Exacerbation of Hangover Symptomology Significantly Corresponds with Heavy and Chronic Alcohol Drinking: A Pilot Study
Abstract
1. Introduction
2. Study Participants and Methods
2.1. Patient Recruitment
2.2. Study Paradigm
2.3. Analysis
3. Results
3.1. Demographics and Drinking Profile
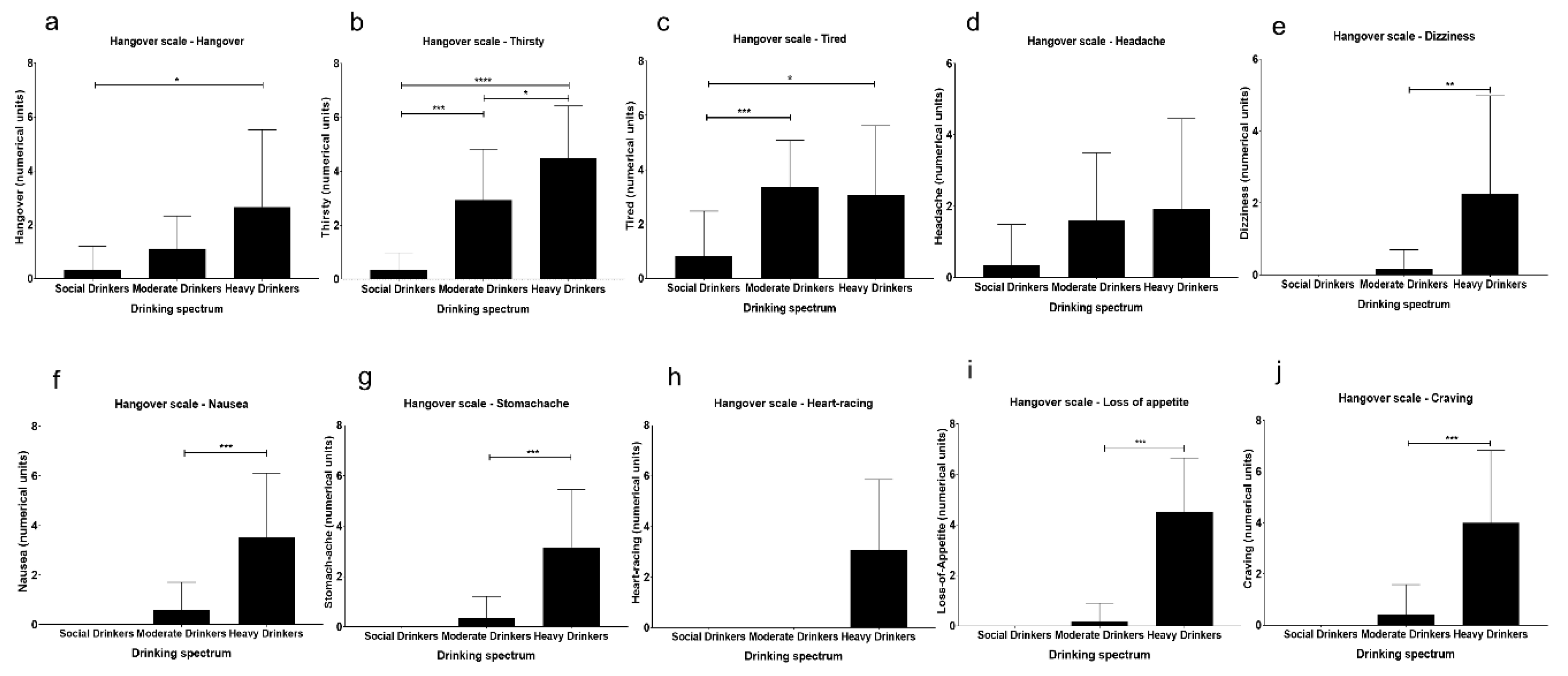
3.2. Assessment of Hangover Symptoms
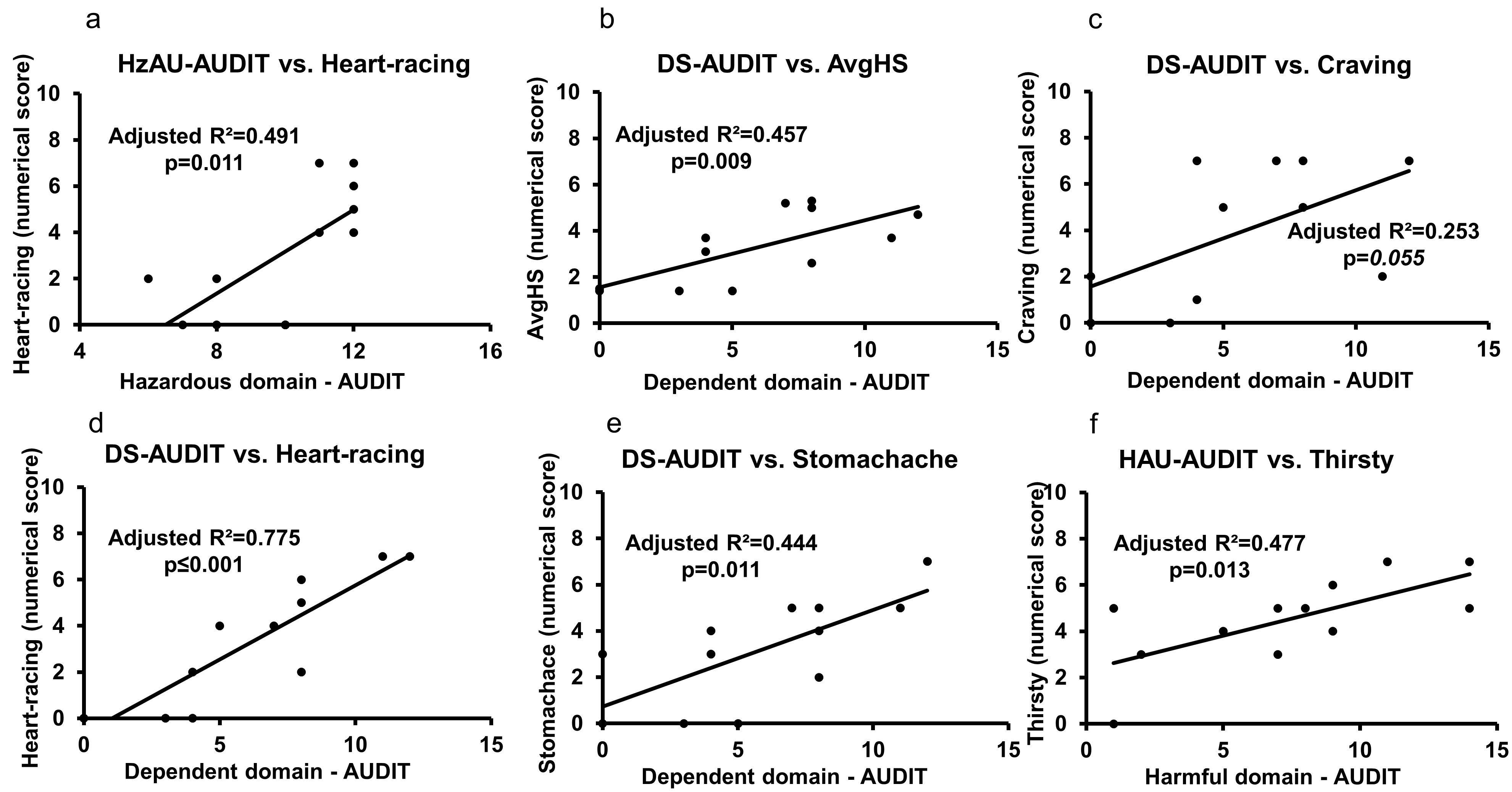
3.3. AUDIT Domains, and Association of Drinking Markers in Heavy Drinkers
3.4. Internal Consistency of Hangover Measures in Heavy Drinkers
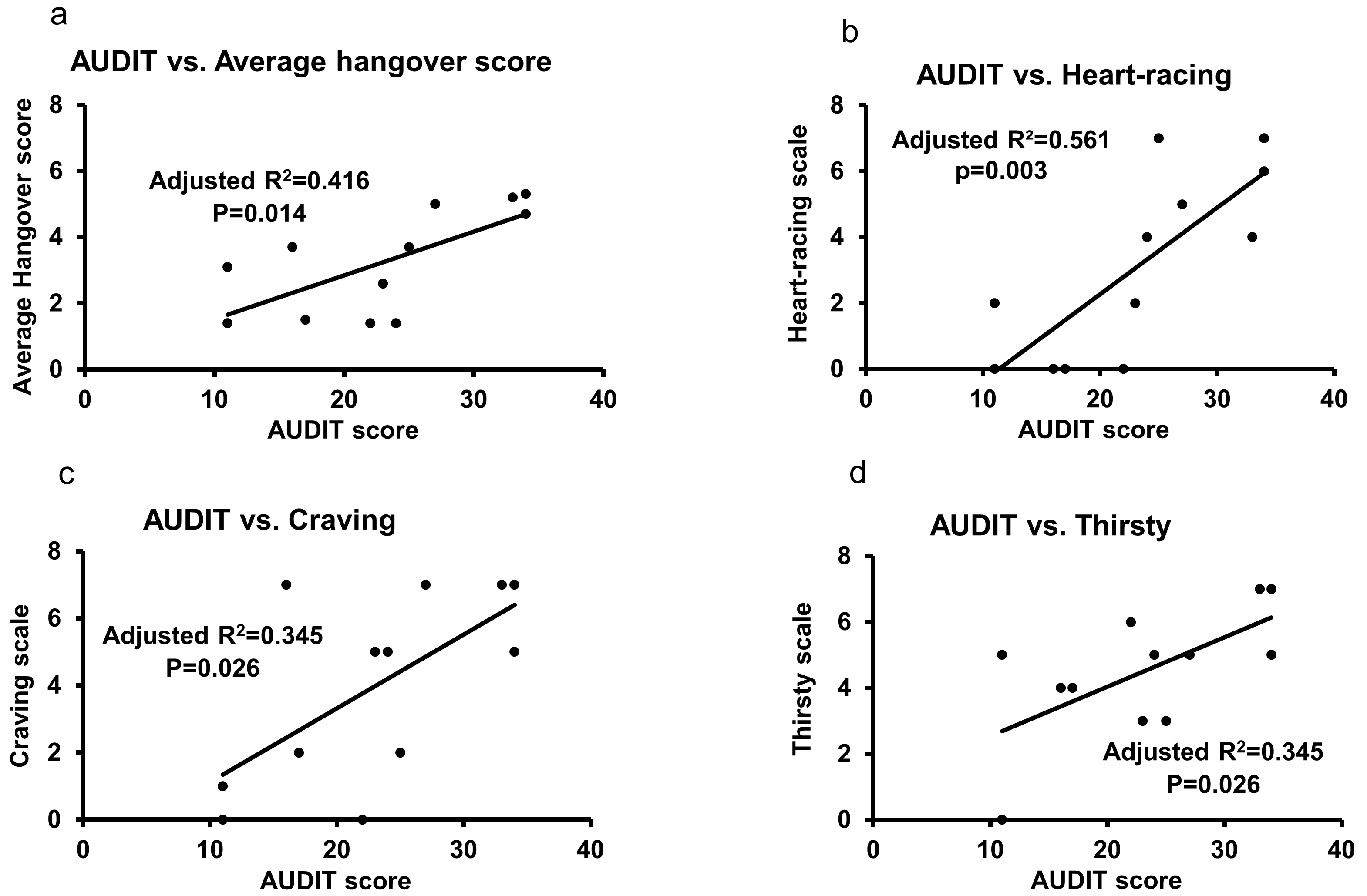
3.5. Association of AUDIT and Hangover Measures in Heavy Drinkers
3.6. Role of AUDIT and Heavy Drinking TLFB Markers on Hangover Symptoms
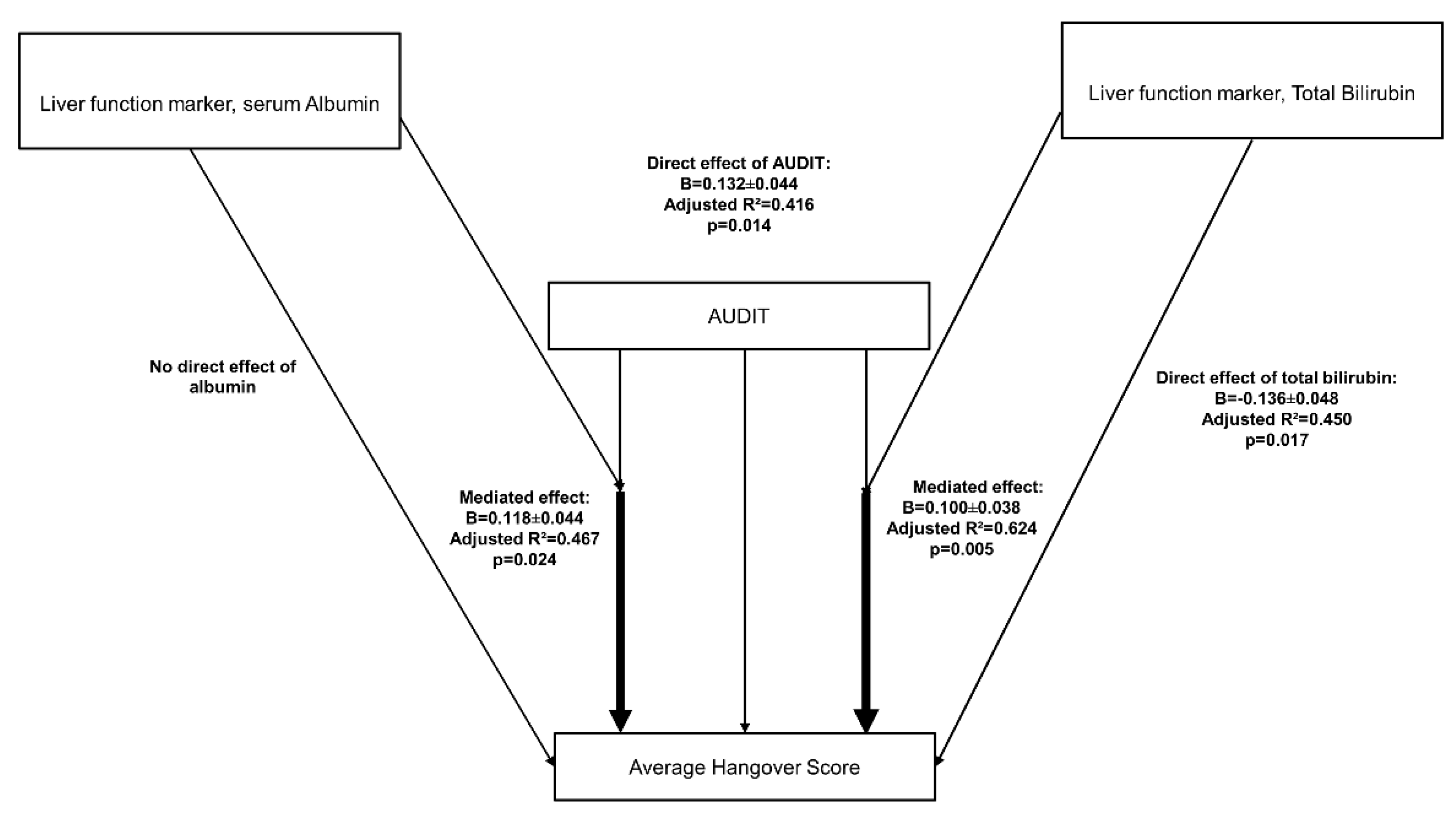
3.7. Role of Liver Dysfunction on Hangover Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stahre, M.; Roeber, J.; Kanny, D.; Brewer, R.D.; Zhang, X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev. Chronic Dis. 2014, 11, E109. [Google Scholar] [CrossRef] [PubMed]
- Abuse, S.; M.H.S. Administration. National Survey on Drug Use and Health; M.H.S.: Everett, WA, USA, 2014. [Google Scholar]
- Van Schrojenstein Lantman, M.; van de Loo, A.J.; Mackus, M.; Verster, J.C. Development of a definition for the alcohol hangover: Consumer descriptions and expert consensus. Curr. Drug Abus. Rev. 2016, 9, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Howland, J.; Rohsenow, D.J.; Greece, J.; Almeida, A.; Minsky, S.J.; Allensworth-Davies, D.; Arnedt, J.T.; Hermos, J. The Incidence and Severity of Hangover the Morning after Moderate Alcohol Intoxication. Addiction 2008, 103. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C.; de Klerk, S.; Bervoets, A.C.; Kruisselbrink, L.D. Can hangover immunity really be claimed? Curr. Drug Abuse Rev. 2013, 6, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Sacks, J.J.; Gonzales, K.R.; Bouchery, E.E.; Tomedi, L.E.; Brewer, R.D. 2010 National and State Costs of Excessive Alcohol Consumption. Am. J. Prev. Med. 2015, 49, e73–e79. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C. The alcohol hangover—A puzzling phenomenon. Alcohol Alcohol. 2008, 43, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Van Schrojenstein Lantman, M.; Mackus, M.; van de Loo, A.J.A.E.; Verster, J.C. The impact of alcohol hangover symptoms on cognitive and physical functioning, and mood. Hum. Psychopharmacol. Clin. Exp. 2017, 32, e2623. [Google Scholar] [CrossRef] [PubMed]
- Penning, R.; McKinney, A.; Verster, J.C. Alcohol hangover symptoms and their contribution to the overall hangover severity. Alcohol Alcohol. 2012, 47, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C.; Alford, C.; Bervoets, A.C.; de Klerk, S.; Grange, J.A.; Hogewoning, A.; Jones, K.; Kruisselbrink, D.L.; Owen, L.; Piasecki, T.M.; et al. Hangover Research Needs: Proceedings of the 5(th) Alcohol Hangover Research Group Meeting. Curr. Drug Abuse Rev. 2013, 6, 245–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verster, J.C.; Stephens, R.; Penning, R.; Rohsenow, D.; McGeary, J.; Levy, D.; McKinney, A.; Finnigan, F.; Piasecki, T.M.; Adan, A.; et al. The Alcohol Hangover Research Group Consensus Statement on Best Practice in Alcohol Hangover Research. Curr. Drug Abus. Rev. 2010, 3, 116–126. [Google Scholar] [CrossRef]
