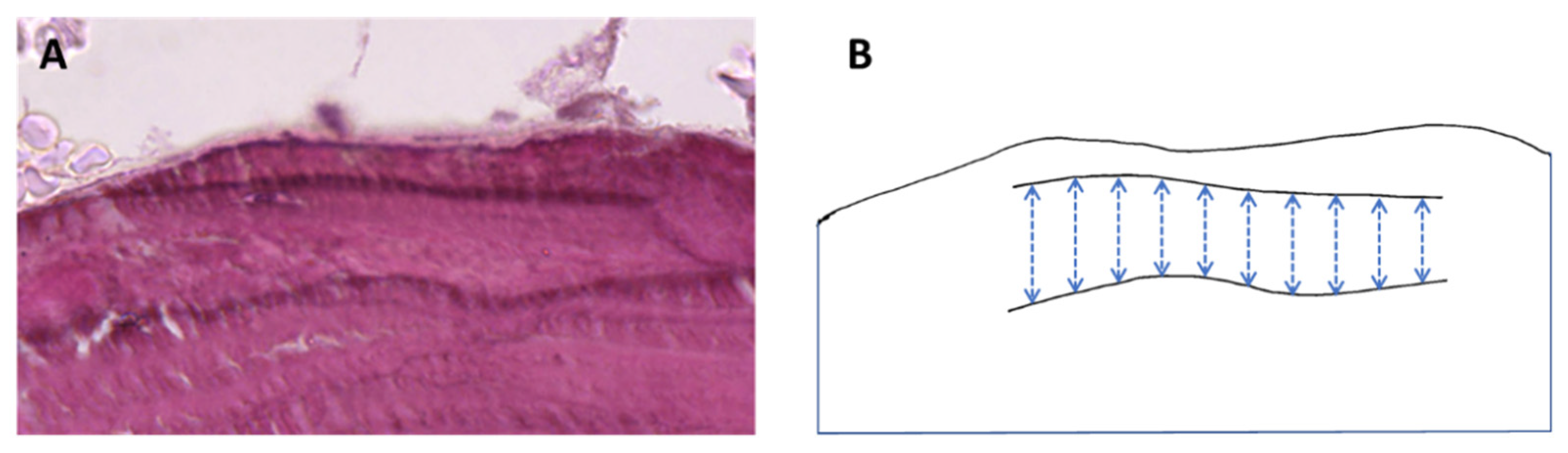
2.5. Bone Biopsy Histology
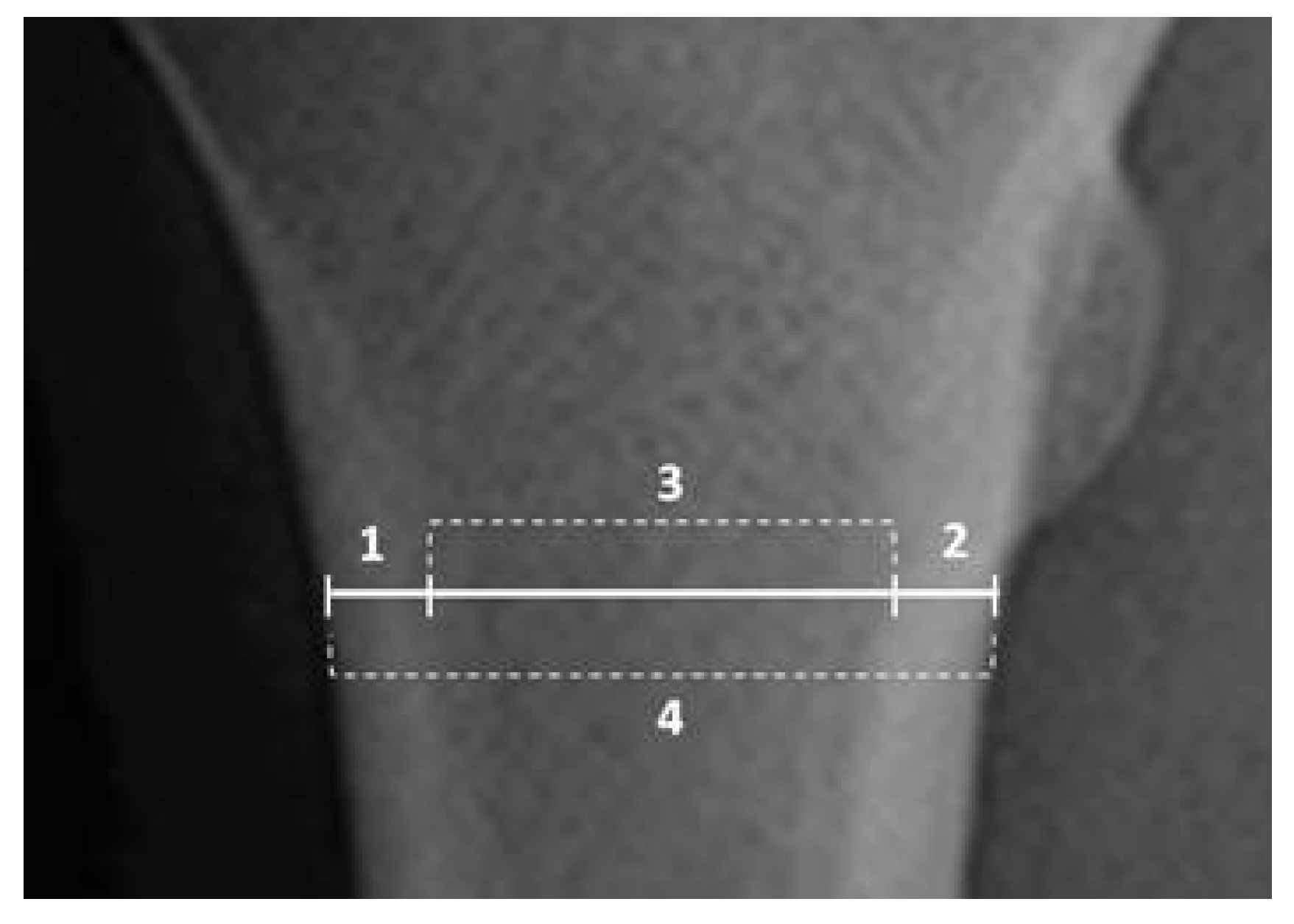
2.6. Rodiographic Cortical Bone Analyses
2.7. Bone mRNA Analyses
2.8. Statistical Analyses
3. Results
3.1. Biochemical Analyses
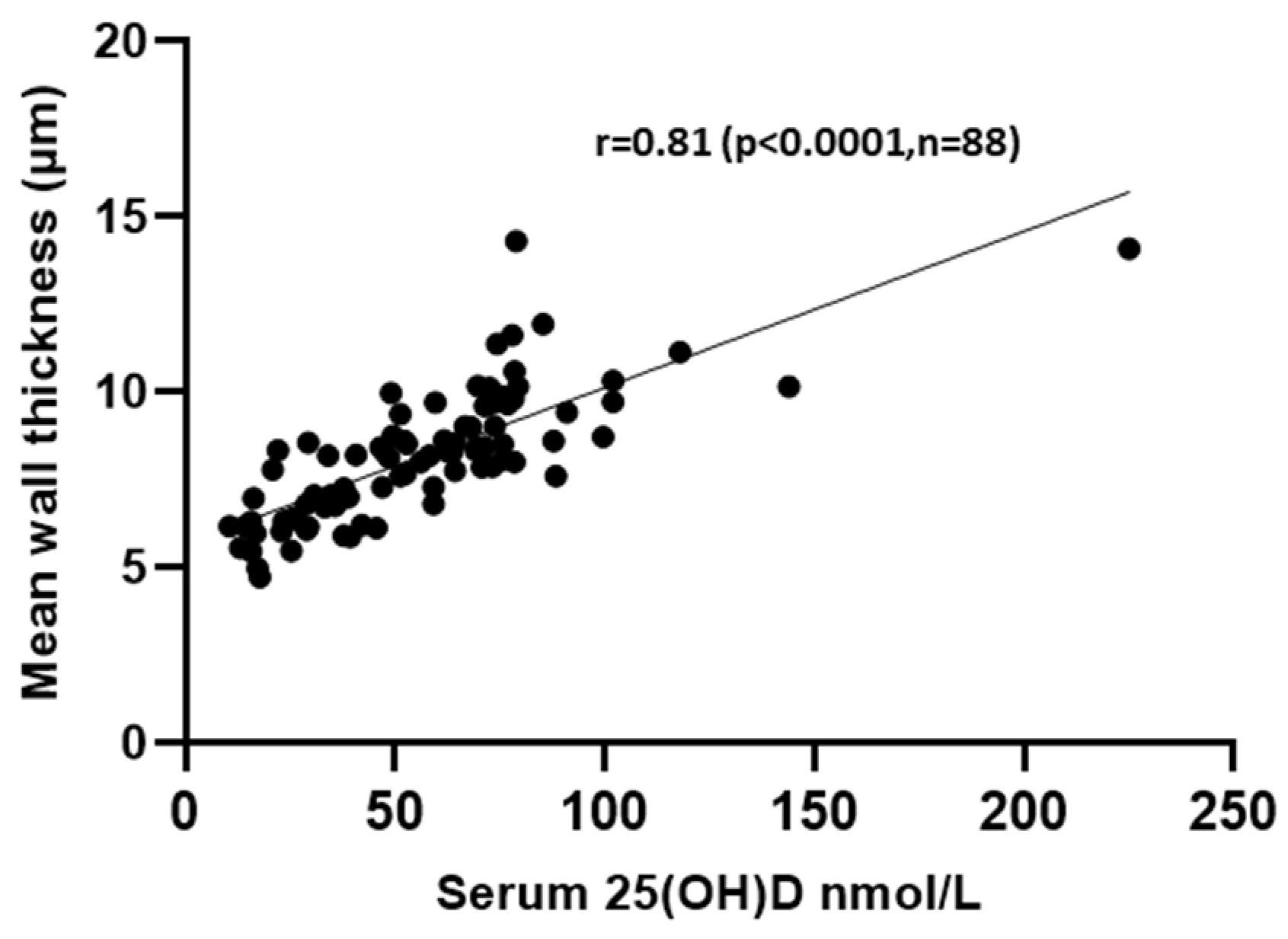
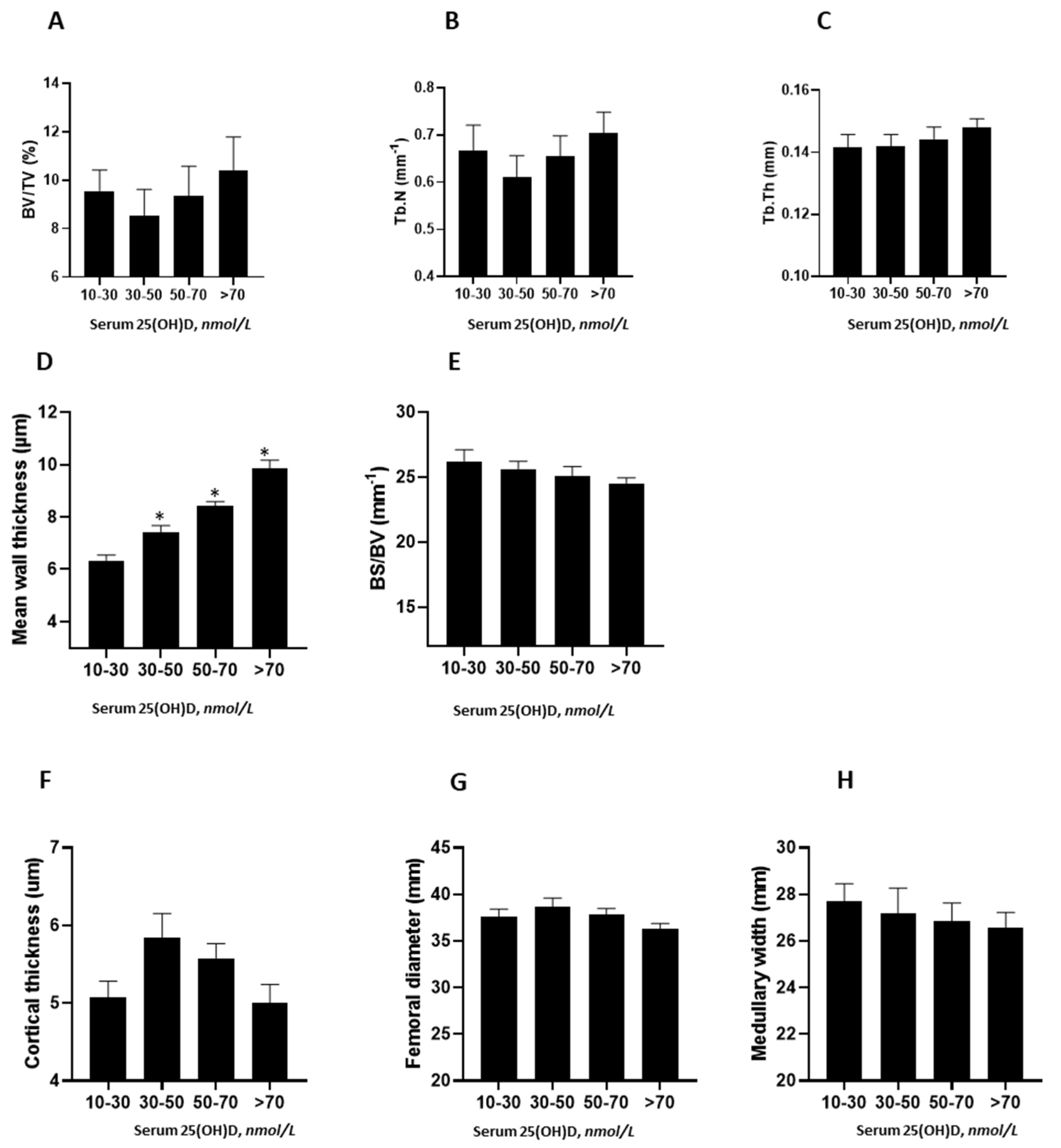
3.2. Relationship between Serum Vitamin D Levels and Bone Structure and Histomorphometric Measures
3.3. Contribution of CYP27B1 and CYP24A1 mRNA Expression in Bone to Histomorphometric Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| 25(OH)D Interval nmol/L (Number of Patients) | Median 25(OH)D Levels (nmol/L) | Test Statistics | p Value (vs. >70 nmol/L) | Adjusted p Value a (vs. >70 nmol/L) |
|---|---|---|---|---|
| 10–30 (21) | 6.17 | −50.31 | <0.001 | <0.001 |
| 30–50 (18) | 7.16 | −34.77 | <0.001 | <0.001 |
| 50–70 (20) | 8.40 | −17.17 | 0.021 | 0.12 |
| >70 (29) | 9.70 |
References
- Reid, I.R.; Bolland, M.J.; Grey, A. Effects of vitamin D supplements on bone mineral density: A systematic review and meta-analysis. Lancet 2014, 383, 146–155. [Google Scholar] [CrossRef]
- Weaver, C.M.; Alexander, D.D.; Boushey, C.J.; Dawson-Hughes, B.; Lappe, J.M.; LeBoff, M.S.; Liu, S.; Looker, A.C.; Wallace, T.C.; Wang, D.D. Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016, 27, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.G.; Zeng, X.T.; Wang, J.; Liu, L. Association Between Calcium or Vitamin D Supplementation and Fracture Incidence in Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA 2017, 318, 2466–2482. [Google Scholar] [CrossRef]
