Targeting Endotypic Traits with Medications for the Pharmacological Treatment of Obstructive Sleep Apnea. A Review of the Current Literature
Abstract
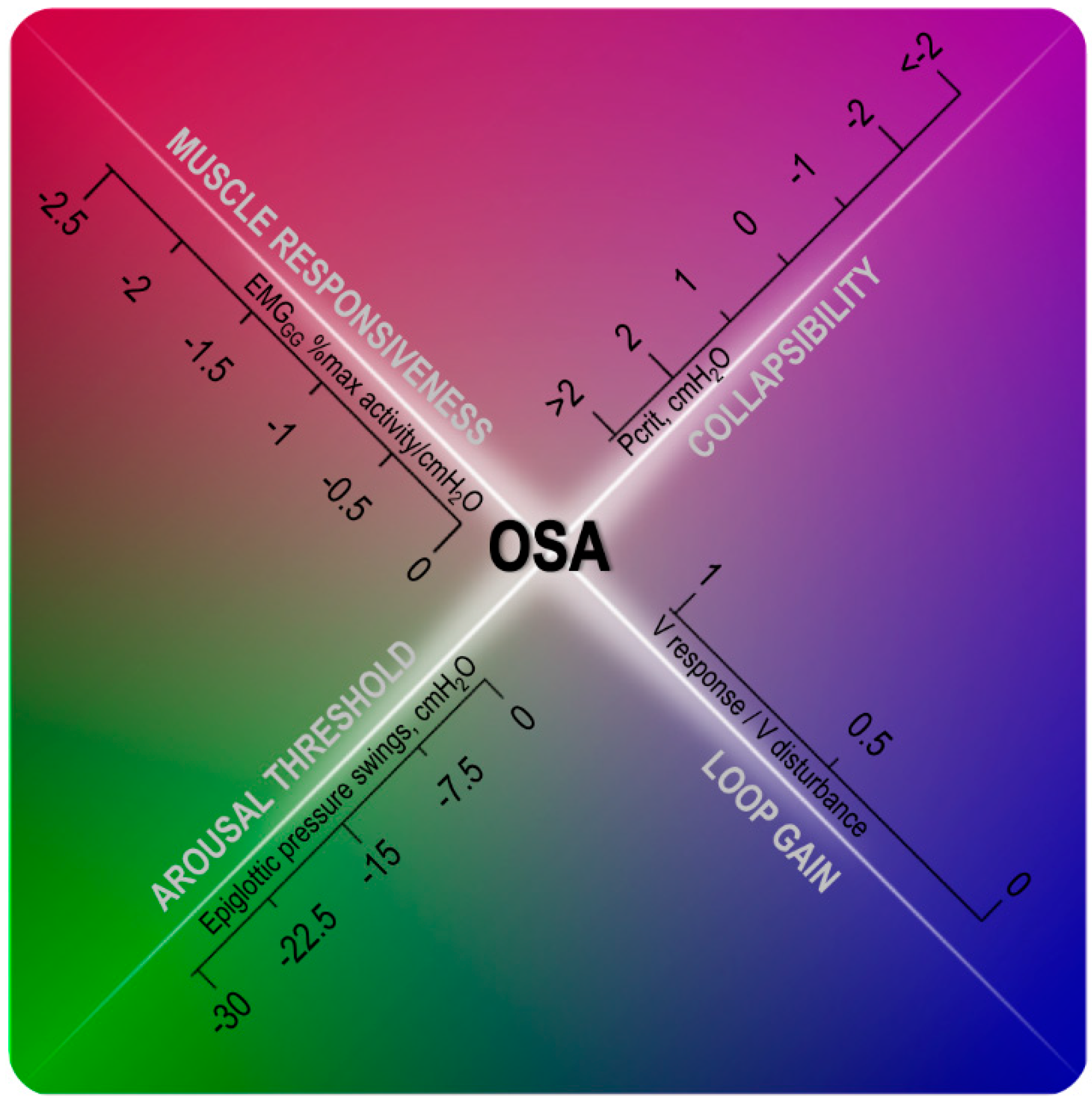
1. Introduction
Personalized Medicine Approach
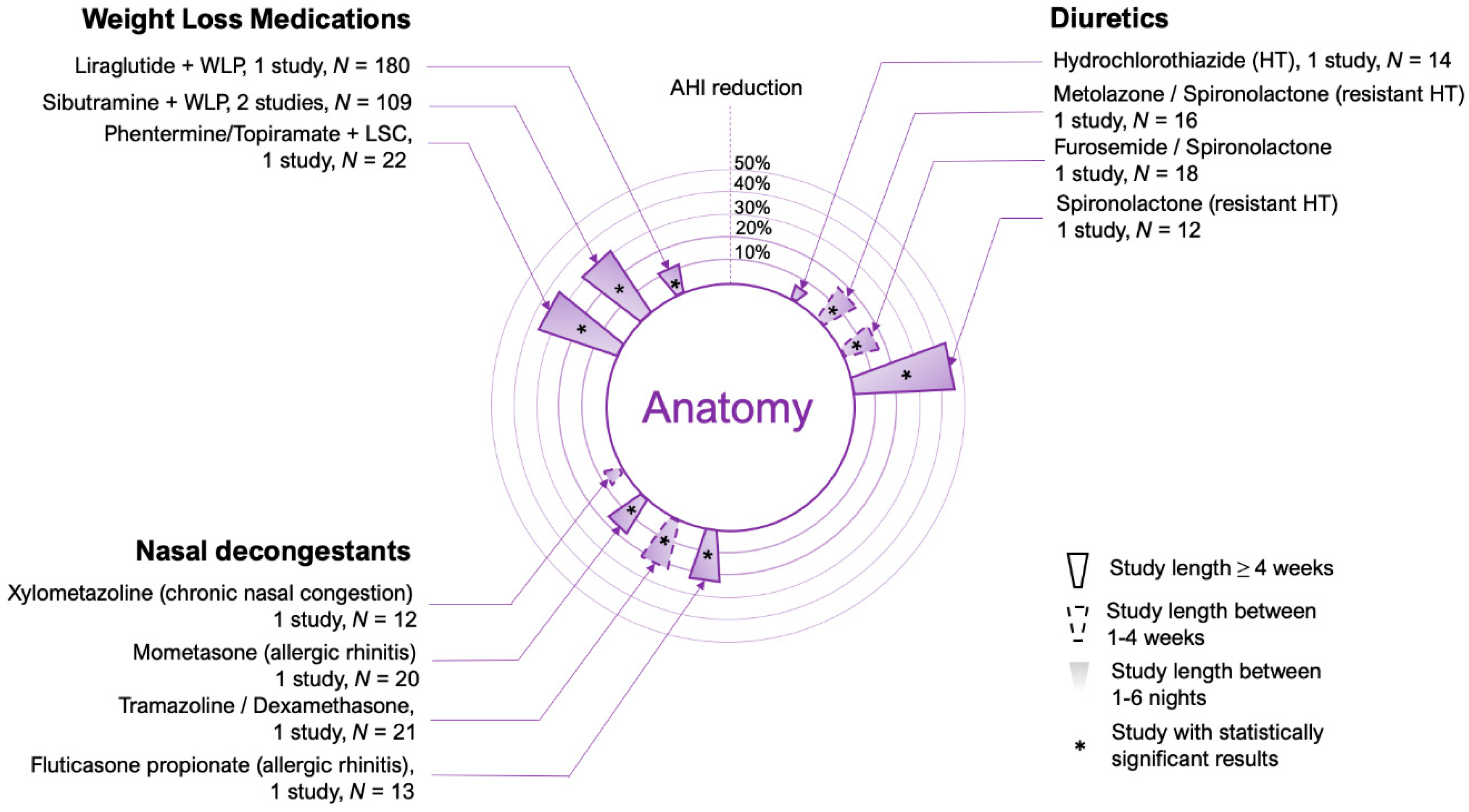
2. Upper Airway Anatomy
2.1. Weight Loss Medications
2.2. Upper Airway Edema
2.3. Nasal Decongestants
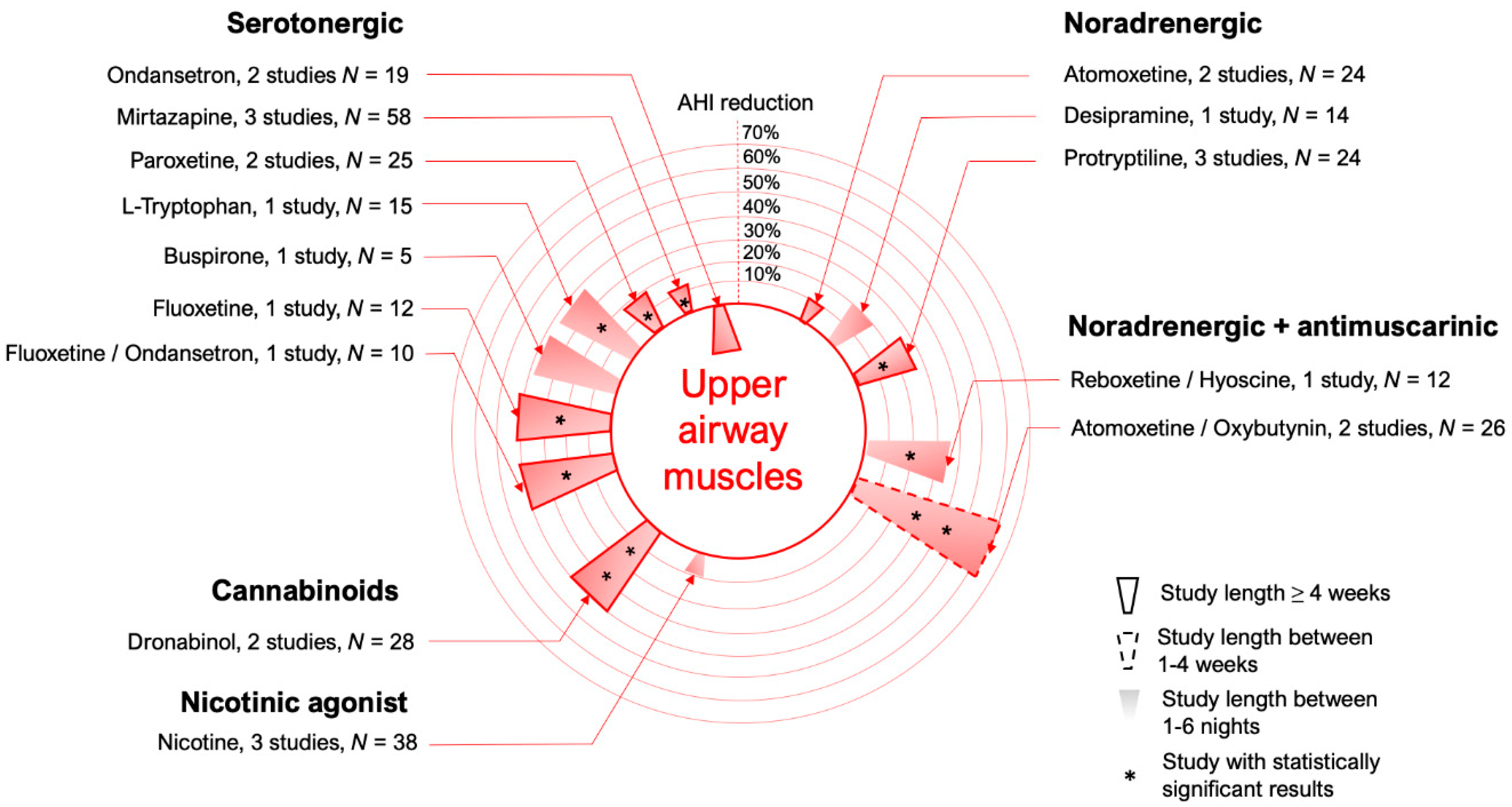
3. Upper Airway Dilator Muscle Activation
3.1. Serotonergic Mechanisms
3.2. Noradrenergic Mechanisms
3.3. K+ Channel Blockers
3.4. Cannabinoids
3.5. Nicotine
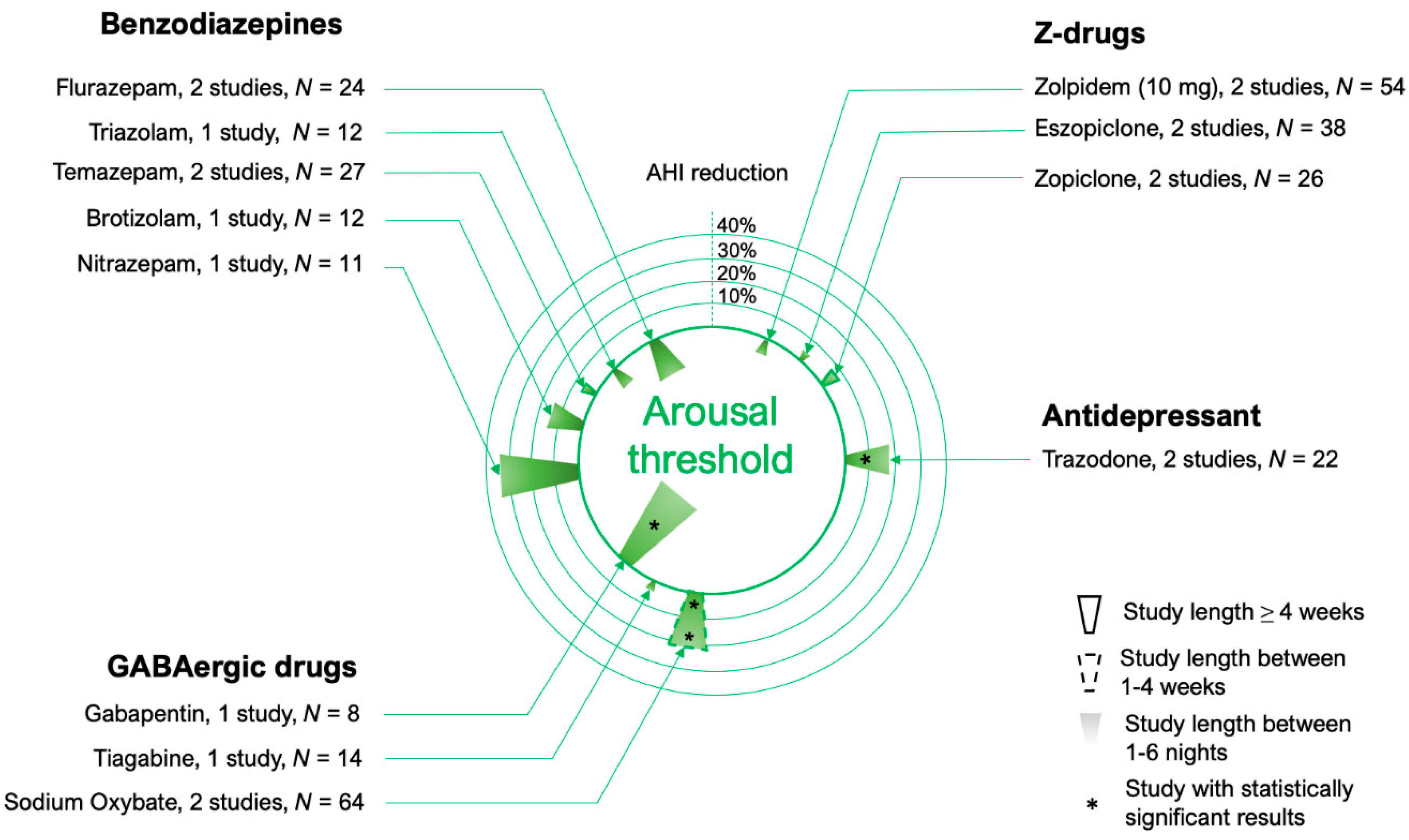
4. Arousal Threshold
4.1. Benzodiazepines
4.2. Z-Drugs
4.3. Other Hypnotics and Sedatives
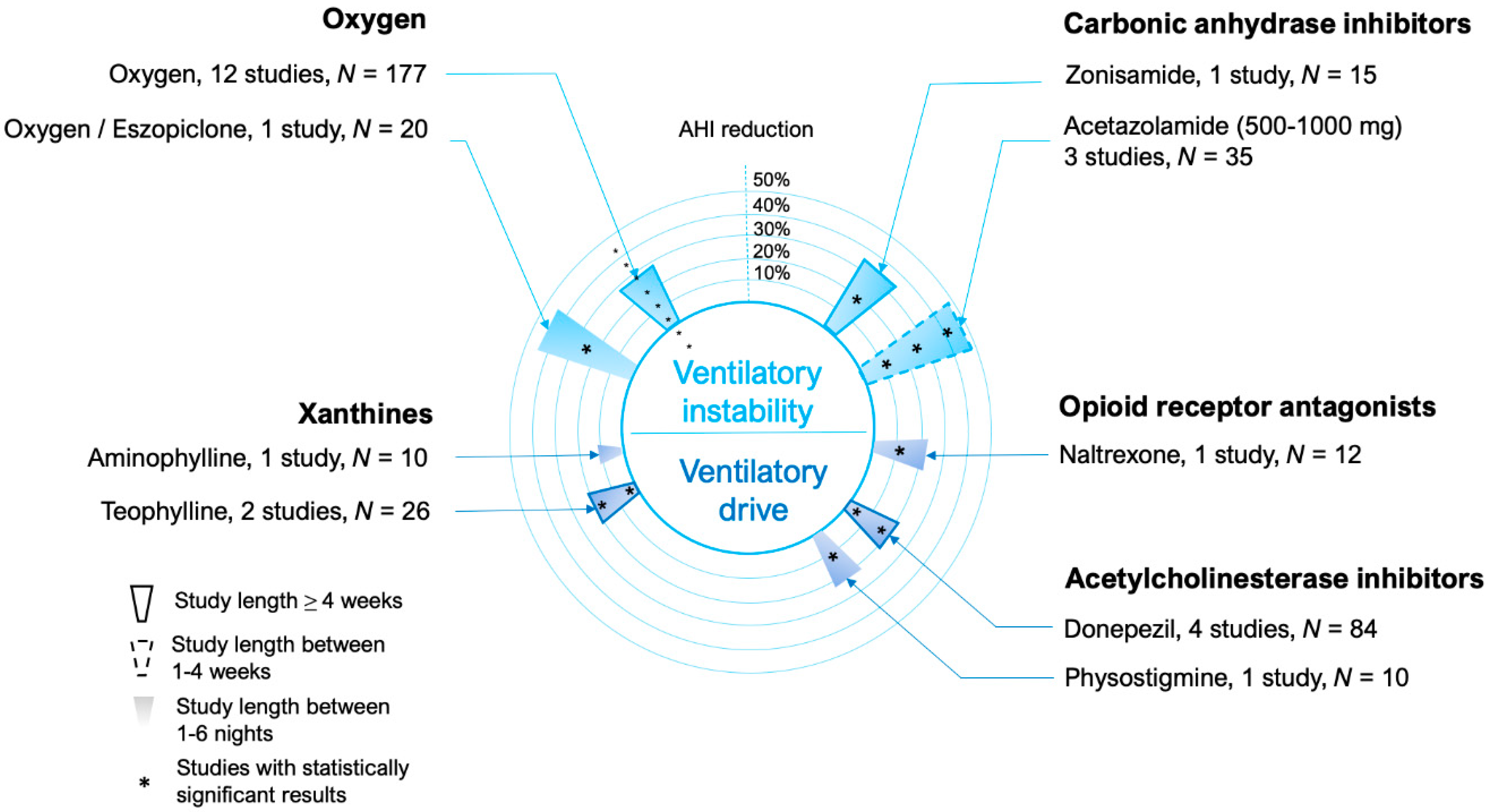
5. Ventilatory Control (Loop Gain)
5.1. Carbonic Anhydrase Inhibitors
5.2. Oxygen
5.3. Carbon Dioxide Rebreathing
6. Other Drugs Acting on Ventilatory Drive
6.1. Xanthines
6.2. Opioid Antagonists
6.3. Acetylcholinesterase Inhibitors
