The Effects of Postoperative Astaxanthin Administration on Nasal Mucosa Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Preparation
2.2. Histological Analysis
2.3. Statistical Analysis
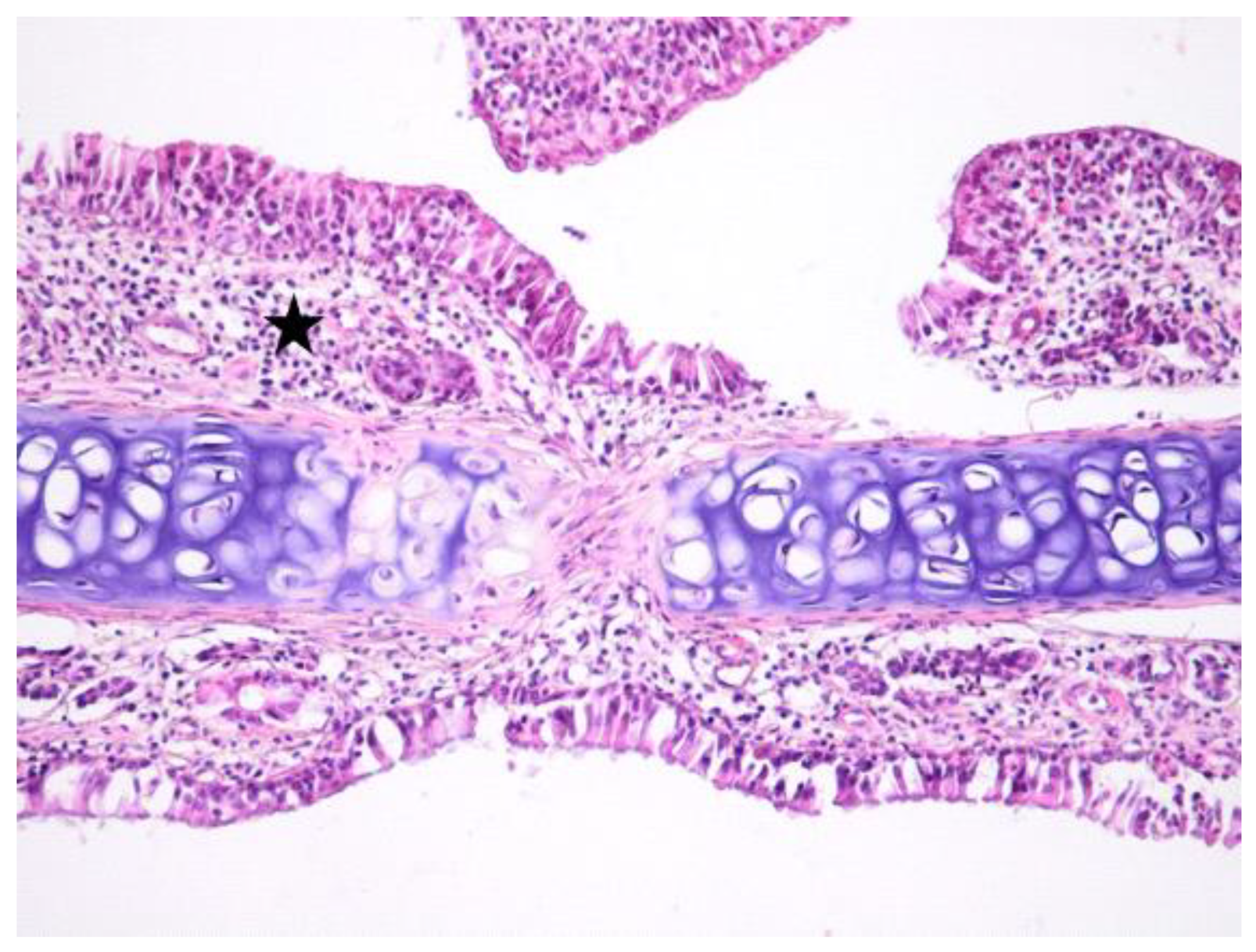
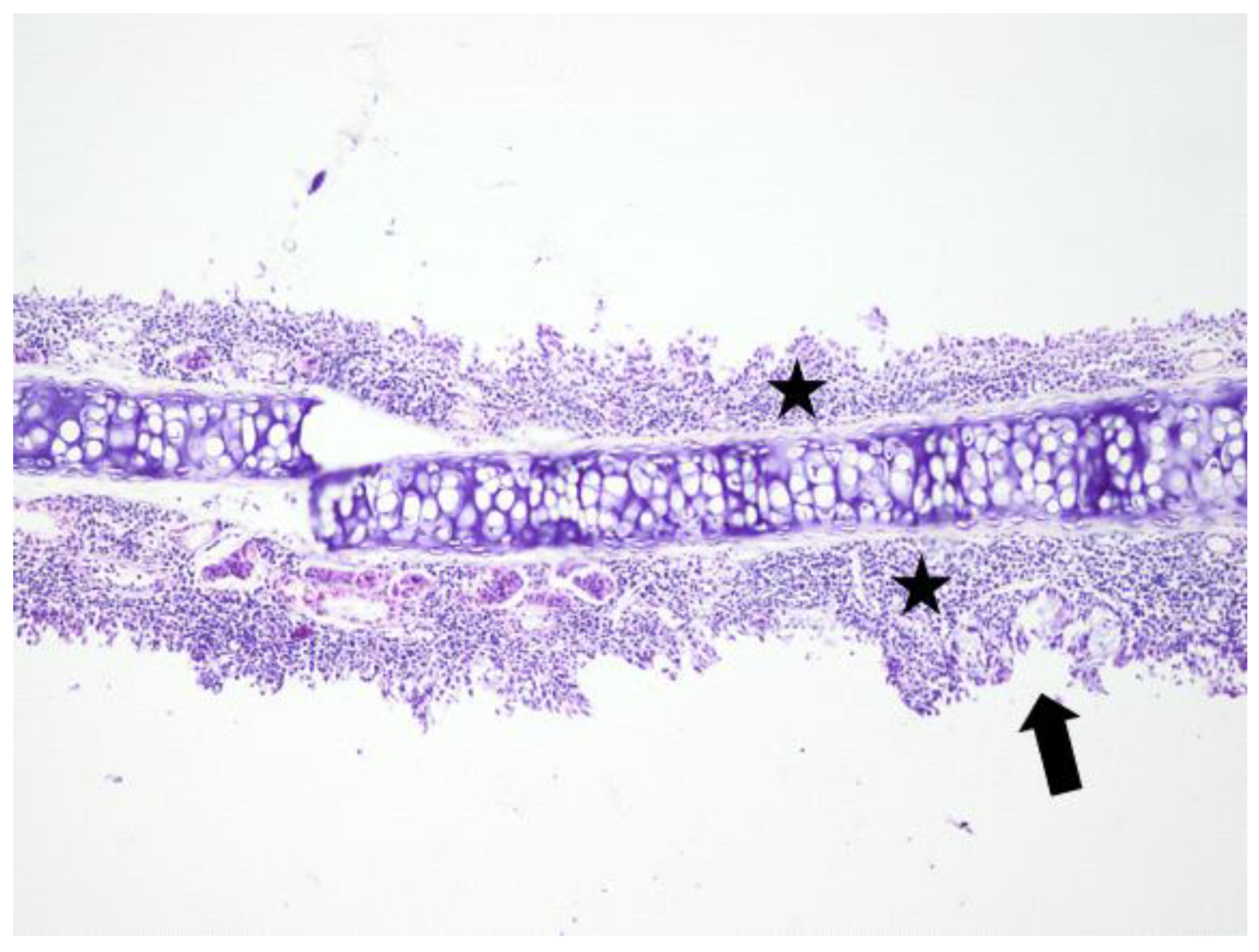
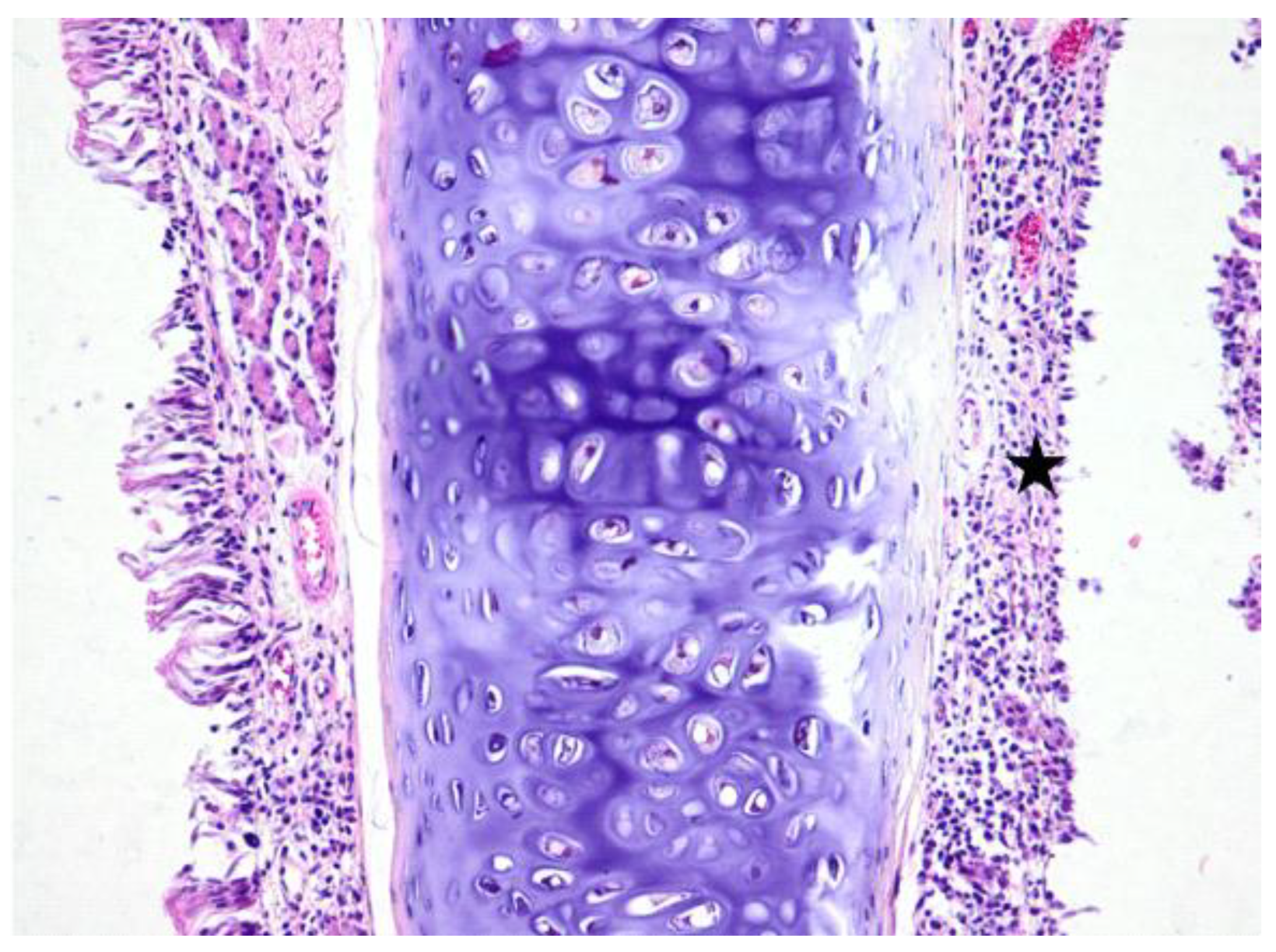
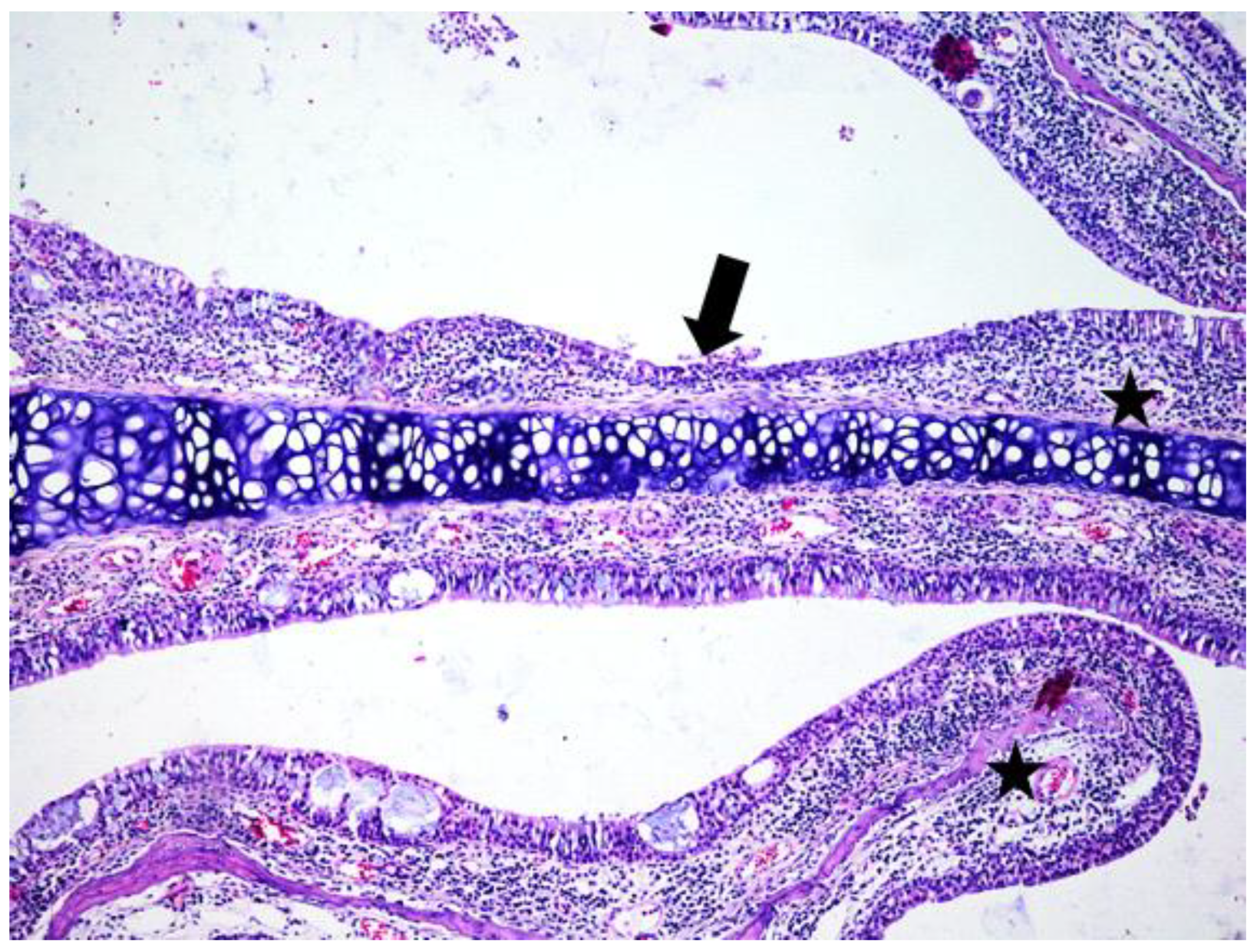
3. Results
3.1. Histologic Modifications in Relationship to Time
3.2. Index Changes in Relationship to Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khalil, H.S.; Nunez, D.A. Functional endoscopic sinus surgery for chronic rhinosinusitis. Cochrane Database Syst. Rev. 2006, 19, CD004458. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.L.; Batra, P.S.; Seiden, A.M.; Hannley, M. Evidence supporting endoscopic sinus surgery in the management of adult chronic rhinosinusitis: A systematic review. Am. J. Rhinol. 2005, 19, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, H.H. Surgical causes of failure in endoscopic sinus surgery. Laryngoscope 1999, 109, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, O.A.; Schlosser, R.J.; Mace, J.C.; Smith, T.L.; Soler, Z.M. Impact of synechiae after endoscopic sinus surgery on long-term outcomes in chronic rhinosinusitis. Laryngoscope 2013, 123, 2615–2619. [Google Scholar] [CrossRef] [PubMed]