- Vatsalya, V.; Stangl, B.L.; Schmidt, V.Y.; Ramchandani, V.A. Characterization of hangover following intravenous alcohol exposure in social drinkers: Methodological and clinical implications. Addict. Biol. 2018, 23, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Wiese, G.J.; Shlipak, M.G.; Browner, W.S. The alcohol hangover. Ann. Intern. Med. 2000, 132, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Kruisselbrink, L.D.; Bervoets, A.C.; de Klerk, S.; van de Loo, A.J.A.E.; Verster, J.C. Hangover resistance in a Canadian university student population. Addict. Behav. Rep. 2017, 5, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Farokhnia, M.; Lee, M.R.; Farinelli, L.A.; Ramchandani, V.A.; Akhlaghi, F.; Leggio, L. Pharmacological manipulation of the ghrelin system and alcohol hangover symptoms in heavy drinking individuals: Is there a link? Pharmacol. Biochem. Behav. 2018, 172, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Sobell, L.C.; Sobell, M.B. Timeline Follow-Back. In Measuring Alcohol Consumption; Springer: Berlin, Germany, 1992; pp. 41–72. [Google Scholar]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; de la Fuente, J.R.; Grant, M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.; Grange, J.A.; Jones, K.; Owen, L. A critical analysis of alcohol hangover research methodology for surveys or studies of effects on cognition. Psychopharmacology 2014, 231, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Rohsenow, D.J.; Howland, J.; Minsky, S.J.; Greece, J.; Almeida, A.; Roehrs, T.A. The Acute Hangover Scale: A New Measure of Immediate Hangover Symptoms. Addict. Behav. 2007, 32, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.E.; Sobell, L.C.; Sobell, M.B.; Pavan, D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985, 15, 61–67. [Google Scholar] [CrossRef]