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sawyer, A.M.; Gooneratne, N.S.; Marcus, C.L.; Ofer, D.; Richards, K.C.; Weaver, T.E. A systematic review of CPAP adherence across age groups: Clinical and empiric insights for developing CPAP adherence interventions. Sleep Med. Rev. 2011, 15, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Weaver, T.E.; Grunstein, R.R. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc. Am. Thorac. Soc. 2008, 5, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining Phenotypic Causes of Obstructive Sleep Apnea. Identification of Novel Therapeutic Targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004. [Google Scholar] [CrossRef]
- Horner, R.L.; Grace, K.P.; Wellman, A. A resource of potential drug targets and strategic decision-making for obstructive sleep apnoea pharmacotherapy. Respirology 2017, 22, 861–873. [Google Scholar] [CrossRef]
- Younes, M. Contributions of Upper Airway Mechanics and Control Mechanisms to Severity of Obstructive Apnea. Am. J. Respir. Crit. Care Med. 2003, 168, 645–658. [Google Scholar] [CrossRef]
- Younes, M. Role of Arousals in the Pathogenesis of Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2004, 169, 623–633. [Google Scholar] [CrossRef]
- Younes, M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J. Appl. Physiol. 2008, 105, 1389–1405. [Google Scholar] [CrossRef]
- Wellman, A.; Eckert, D.J.; Jordan, A.S.; Edwards, B.A.; Passaglia, C.L.; Jackson, A.C.; Gautam, S.; Owens, R.L.; Malhotra, A.; White, D.P. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J. Appl. Physiol. 2011, 110, 1627–1637. [Google Scholar]
- Wellman, A.; Edwards, B.A.; Sands, S.A.; Owens, R.L.; Nemati, S.; Butler, J.; Passaglia, C.L.; Jackson, A.C.; Malhotra, A.; White, D.P. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J. Appl. Physiol. 2013, 114, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.A.; Eckert, D.J.; McSharry, D.G.; Sands, S.A.; Desai, A.; Kehlmann, G.; Bakker, J.P.; Genta, P.R.; Owens, R.L.; White, D.P.; et