- Vanderschueren, D.; Pye, S.R.; O’Neill, T.W.; Lee, D.M.; Jans, I.; Billen, J.; Gielen, E.; Laurent, M.; Claessens, F.; Adams, J.E.; et al. Active vitamin D (1,25-dihydroxyvitamin D) and bone health in middle-aged and elderly men: The European Male Aging Study (EMAS). J. Clin. Endocrinol. Metab. 2013, 98, 995–1005. [Google Scholar] [CrossRef][Green Version]
- Anderson, P.H.; Atkins, G.J. The skeleton as an intracrine organ for vitamin D metabolism. Mol. Asp. Med. 2008, 29, 397–406. [Google Scholar] [CrossRef]
- Turner, A.G.; Hanrath, M.A.; Morris, H.A.; Atkins, G.J.; Anderson, P.H. The local production of 1,25(OH)2D3 promotes osteoblast and osteocyte maturation. J. Steroid Biochem. Mol. Biol. 2014, 144, 114–118. [Google Scholar] [CrossRef]
- Nordin, B.E.; Need, A.G.; Hartley, T.F.; Philcox, J.C.; Wilcox, M.; Thomas, D.W. Improved method for calculating calcium fractions in plasma: Reference values and effect of menopause. Clin. Chem. 1989, 35, 14–17. [Google Scholar]
- Helfrich, M.H.; Stuart, R. Bone Research Protocols, 2nd ed.; Humana Press: New York, NY, USA, 2012. [Google Scholar]
- Lips, P.; Courpron, P.; Meunier, P.J. Mean wall thickness of trabecular bone packets in the human iliac crest: Changes with age. Calcif. Tissue Res. 1978, 26, 13–17. [Google Scholar] [CrossRef]
- Lai, J.K.C.; Lucas, R.M.; Clements, M.S.; Roddam, A.W.; Banks, E. Hip fracture risk in relation to vitamin D supplementation and serum 25-hydroxyvitamin D levels: A systematic review and meta-analysis of randomised controlled trials and observational studies. BMC Public Health 2010, 10, 1–17. [Google Scholar] [CrossRef]