- Conigrave, M.K.; Saunders, J.B.; Reznik, R.B. Predictive capacity of the AUDIT questionnaire for alcohol-related harm. Addiction 1995, 90, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, M.T.; Robertson, B.M.; Epler, A.J. Hangover and Risk for Alcohol Use Disorders: Existing Evidence and Potential Mechanisms. Curr. Drug. Abus. Rev. 2010, 3, 92–102. [Google Scholar] [CrossRef]
- Tryba, M. Alpha2-adrenoceptor agonists in intensive care medicine: Prevention and treatment of withdrawal. Best Pract. Res. Clin. Anaesthesiol. 2000, 14, 459–470. [Google Scholar] [CrossRef]
- Piasecki, T.M.; Sher, K.J.; Slutske, W.S.; Jackson, K.M. Hangover frequency and risk for alcohol use disorders: Evidence from a longitudinal high-risk study. J. Abnorm. Psychol. 2005, 114, 223. [Google Scholar] [CrossRef] [PubMed]
- Hunt-Carter, E.; Slutske, W.; Piasecki, T. Characteristics and correlates of drinking to relieve hangover in a college sample. In Alcoholism-Clinical and Experimental Research; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Epler, A.J.; Tomko, R.L.; Piasecki, T.M.; Wood, P.K.; Sher, K.J.; Shiffman, S.; Heath, A.C. Does hangover influence the time to next drink? An investigation using ecological momentary assessment. Alcohol. Clin. Exp. Res. 2014, 38, 1461–1469. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McKinney, A.; Coyle, K. Alcohol hangover effects on measures of affect the morning after a normal night’s drinking. Alcohol. Alcohol. 2006, 41, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Slutske, S.W.; Piasecki, T.M.; Hunt-Carter, E.E. Development and initial validation of the Hangover Symptoms Scale: Prevalence and correlates of Hangover Symptoms in college students. Alcohol. Clin. Exp. Res. 