al. Clinical Predictors of the Respiratory Arousal Threshold in Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2014, 190, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Sands, S.A.; Edwards, B.A.; Terrill, P.I.; Taranto-Montemurro, L.; Azarbarzin, A.; Marques, M.; Hess, L.B.; White, D.P.; Wellman, A. Phenotyping Pharyngeal Pathophysiology using Polysomnography in Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2018, 197, 1187–1197. [Google Scholar] [PubMed]
- Sands, S.A.; Terrill, P.I.; Edwards, B.A.; Taranto Montemurro, L.; Azarbarzin, A.; Marques, M.; de Melo, C.M.; Loring, S.H.; Butler, J.P.; White, D.P.; et al. Quantifying the Arousal Threshold Using Polysomnography in Obstructive Sleep Apnea. Sleep 2018, 41, zsx183. [Google Scholar] [CrossRef] [PubMed]
- Terrill, P.I.; Edwards, B.A.; Nemati, S.; Butler, J.P.; Owens, R.L.; Eckert, D.J.; White, D.P.; Malhotra, A.; Wellman, A.; Sands, S.A. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur. Respir. J. 2015, 45, 408–418. [Google Scholar] [CrossRef]
- Kapur, V.K.; Baldwin, C.M.; Resnick, H.E.; Gottlieb, D.J.; Nieto, F.J. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep 2005, 28, 472–478. [Google Scholar] [CrossRef]
- Azarbarzin, A.; Sands, S.A.; Stone, K.L.; Taranto-Montemurro, L.; Messineo, L.; Terrill, P.I.; Ancoli-Israel, S.; Ensrud, K.; Purcell, S.; White, D.P.; et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur. Heart J. 2019, 40, 1149–1157. [Google Scholar]
- Winslow, D.H.; Bowden, C.H.; DiDonato, K.P.; McCullough, P.A. A Randomized, Double-Blind, Placebo-Controlled Study of an Oral, Extended-Release Formulation of Phentermine/Topiramate for the Treatment of Obstructive Sleep Apnea in Obese Adults. Sleep 2012, 35, 1529–1539. [Google Scholar]
- Yokoyama, O.; Yamaguchi, A.; Yoshida, M.; Yamanishi, T.; Ishizuka, O.; Seki, N.; Takahashi, S.; Yamaguchi, O.; Higo, N.; Minami, H.; et al. Once-daily oxybutynin patch improves