- Lips, P.; Bouillon, R.; van Schoor, N.M.; Vanderschueren, D.; Verschueren, S.; Kuchuk, N.; Milisen, K.; Boonen, S. Reducing fracture risk with calcium and vitamin D. Clin. Endocrinol. 2010, 73, 277–285. [Google Scholar] [CrossRef]
- Need, A.G.; O’Loughlin, P.D.; Morris, H.A.; Coates, P.S.; Horowitz, M.; Nordin, B.E. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J. Bone Miner. Res. 2008, 23, 1859–1863. [Google Scholar] [CrossRef]
- Marques, A.; Lucas, R.; Simoes, E.; Verstappen, S.M.M.; Jacobs, J.W.G.; da Silva, J.A.P. Do we need bone mineral density to estimate osteoporotic fracture risk? A 10-year prospective multicentre validation study. RMD Open 2017, 3, e000509. [Google Scholar] [CrossRef]
- Levin, A.; Bakris, G.L.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.A.; Andress, D.L. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007, 71, 31–38. [Google Scholar] [CrossRef]
- Yoon, B.-H.; Kim, J.G.; Lee, Y.-K.; Ha, Y.-C.; Koo, K.-H.; Kim, J.H. Femoral head trabecular micro-architecture in patients with osteoporotic hip fractures: Impact of bisphosphonate treatment. Bone 2017, 105, 148–153. [Google Scholar] [CrossRef]
- Yang, D.; Anderson, P.H.; Turner, A.G.; Morris, H.A.; Atkins, G.J. Comparison of the biological effects of exogenous and endogenous 1,25-dihydroxyvitamin D3 on the mature osteoblast cell line MLO-A5. J. Steroid Biochem. Mol. Biol. 2016, 164, 374–378. [Google Scholar] [CrossRef]
- Anderson, P.H. Vitamin D Activity and Metabolism in Bone. Curr. Osteoporos. Rep. 2017, 15, 443–449. [Google Scholar] [CrossRef]