2003, 27, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.D.; Harford, T.C.; Grant, B.F. Family history as a predictor of alcohol dependence. Alcohol. Clin. Exp. Res. 1992, 16, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Newlin, B.D.; Pretorius, M.B. Sons of alcoholics report greater hangover symptoms than sons of nonalcoholics: A pilot study. Alcohol. Clin. Exp. Res. 1990, 14, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Vatsalya, V.; Gowin, J.L.; Schwandt, M.L.; Momenan, R.; Coe, M.A.; Cooke, M.E.; Hommer, D.W.; Bartlett, S.; Heilig, M.; Ramchandani, V.A. Effects of varenicline on neural correlates of alcohol salience in heavy drinkers. Int. J. Neuropsychopharmacol. 2015, 18. [Google Scholar] [CrossRef] [PubMed]
- Vatsalya, V.; Song, M.; Schwandt, M.L.; Cave, M.C.; Barve, S.S.; George, D.T.; Ramchandani, V.A.; McClain, C.J. Effects of sex, drinking history, and omega-3 and omega-6 fatty acids dysregulation on the onset of liver injury in very heavy drinking alcohol-dependent patients. Alcohol. Clin. Exp. Res. 2016, 40, 2085–2093. [Google Scholar] [CrossRef] [PubMed]

| Measures | Heavy Drinkers (n = 12) | Moderate Drinkers (n = 17) | Social Drinkers (n = 12) | Heavy vs. Moderate Drinking Group Significance |
|---|---|---|---|---|
| Demographics and Family History of Alcoholism | ||||
| Sex (M or F) | n(M) = 9, n(F) = 3 | n(M) = 6, n(F) = 11 | n(M) = 5, n(F) = 7 | NA |
| Age (years.) a,b | 49.8 ± 9.8 | 25.1± 3.1 | 31.6 ± 10.6 | ≤0.001 |
| BMI a | 30.6 ± 7.6 | 25.1 ± 4.7 | 25.0 ± 3.1 | 0.023 |
| FHA | FHP, n = 11; FHN, n = 1 | FHP, n = 8; FHN, n = 9 | FHP, n = 5; FHN, n = 7 | NA |
| Heavy Drinking Markers | ||||
| TD90 a,b | 1153.92 ± 765.56 | 74.41 ± 31.39 | 23.67 ± 18.14 | ≤0.001 |