- Watelet, J.B.; Bachert, C.; Gevaert, P.; Van Cauwenberge, P. Wound healing of the respiratory mucosa: A review. Am. J. Rhinol. 2002, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wigand, M.E.; Göde, U.; Linger, F.; Dunker, I. Normal wound healing of the paranasal sinusis: Clinical and experimental investigations. Eur Arch. Otorhinolaryngol. 1991, 248, 390–394. [Google Scholar]
- Valentine, R.; Wormald, P.J. Nasal dressings after endoscopic sinus surgery: What and why? Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Khalmuratova, R.; Jeon, S.Y.; Kim, D.W.; Kim, J.P.; Ahn, S.K.; Park, J.J.; Hur, D.G. Wound Healing of Nasal Mucosa in a Rat. Am. J. Rhinol. Allergy 2009, 23, 33–37. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, D.; Cowin, A.; Adams, D.; Wormald, P.J. The effect of an expandable polyvinyl acetate (Merocel) pack on the healing of the nasal mucosa of sheep. Am. J. Rhinol. 2005, 19, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Bleier, B.S.; Palmer, J.N.; Gratton, M.A.; Cohen, N.A. In vivo laser tissue welding in the rabbit maxillary sinus. Am. J. Rhinol. 2008, 22, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Stumpe, M.R.; Handorf, C.; Thompson, J.W.; Chandra, R.K. Absorbable biomaterials used within paranasal sinuses: An animal study. Am. J. Rhinol. 