- Anderson, P.H.; Atkins, G.J.; Turner, A.G.; Kogawa, M.; Findlay, D.M.; Morris, H.A. Vitamin D metabolism within bone cells: Effects on bone structure and strength. Mol. Cell. Endocrinol. 2011, 347, 42–47. [Google Scholar] [CrossRef]
- Kogawa, M.; Findlay, D.M.; Anderson, P.H.; Atkins, G.J. Modulation of osteoclastic migration by metabolism of 25(OH)-vitamin D3. J. Steroid Biochem. Mol. Biol. 2013, 136, 59–61. [Google Scholar] [CrossRef]
- Kogawa, M.; Findlay, D.M.; Anderson, P.H.; Ormsby, R.; Vincent, C.; Morris, H.A.; Atkins, G.J. Osteoclastic metabolism of 25(OH)-vitamin D3: A potential mechanism for optimization of bone resorption. Endocrinology 2010, 151, 4613–4625. [Google Scholar] [CrossRef]
- Lidor, C.; Sagiv, P.; Amdur, B.; Gepstein, R.; Otremski, I.; Hallel, T.; Edelstein, S. Decrease in bone levels of 1,25-dihydroxyvitamin D in women with subcapital fracture of the femur. Calcif. Tissue Int. 1993, 52, 146–148. [Google Scholar] [CrossRef]
- Ormsby, R.T.; Findlay, D.M.; Kogawa, M.; Anderson, P.H.; Morris, H.A.; Atkins, G.J. Analysis of vitamin D metabolism gene expression in human bone: Evidence for autocrine control of bone remodelling. J. Steroid Biochem. Mol. Biol. 2014, 144, 110–113. [Google Scholar] [CrossRef]
- Atkins, G.J.; Anderson, P.H.; Findlay, D.M.; Welldon, K.J.; Vincent, C.; Zannettino, A.C.W.; O’Loughlin, P.D.; Morris, H.A. Metabolism of vitamin D3 in human osteoblasts: Evidence for autocrine and paracrine activities of 1α,25-dihydroxyvitamin D3. Bone 2007, 40, 1517–1528. [Google Scholar] [CrossRef]