| HDD90 a | 67.33 ± 34.88 | 4.82 ± 9.47 | 0.58 ± 1.38 | ≤0.001 |
| NDD90 a | 72.5 ± 24.4 | 31.47 ± 16.62 | 18.25 ± 18.70 | ≤0.001 |
| AvgDPD90 a,b | 13.57 ± 8.1 | 2.5 ± 0.99 | 1.4 ± 0.72 | ≤0.001 |
| AUDIT Scores and Its Domains | ||||
| AUDIT | 23.08 ± 8.19 | NC | NC | NA |
| AUDIT>20 | 10/12 | NC | NC | NA |
| HzAU | 9.92 ± 2.15 | NC | NC | NA |
| DS | 5.83 ± 3.86 | NC | NC | NA |
| HAU | 7.33 ± 4.5 | NC | NC | NA |
| Symptoms | Social Drinkers (SD) n = 12 | Moderate Drinkers (MD) n = 17 | Heavy Drinkers (HD) n = 12 | MD vs. HD Effects (Adjusted R2) |
|---|---|---|---|---|
| Hangover a | 0.33 ± 0.89 | 1.12 ± 1.22 | 2.67 ± 2.87 | p = 0.056; R2 = 0.129 |
| Thirsty a,b | 0.33 ± 0.65 | 2.94 ± 1.89 | 4.50 ± 1.93 | p = 0.039; R2 = 0.149 |
| Tired a,b | 0.83 ± 1.64 | 3.35 ± 1.73 | 3.08 ± 2.54 | p = 0.736; R2 = 0.004 |
| Headache | 0.33 ± 1.16 | 1.59 ± 1.91 | 1.92 ± 2.54 | p = 0.693; R2 = 0.006 |
| Dizziness a | 0.00 | 0.18 ± 0.53 | 2.25 ± 0.53 | p = 0.005; R2 = 0.259 |
| Nausea a | 0.00 | 0.59 ± 1.12 | 3.50 ± 2.61 | p ≤ 0.001; R2 = 0.214 |
| Stomachache a | 0.00 | 0.35 ± 0.86 | 3.17 ± 2.29 | p ≤ 0.001; R2 = 0.445 |
| Heart-racing a | 0.00 | 0.00 | 3.08 ± 2.78 | p ≤ 0.001; R2 = 0.441 |
| Loss of Appetite a | 0.00 | 0.18 ± 0.73 | 4.50 ± 2.15 | p ≤ 0.001; R2 = 0.689 |
| Craving a | 0.00 | 0.41 ± 1.18 | 4.0 ± 2.83 | p ≤ 0.001; R2 = 0.451 |
| AvgHS a,b | 0.18 ± 0.41 | 1.14 ± 0.64 | 3.3 ± 1.58 | p ≤ 0.001; R2 = 0.489 |
| Measures | Heavy Drinkers (n = 12) | Moderate Drinkers (n = 17) | Social Drinkers (n = 12) | Heavy vs. Moderate Drinking Group Significance |
|---|---|---|---|---|
| ALT (U/L) | 73.83 ± 61.00 | 18.82 ± 7.95 | 28.5 ± 28.77 | 0.001 |