2008, 22, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Khalmuratova, R.; Kim, D.W.; Jeon, S.Y. Effect of Dexamethasone on Wound Healing of the Septal Mucosa in the Rat. Am. J. Rhinol. Allergy 2011, 25, e112–e116. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-modulating Technologies for Augmentation of the Healing Process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.O.; Park, S.J.; Yoon, S.Y.; Yun, C.H.; Chung, A.S. Sustained production of HO activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3 kinase/NF-kappa B pathway. J. Biol. Chem. 2002, 277, 30271–30282. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khanna, S.; Nallu, K.; Hunt, T.K.; Sen, C.K. Dermal wound healing is subject to redox control. Mol. Ther. 2006, 13, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin, Oxidative Stress, Inflammation and Cardiovascular Disease. Future Cardiol. 2009, 5, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Tjokroprawiro, A. Astaxanthin—Oxidative Stress—Diabetes Mellitus. From Basics to Clinics and from General to Specific. Folia Med. Indones. 2008, 44, 293–299. [Google Scholar]
- Zhang, X.S.; Zhang, X.; Wu, Q.; Li, W.; Wang, C.X.; Xie, G.B.; Zhou, M.L. Astaxanthin Offers Neuroprotection and Reduces Neuroinflammation in Experimental Subarachnoid Hemorrhage. J. Surg. Res. 2014, 192, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H.; Sun, S.; Iijima, K.; Gross, M.D. Antitumor Activity of Astaxanthin and Its Mode of Action. Nutr. Cancer 2000, 36, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, M.; Hirano, S.; Hiwatashi, N.; Tateya, I.; Kanemaru, S.I.; Nakamura, T.; Ito, J. Effect of Astaxanthin on Vocal Fold Wound Healing. Laryngoscope 2014, 124, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Prophet, E.B.; Mills, B.; Arrington, J.B.; Sobin, L.H. Laboratory Methods in Histotechnology; American Registry of Pathology: Washington, DC, USA, 1995. [Google Scholar]
- Kittel, B.; Ruehl-Fehlert, C.; Morawietz, G.; Klapwijk, J.; Elwell, M.R.; Lenz, B.; Wadsworth, P.F. Wadsworth. Revised Guides for Organ Sampling and Trimming in Rats and Mice—Part 2. Exp. Toxicol. Pathol. 2004, 55, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, M.; Hirano, S.; Ohno, S.; Tateya, I.; Kanemaru, S.I.; Nakamura, T.; Ito, J. Expression of reactive oxygen species during wound healing of vocal folds in a rat model. Ann. Otol. Rhinol. Laryngol. 