- Bislev, L.S.; Langagergaard Rodbro, L.; Rolighed, L.; Sikjaer, T.; Rejnmark, L. Bone Microstructure in Response to Vitamin D3 Supplementation: A Randomized Placebo-Controlled Trial. Calcif. Tissue Int. 2019, 104, 160–170. [Google Scholar] [CrossRef]
| Median (IQ Range) | Range | |
|---|---|---|
| Age, years | 86.5 (80.3–90.3) | 63.0–102.7 |
| eGFR, mL/min/1.73 m2 | 70 (47–84) | 11.0–90+ |
| Ionized Ca, mmol/L | 1.19 (1.15–1.23) | 1.03–1.37 |
| 25(OH)D, nmol/L | 52.7 (30.4–73.4) | 10–225 |
| 1,25(OH)2D, pmol/L | 93.8 (67.1–129.8) | 18.4–229 |
| PTH, pmol/L | 4.9 (3.28–9.43) | 1.1–32.7 |
| Osteocalcin, nmol/L | 2.41 (1.21–3.24) | 0.33–21.8 |
| BS-ALP, µg/L | 13.3 (9.78–17.23) | 3.45–36.30 |
| Dependent Variable | Independent Variables | Standardized Coefficients (beta) | p-Value |
|---|---|---|---|
| Serum 1,25(OH)2D | 25(OH)D | 0.332 | <0.001 |
| +PTH | 0.272 | 0.006 | |
| +eGFR | 0.439 | <0.001 | |
| +Constant | +0.187 | 0.992 | |
| Combined R2 = 0.235 | <0.001 |
| Dependent Variable | Independent Variables | Standardized Coefficients (beta) | p-Value |
|---|---|---|---|
| MWT | 25(OH)D | 0.745 | <0.001 |
| +1,25(OH)2D | +0.095 | 0.190 (NS) | |
| +5.4 | |||
| Adjusted R2 = 0.602 | p < 0.001 |
| Dependent Variable | Independent Variables | Standardized Coefficients (beta) | p-Value |
|---|---|---|---|
| MWT | 25(OH)D | 0.743 | <0.001 |
| +CYP27B1 | +0.043 | 0.655 (NS) | |
| +CYP24A1 | +0.064 | 0.504 (NS) | |
| +5.1 | |||
| Adjusted R2 = 0.54 | p < 0.001 |
| Dependent Variable | Independent Variables | Standardized Coefficients (beta) | p-Value |
|---|---|---|---|
| BS/BV | 25(OH)D | −0.294 | 0.012 |
| +CYP27B1 | −0.208 | 0.115 | |
| +CYP24A1 | +0.306 | 0.021 | |