| AST (U/L) | 174.75 ± 82.11 | 24.53 ± 6.27 | 28.7 ± 13.92 | ≤0.001 |
| AST:ALT ratio | 3.53 ± 2.56 | 1.39 ± 0.48 | 1.03 ± 0.56 | 0.002 |
| Total Bilirubin (μmol/L) | 9.72 ± 7.77 | 0.55 ± 0.35 | 0.610 ± 0.31 | ≤0.001 |
| Albumin (g/dL) | 2.66 ± 0.29 | 4.17 ± 0.26 | 4.08 ± 0.24 | ≤0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vatsalya, V.; Hassan, H.Z.; Kong, M.; Stangl, B.L.; Schwandt, M.L.; Schmidt-Teron, V.Y.; Verster, J.C.; Ramchandani, V.A.; McClain, C.J. Exacerbation of Hangover Symptomology Significantly Corresponds with Heavy and Chronic Alcohol Drinking: A Pilot Study. J. Clin. Med. 2019, 8, 1943. https://doi.org/10.3390/jcm8111943
Vatsalya V, Hassan HZ, Kong M, Stangl BL, Schwandt ML, Schmidt-Teron VY, Verster JC, Ramchandani VA, McClain CJ. Exacerbation of Hangover Symptomology Significantly Corresponds with Heavy and Chronic Alcohol Drinking: A Pilot Study. Journal of Clinical Medicine. 2019; 8(11):1943. https://doi.org/10.3390/jcm8111943
Chicago/Turabian StyleVatsalya, Vatsalya, Hamza Z. Hassan, Maiying Kong, Bethany L. Stangl, Melanie L. Schwandt, Veronica Y. Schmidt-Teron, Joris C. Verster, Vijay A. Ramchandani, and Craig J. McClain. 2019. "Exacerbation of Hangover Symptomology Significantly Corresponds with Heavy and Chronic Alcohol Drinking: A Pilot Study" Journal of Clinical Medicine 8, no. 11: 1943. https://doi.org/10.3390/jcm8111943
APA StyleVatsalya, V., Hassan, H. Z., Kong, M., Stangl, B. L., Schwandt, M. L., Schmidt-Teron, V. Y., Verster, J. C., Ramchandani, V. A., & McClain, C. J. (2019). Exacerbation of Hangover Symptomology Significantly Corresponds with Heavy and Chronic Alcohol Drinking: A Pilot Study. Journal of Clinical Medicine, 8(11), 1943. https://doi.org/10.3390/jcm8111943