2012, 121, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Rowe-Jones, J.M.; Medcalf, M.; Durham, S.R. Functional endoscopic sinus surgery: 5 years follow up and results of a prospective, randomized, stratified, double-blind, placebo controlled study of postoperative fluticasone propionate aqueous nasal spray. Rhinology 2005, 43, 2–10. [Google Scholar] [PubMed]
- Jorissen, M.; Bachert, C. Effect of corticosteroids on wound healing after endoscopic sinus surgery. Rhinology 2009, 47, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, M.; Hirano, S.; Ohno, S.; Tateya, I.; Kanemaru, S.I.; Nakamura, T.; Ito, J. Effects of Topically Applied Dexamethasone on Mucosal Wound Healing Using a Drug-Releasing Stent. Laryngoscope 2012, 118, 2073–2077. [Google Scholar]
- Wright, E.D.; Agrawal, S. Impact of perioperative systemic steroids on surgical outcomes in patients with chronic rhinosinusitis with polyposis: Evaluation with the novel Perioperative Sinus Endoscopy (POSE) scoring system. Laryngoscope 2007, 117, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, H.H. Corticosteroid therapy during endoscopic sinus surgery in children: Is there a need for a second look? Arch. Otolaryngol. Head Neck Surg. 2001, 127, 188–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Testa, D.; Marcuccio, G.; Panin, G.; Bianco, A.; Tafuri, D.; Thyrion, F.Z.; Nunziata, M.; Piombino, P.; Guerra, G.; Motta, G. Nasal mucosa healing after endoscopic sinus surgery in chronic rhinosinusitis of elderly patients: Role of topic alpha-tocopherol acetate. Aging Clin. Exp. Res. 2017, 29 (Suppl. 1), 191–195. [Google Scholar] [CrossRef] [PubMed]