| +27.5 | |||
| Adjusted R2 = 0.104 | 0.016 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, D.K.; Sawyer, R.K.; Robertson, T.S.; Stamenkov, R.; Solomon, L.B.; Atkins, G.J.; Clifton, P.M.; Morris, H.A.; Anderson, P.H. Elevated Serum 25-Hydroxyvitamin D Levels Are Associated with Improved Bone Formation and Micro-Structural Measures in Elderly Hip Fracture Patients. J. Clin. Med. 2019, 8, 1988. https://doi.org/10.3390/jcm8111988
Sharma DK, Sawyer RK, Robertson TS, Stamenkov R, Solomon LB, Atkins GJ, Clifton PM, Morris HA, Anderson PH. Elevated Serum 25-Hydroxyvitamin D Levels Are Associated with Improved Bone Formation and Micro-Structural Measures in Elderly Hip Fracture Patients. Journal of Clinical Medicine. 2019; 8(11):1988. https://doi.org/10.3390/jcm8111988
Chicago/Turabian StyleSharma, Deepti K., Rebecca K. Sawyer, Thomas S. Robertson, Roumen Stamenkov, Lucian B. Solomon, Gerald J. Atkins, Peter M. Clifton, Howard A. Morris, and Paul H. Anderson. 2019. "Elevated Serum 25-Hydroxyvitamin D Levels Are Associated with Improved Bone Formation and Micro-Structural Measures in Elderly Hip Fracture Patients" Journal of Clinical Medicine 8, no. 11: 1988. https://doi.org/10.3390/jcm8111988
APA StyleSharma, D. K., Sawyer, R. K., Robertson, T. S., Stamenkov, R., Solomon, L. B., Atkins, G. J., Clifton, P. M., Morris, H. A., & Anderson, P. H. (2019). Elevated Serum 25-Hydroxyvitamin D Levels Are Associated with Improved Bone Formation and Micro-Structural Measures in Elderly Hip Fracture Patients. Journal of Clinical Medicine, 8(11), 1988. https://doi.org/10.3390/jcm8111988