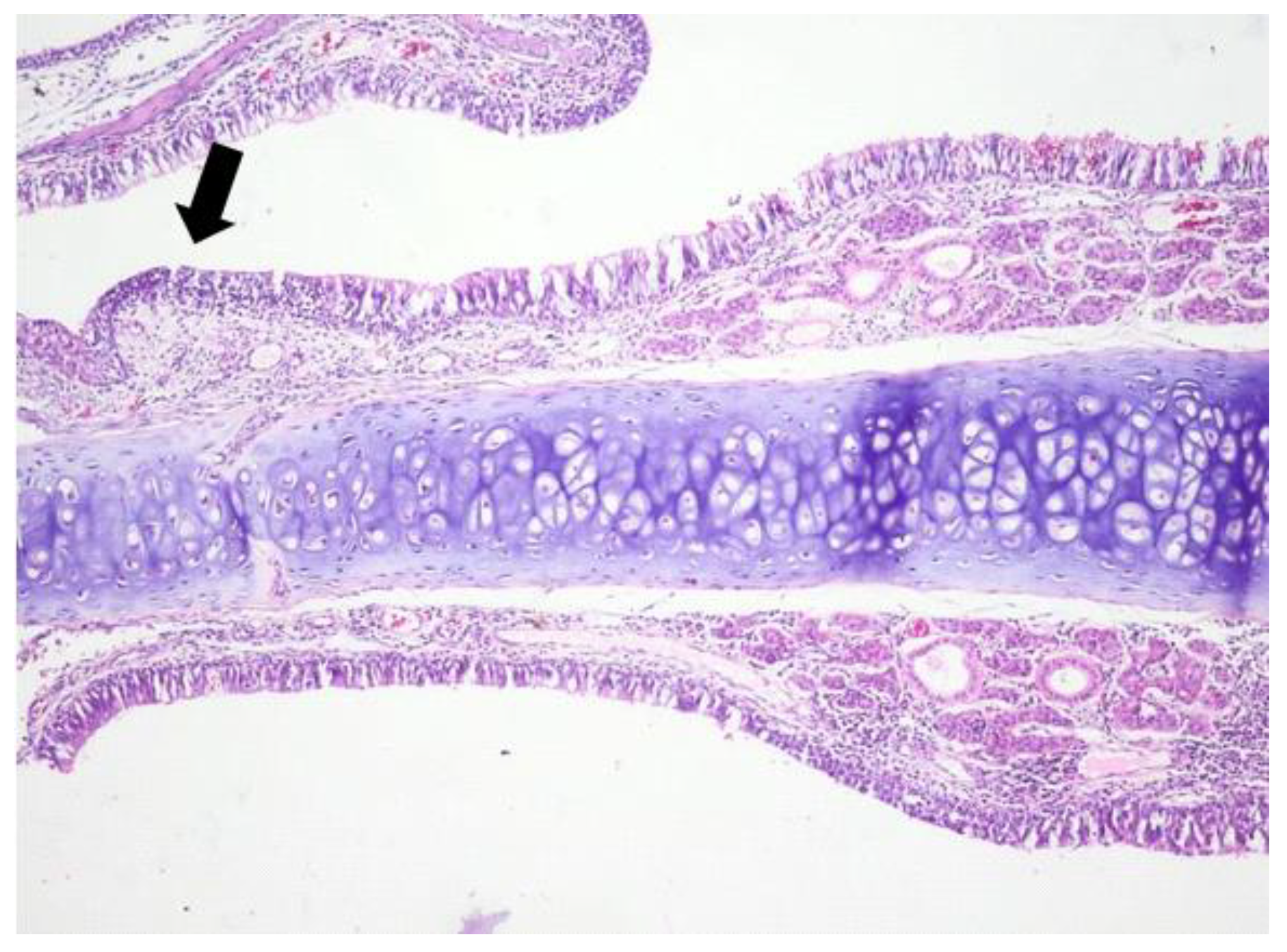
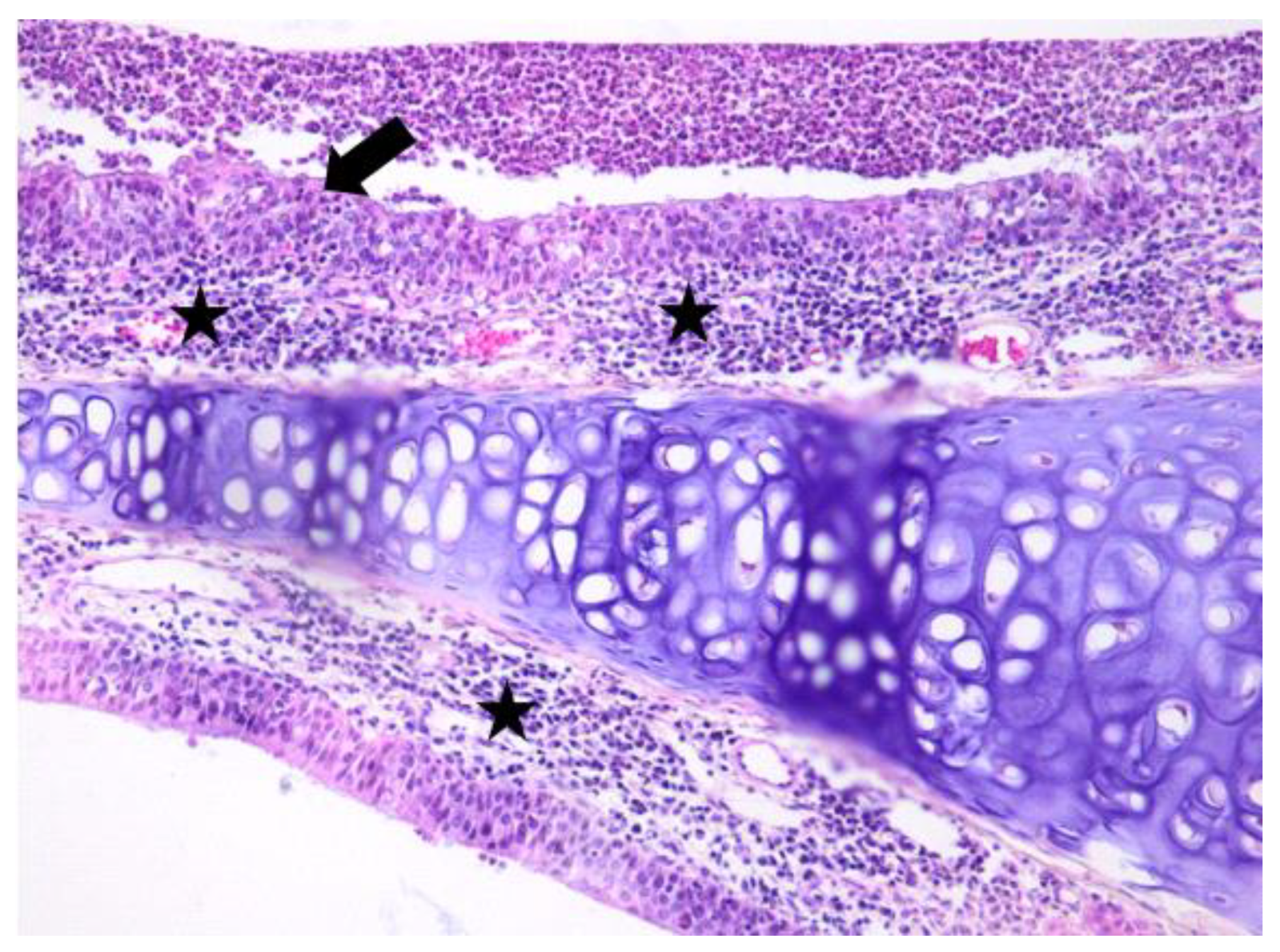
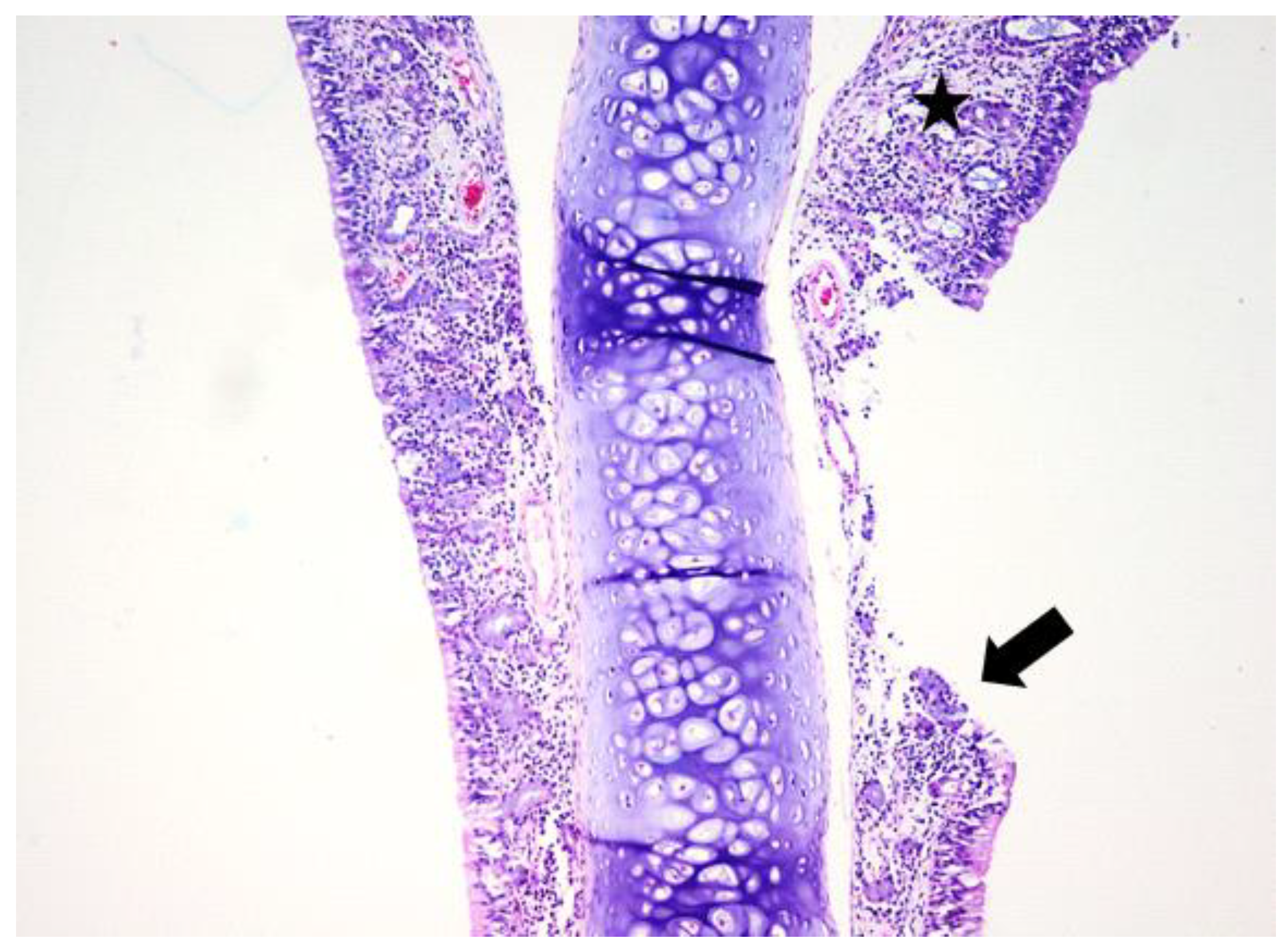
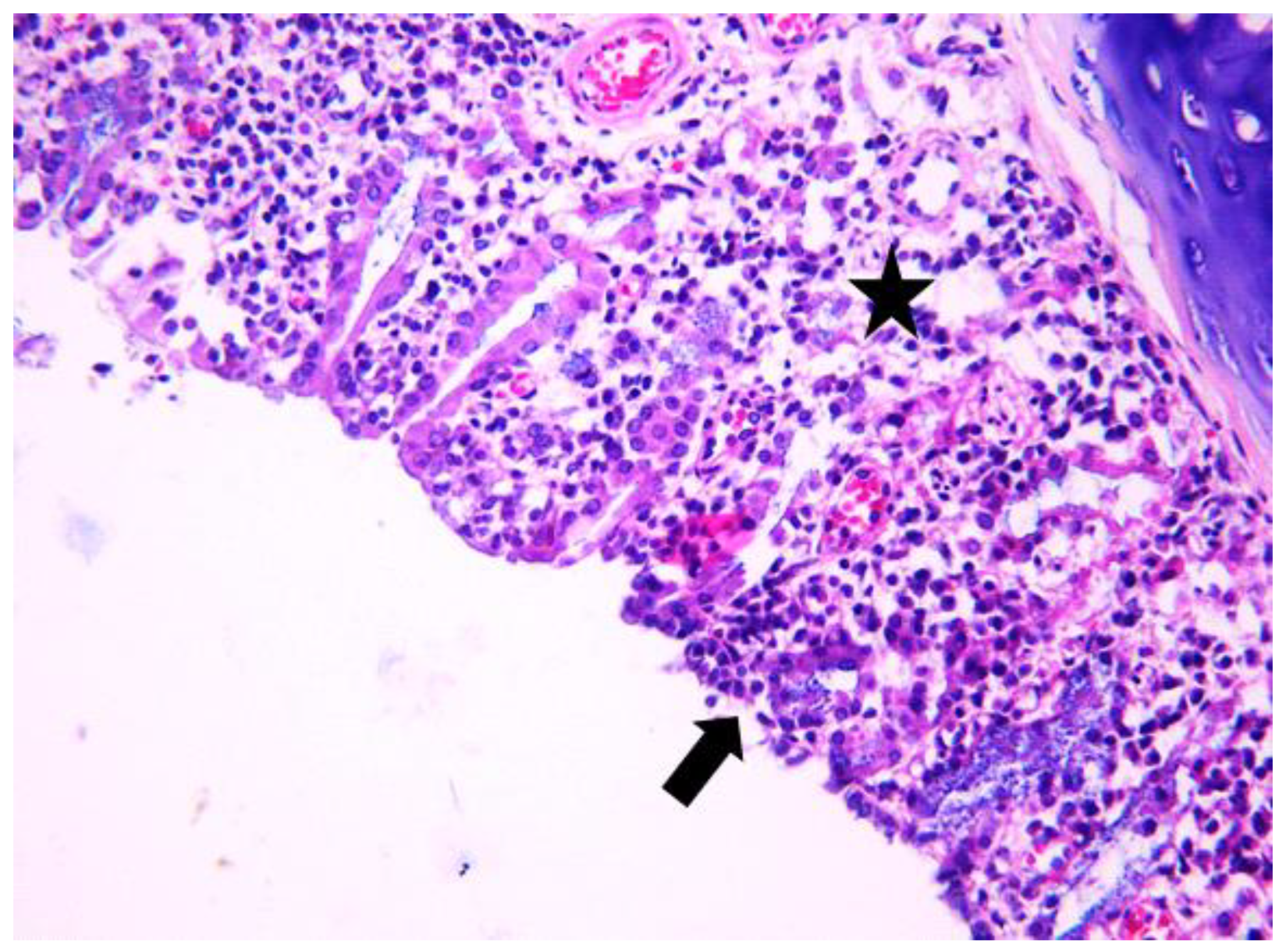
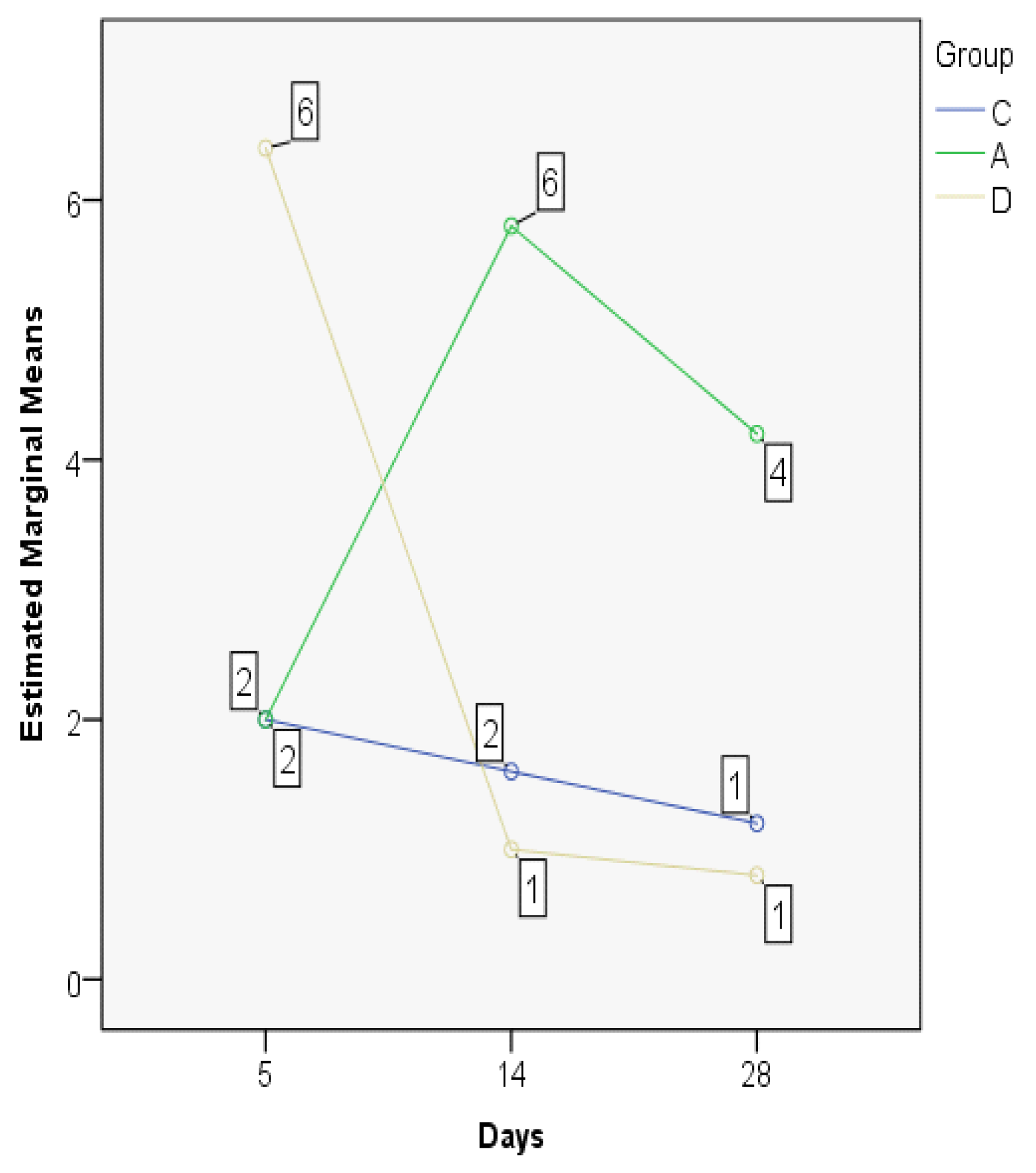
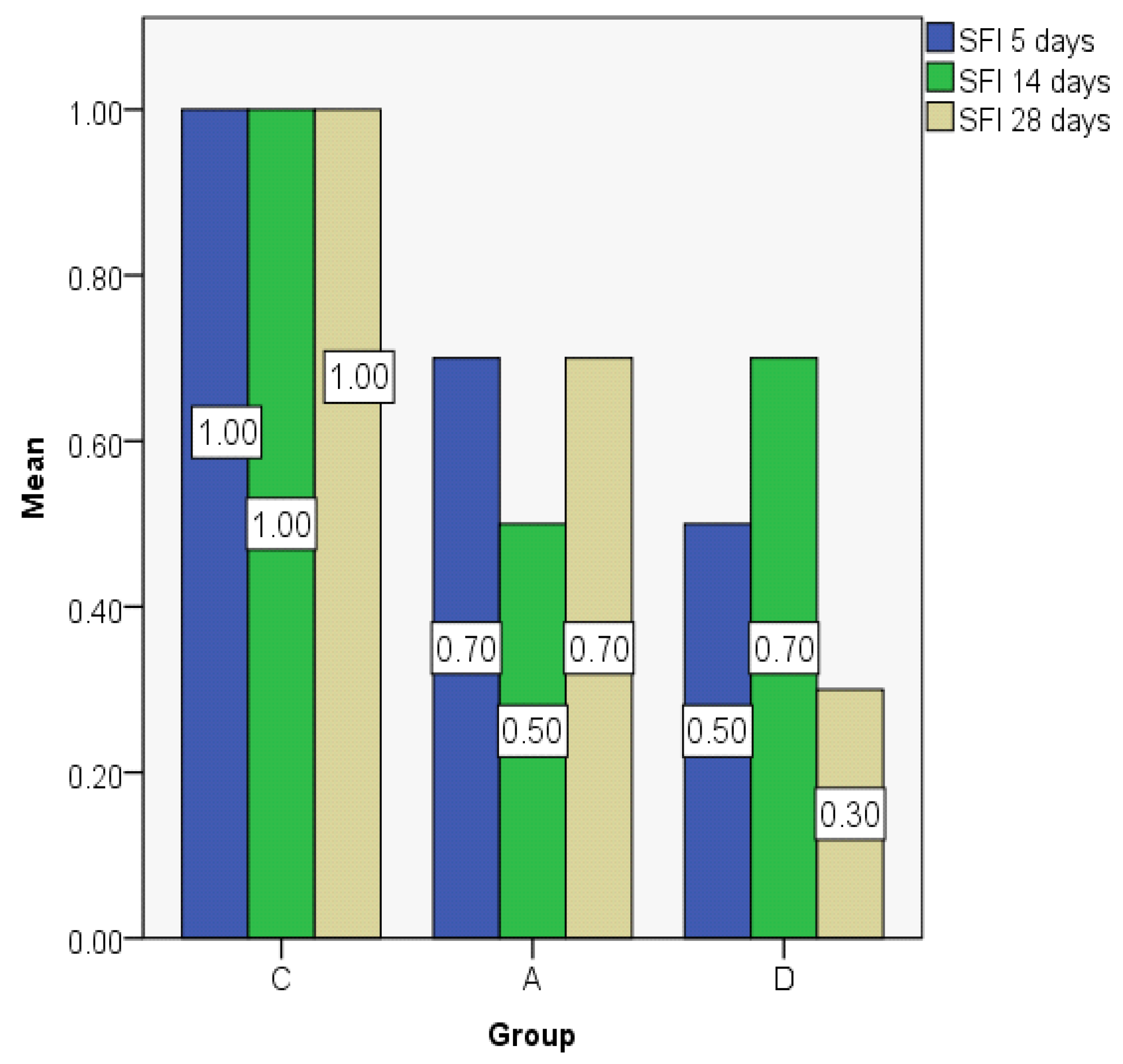
| Group | Mean | Median | Std. Deviation | Minim | Maxim | Range | IQR | |
|---|---|---|---|---|---|---|---|---|
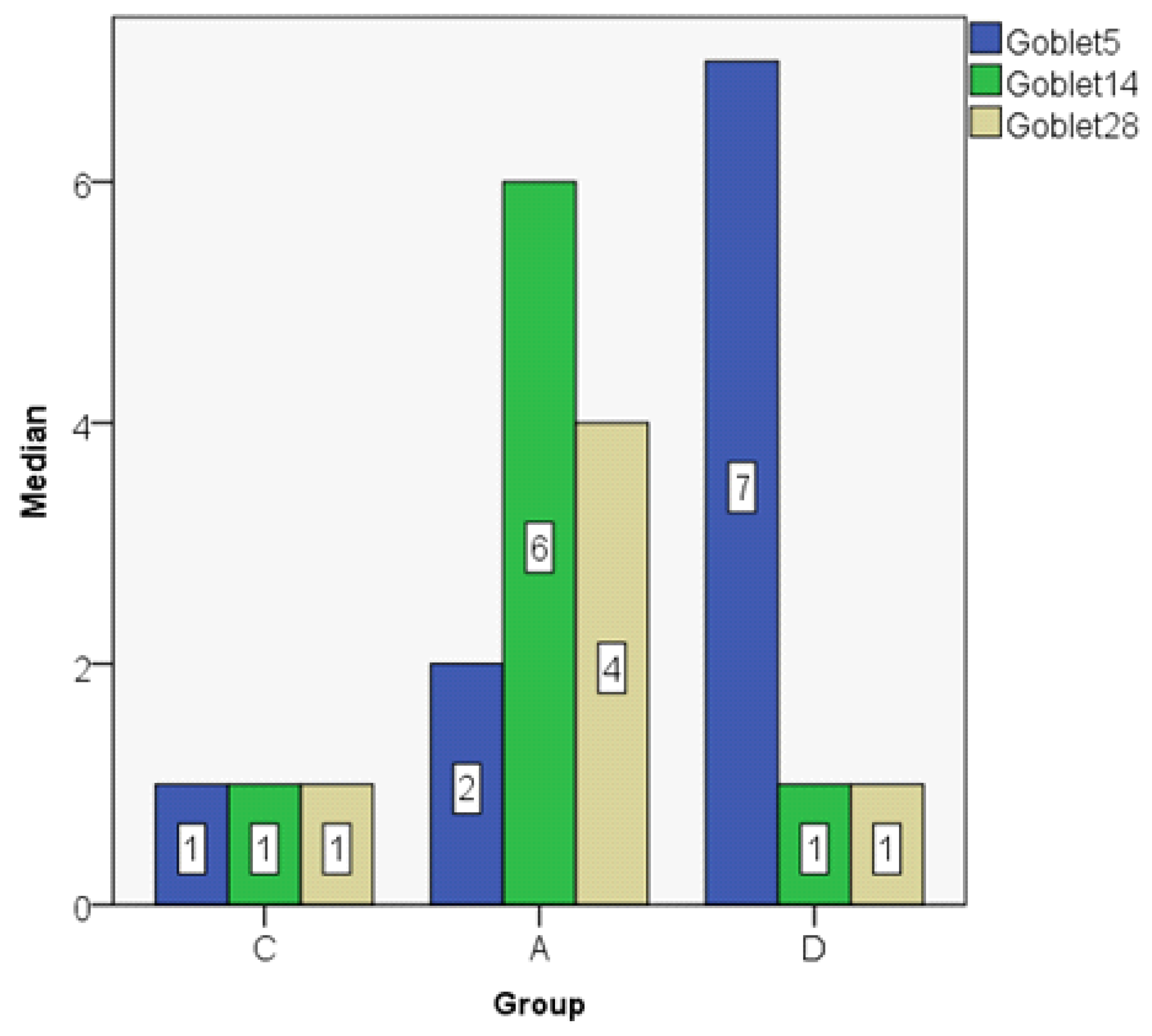
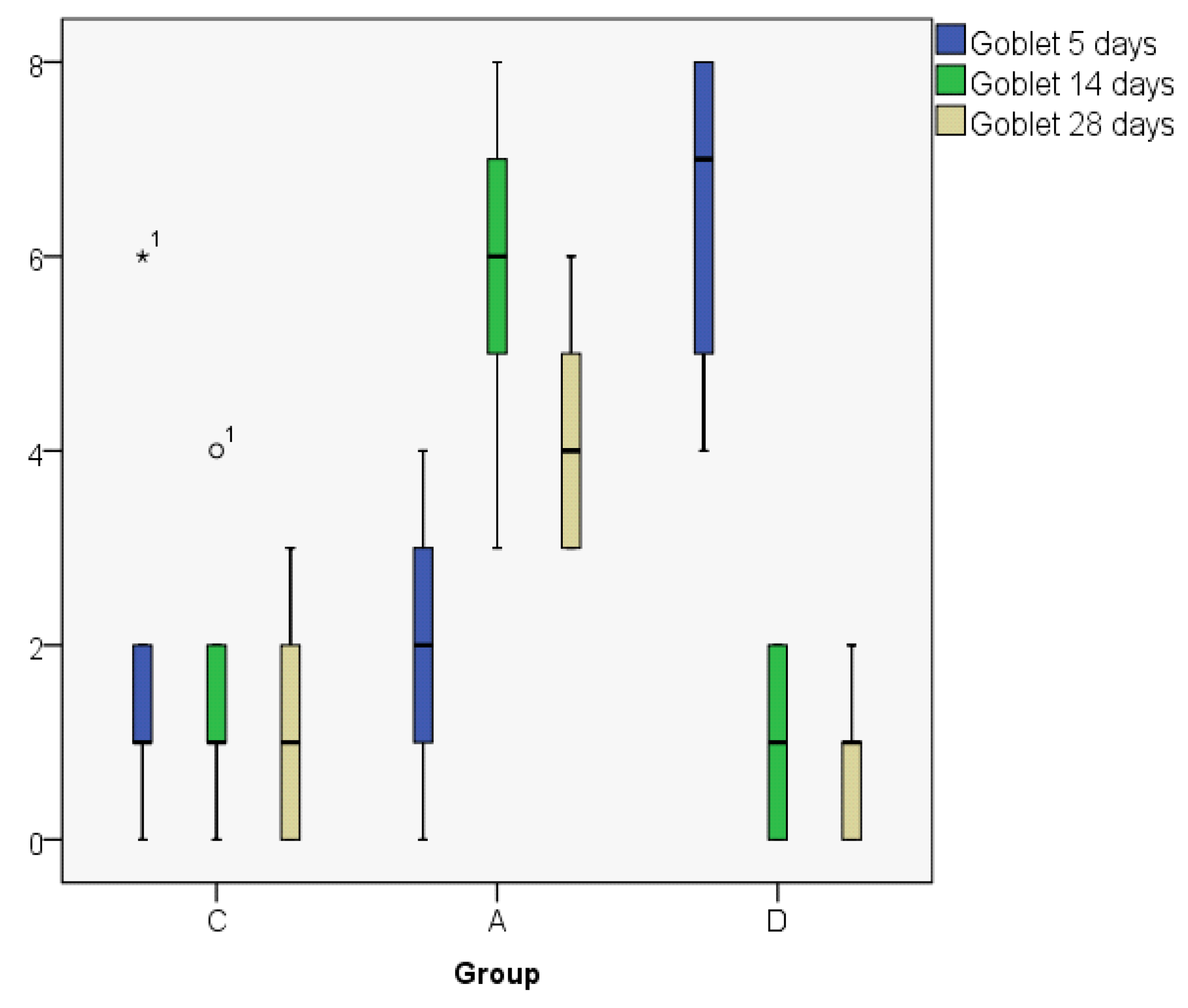
| Goblet5 | C | 2.00 | 1.00 | 2.345 | 0 | 6 | 6 | 4 |
| A | 2.00 | 2.00 | 1.581 | 0 | 4 | 4 | 3 | |
| D | 6.40 | 7.00 | 1.817 | 4 | 8 | 4 | 4 | |
| Goblet14 | C | 1.60 | 1.00 | 1.517 | 0 | 4 | 4 | 3 |
| A | 5.80 | 6.00 | 1.924 | 3 | 8 | 5 | 4 | |
| D | 1.00 | 1.00 | 1.000 | 0 | 2 | 2 | 2 | |
| Goblet28 | C | 1.20 | 1.00 | 1.304 | 0 | 3 | 3 | 3 |
| A | 4.20 | 4.00 | 1.304 | 3 | 6 | 3 | 3 | |
| D | 0.80 | 1.00 | 0.837 | 0 | 2 | 2 | 2 | |
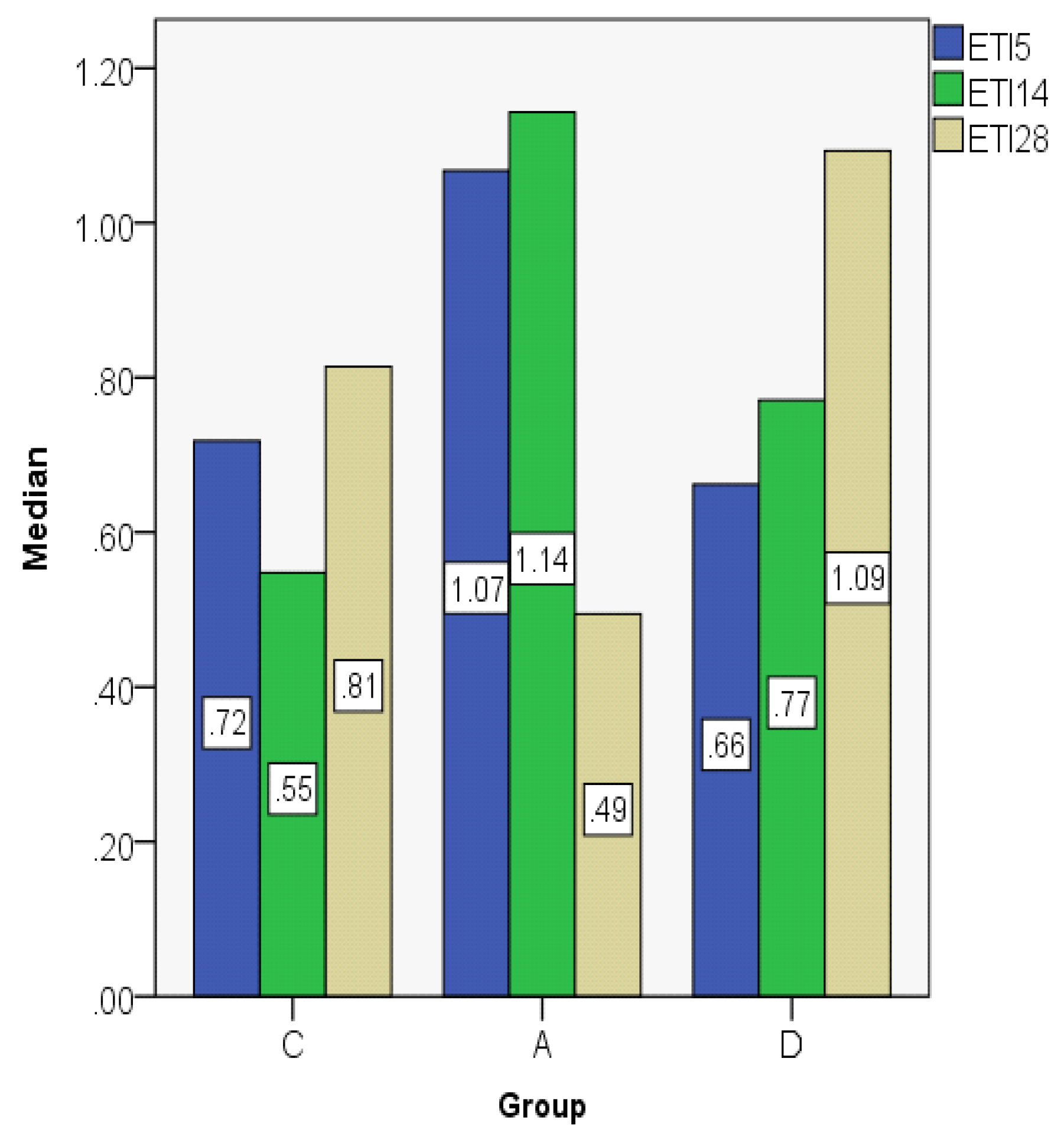
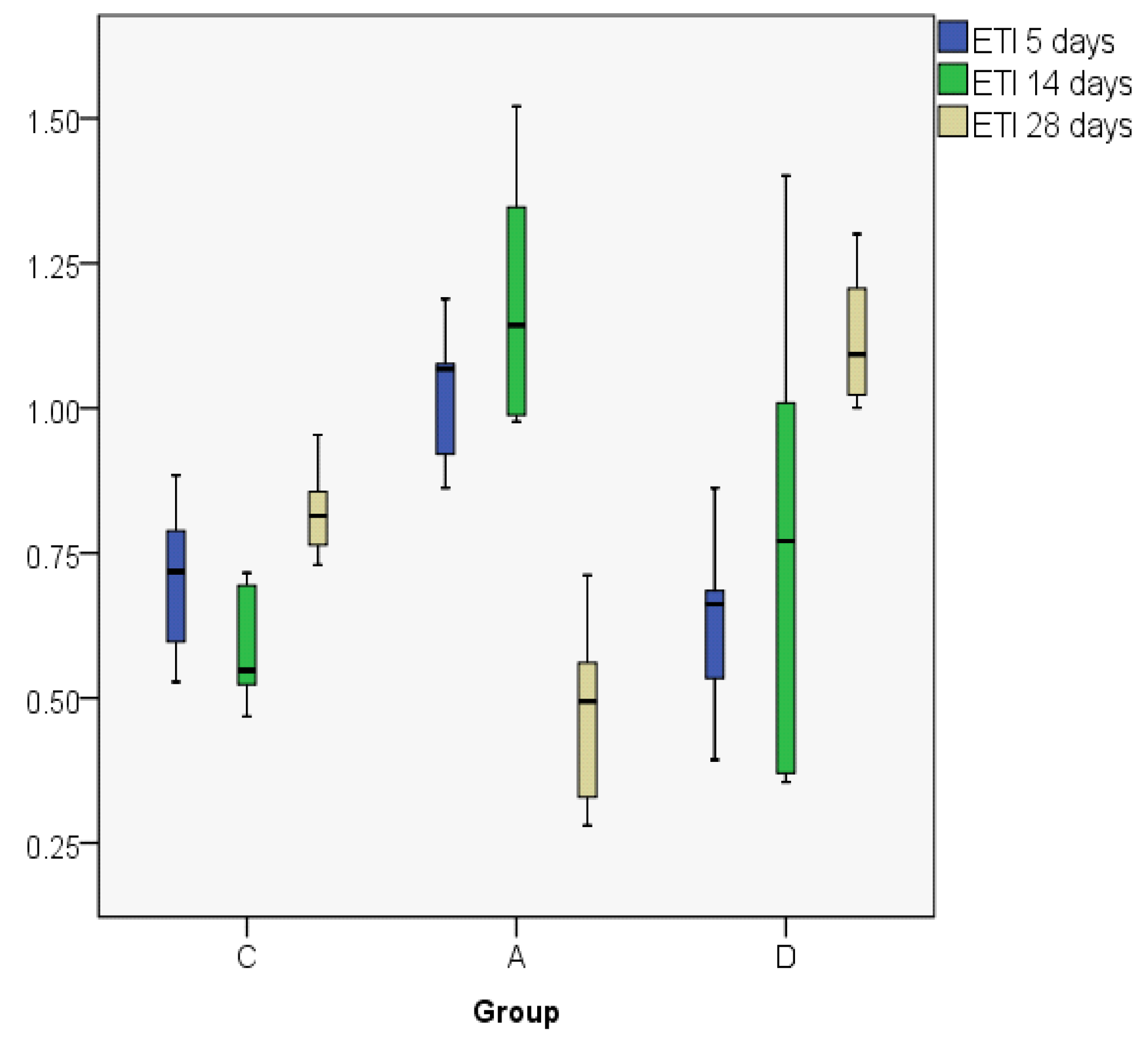
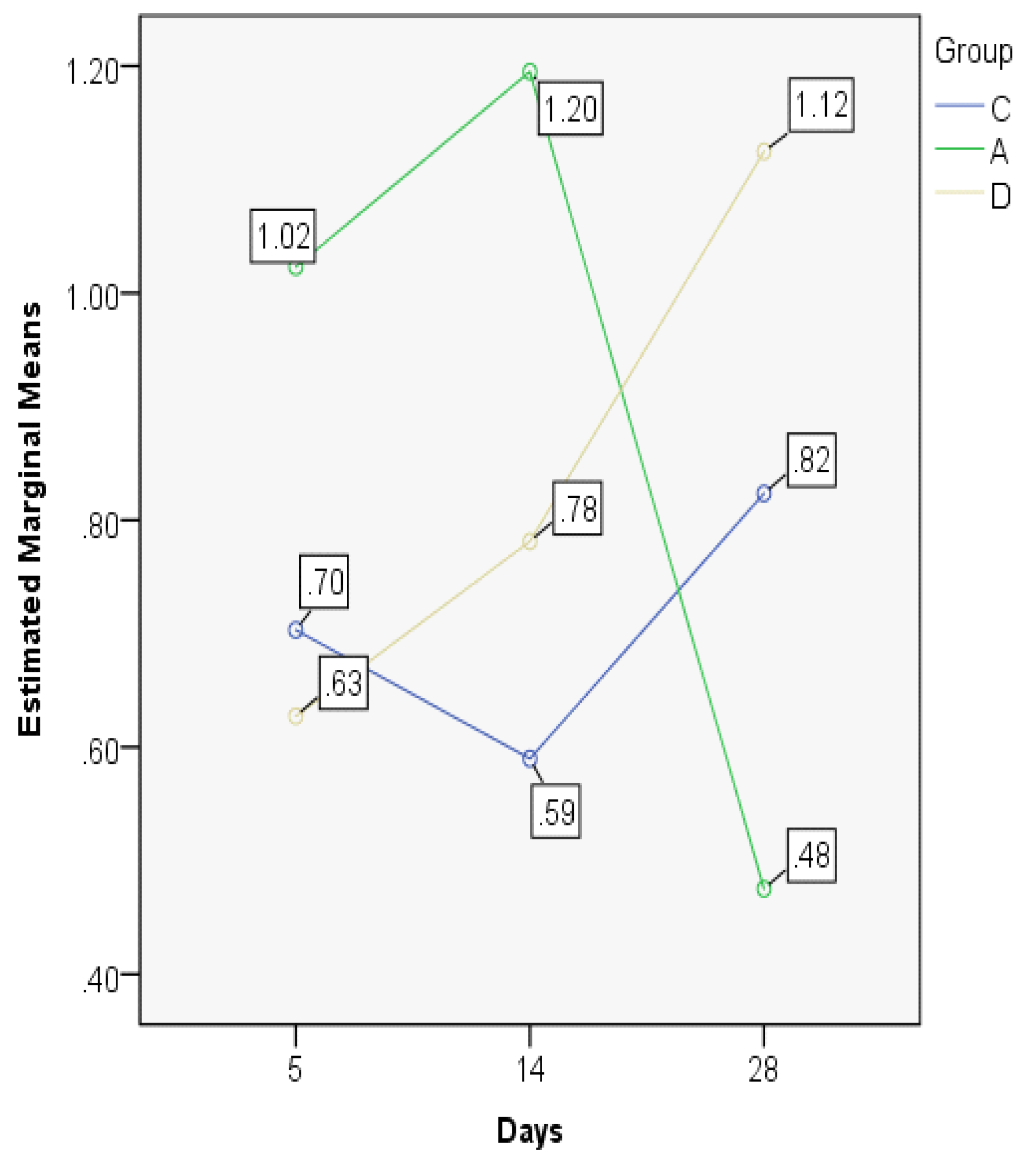
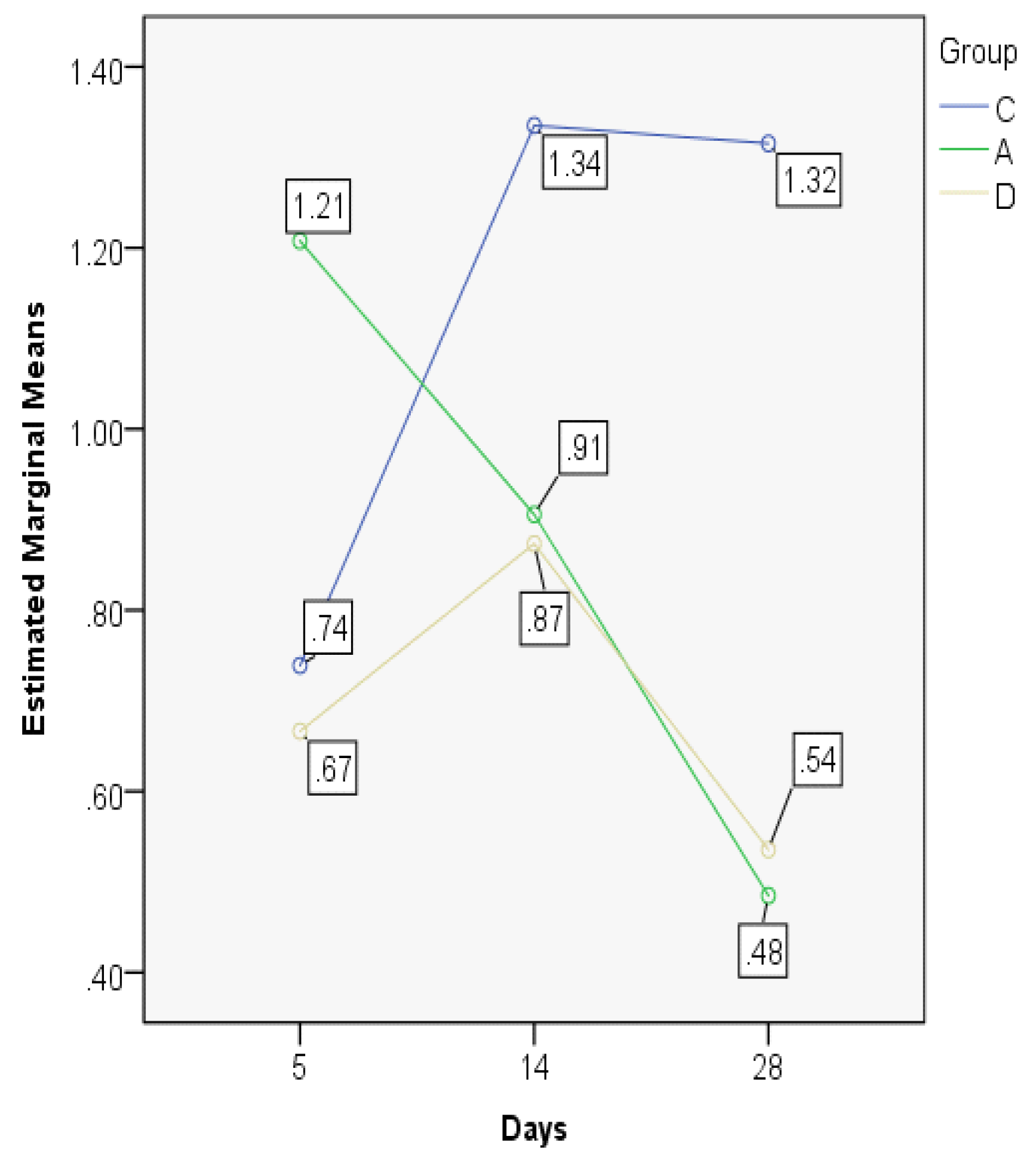
| ETI5 | C | 0.7032 | 0.7182 | 0.14353 | 0.53 | 0.88 | 0.36 | 0.27 |
| A | 1.0231 | 1.0674 | 0.13079 | 0.86 | 1.19 | 0.33 | 0.24 | |
| D | 0.6274 | 0.6620 | 0.17541 | 0.39 | 0.86 | 0.47 | 0.31 | |
| ETI14 | C | 0.5898 | 0.5478 | 0.10927 | 0.47 | 0.72 | 0.25 | 0.21 |
| A | 1.1950 | 1.1429 | 0.23580 | 0.98 | 1.52 | 0.54 | 0.45 | |
| D | 0.7812 | 0.7708 | 0.44348 | 0.35 | 1.40 | 1.05 | 0.84 | |
| ETI28 | C | 0.8236 | 0.8142 | 0.08743 | 0.73 | 0.95 | 0.22 | 0.16 |
| A | 0.4753 | 0.4942 | 0.17553 | 0.28 | 0.71 | 0.43 | 0.33 | |
| D | 1.1247 | 1.0930 | 0.12677 | 1.00 | 1.30 | 0.30 | 0.24 | |
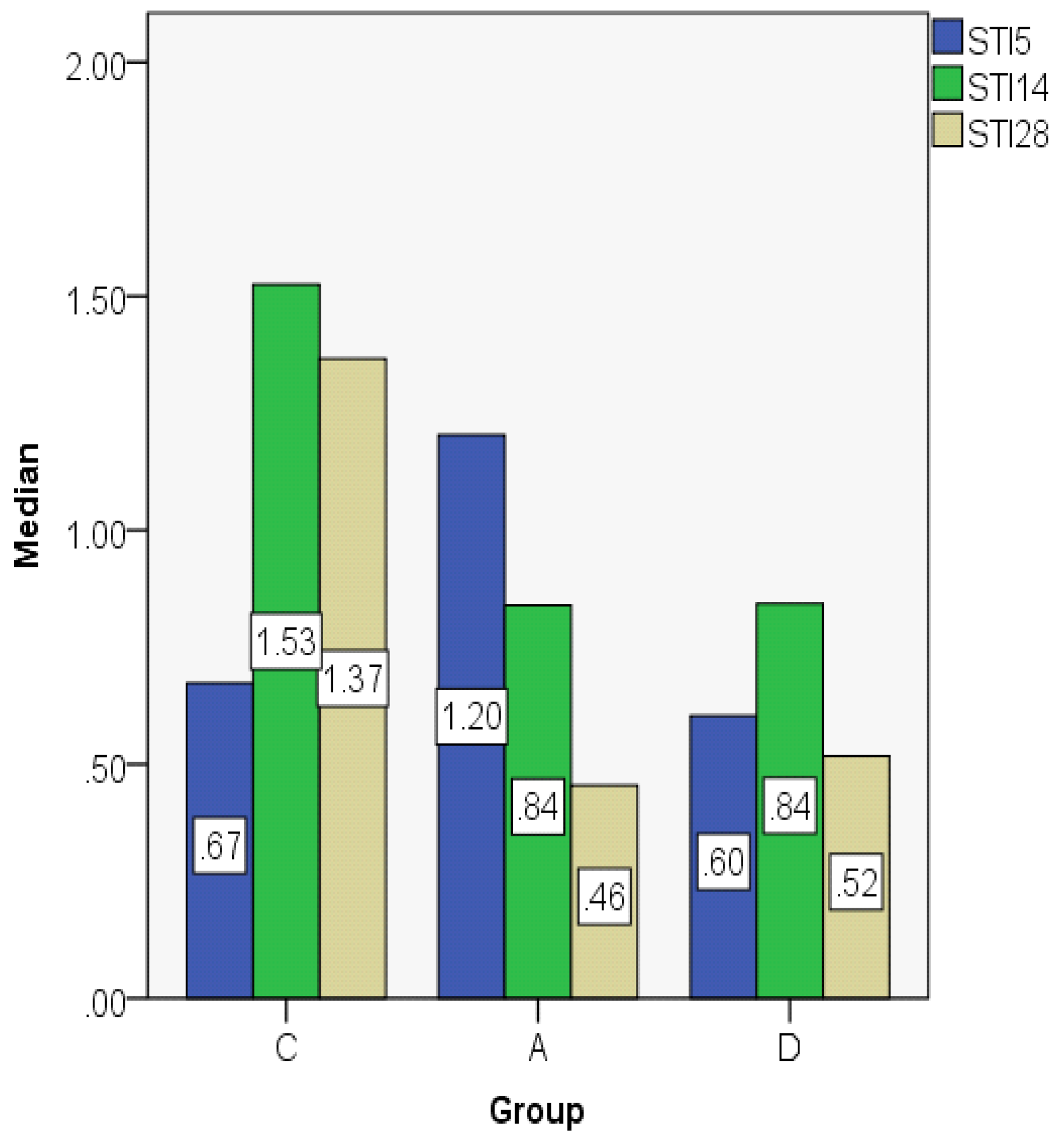
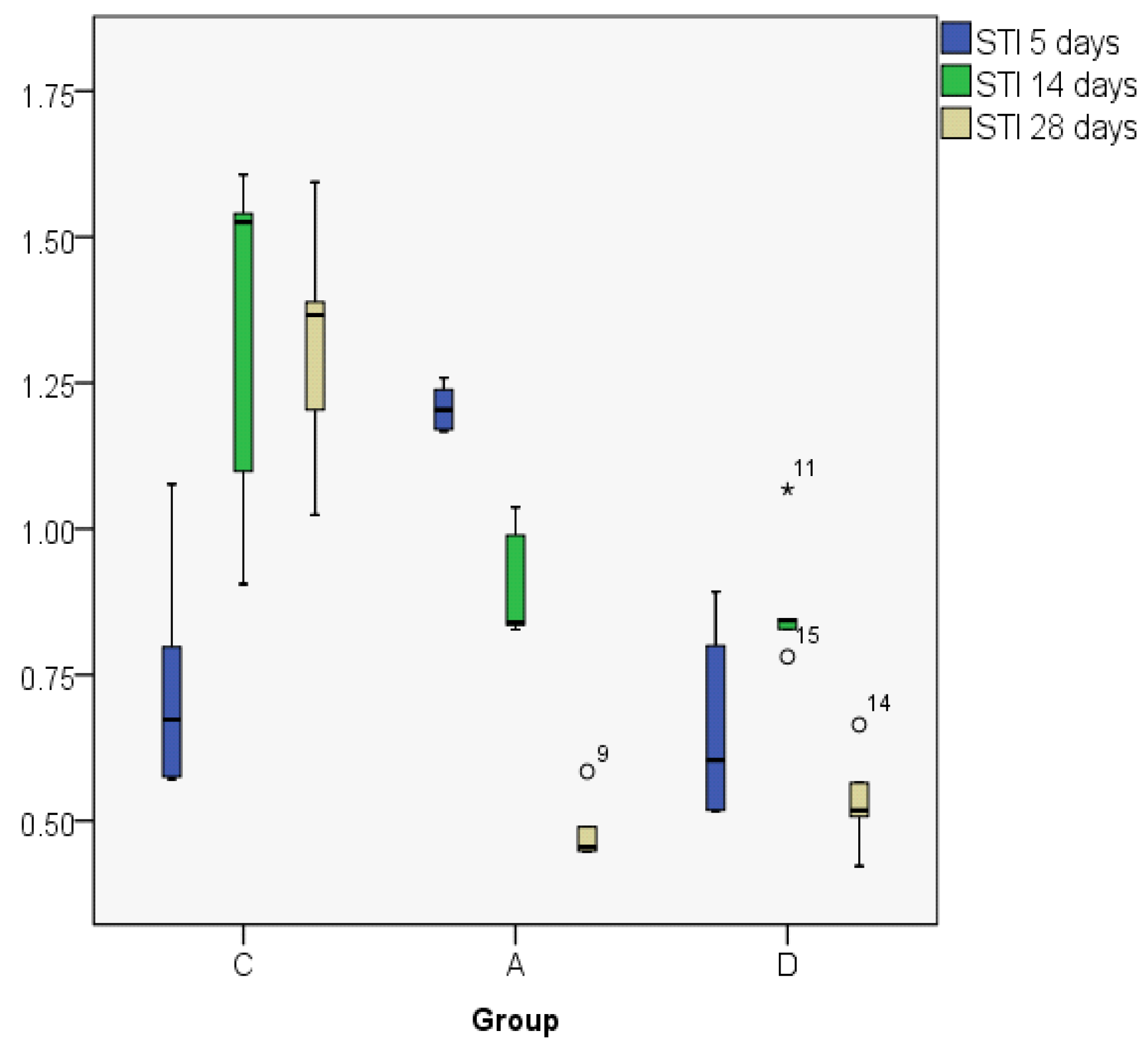
| STI5 | C | 0.7389 | 0.6732 | 0.21001 | 0.57 | 1.08 | 0.50 | 0.36 |
| A | 1.2077 | 1.2038 | 0.04060 | 1.17 | 1.26 | 0.09 | 0.08 | |
| D | 0.6663 | 0.6038 | 0.17112 | 0.52 | 0.89 | 0.38 | 0.33 | |
| STI14 | C | 1.3352 | 1.5252 | 0.31325 | 0.91 | 1.61 | 0.70 | 0.57 |
| A | 0.9056 | 0.8392 | 0.09938 | 0.83 | 1.04 | 0.21 | 0.18 | |
| D | 0.8732 | 0.8434 | 0.11184 | 0.78 | 1.07 | 0.29 | 0.15 | |
| STI28 | C | 1.3153 | 1.3664 | 0.21380 | 1.02 | 1.59 | 0.57 | 0.38 |
| A | 0.4848 | 0.4552 | 0.05832 | 0.45 | 0.58 | 0.14 | 0.09 | |
| D | 0.5354 | 0.5176 | 0.08858 | 0.42 | 0.66 | 0.24 | 0.15 |
| Test Statistics a,b | |||
|---|---|---|---|
| Chi-Square | df | Asymp. Sig. | |
| Goblet5 | 7.715 | 2 | 0.021 |
| Goblet14 | 9.013 | 2 | 0.011 |
| Goblet28 | 9.073 | 2 | 0.011 |
| ETI5 | 8.180 | 2 | 0.017 |
| ETI14 | 6.020 | 2 | 0.049 |
| ETI28 | 12.500 | 2 | 0.002 |
| STI5 | 9.500 | 2 | 0.009 |
| STI14 | 7.280 | 2 | 0.026 |
| STI28 | 9.780 | 2 | 0.008 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manciula, L.-G.; Berce, C.; Tabaran, F.; Trombitaș, V.; Albu, S. The Effects of Postoperative Astaxanthin Administration on Nasal Mucosa Wound Healing. J. Clin. Med. 2019, 8, 1941. https://doi.org/10.3390/jcm8111941
Manciula L-G, Berce C, Tabaran F, Trombitaș V, Albu S. The Effects of Postoperative Astaxanthin Administration on Nasal Mucosa Wound Healing. Journal of Clinical Medicine. 2019; 8(11):1941. https://doi.org/10.3390/jcm8111941
Chicago/Turabian StyleManciula, Lavinia-Gianina, Cristian Berce, Flaviu Tabaran, Veronica Trombitaș, and Silviu Albu. 2019. "The Effects of Postoperative Astaxanthin Administration on Nasal Mucosa Wound Healing" Journal of Clinical Medicine 8, no. 11: 1941. https://doi.org/10.3390/jcm8111941
APA StyleManciula, L.-G., Berce, C., Tabaran, F., Trombitaș, V., & Albu, S. (2019). The Effects of Postoperative Astaxanthin Administration on Nasal Mucosa Wound Healing. Journal of Clinical Medicine, 8(11), 1941. https://doi.org/10.3390/jcm8111941