nocturia and sleep quality in Japanese patients with overactive bladder: Post-hoc analysis of a phase III randomized clinical trial. Int. J. Urol. 2015, 22, 684–688. [Google Scholar] [CrossRef]
- Isono, S. Obesity and obstructive sleep apnoea: Mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology 2012, 17, 32–42. [Google Scholar]
- Ito, E.; Tsuiki, S.; Maeda, K.; Okajima, I.; Inoue, Y. Oropharyngeal Crowding Closely Relates to Aggravation of OSA. Chest 2016, 150, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Peterson, E.; Yaremchuk, K.L. The Role of Tonsillectomy in Adults with Tonsillar Hypertrophy and Obstructive Sleep Apnea. Otolaryngol. Neck Surg. 2017, 157, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, A.; Gabriel, J.M.; White, L.H.; Montemurro, L.T.; Kasai, T.; Bradley, T.D. A Randomized, Double Crossover Study to Investigate the Influence of Saline Infusion on Sleep Apnea Severity in Men. Sleep 2014, 37, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, M.; Schleimer, R.P.; Keshavarzian, A. Sleep disruption in chronic rhinosinusitis. Expert Rev. Anti-Infect. Ther. 2017, 15, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Hudgel, D.W.; Patel, S.R.; Ahasic, A.M.; Bartlett, S.J.; Bessesen, D.H.; Coaker, M.A.; Fiander, P.M.; Grunstein, R.R.; Gurubhagavatula, I.; Kapur, V.K.; et al. The Role of Weight Management in the Treatment of Adult Obstructive Sleep Apnea. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e70–e87. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of obstructive sleep apnea: A population health perspective. Am. J. Respir. Crit. Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef]
- Wong, A.-M.; Barnes, H.N.; Joosten, S.A.; Landry, S.A.; Dabscheck, E.; Mansfield, D.R.; Dharmage, S.C.; Senaratna, C.V.; Edwards, B.A.; Hamilton, G.S. The effect of surgical weight loss on obstructive sleep apnoea: A systematic review and meta-analysis. Sleep Med. Rev. 2018, 42, 85–99. [Google Scholar] [CrossRef]
- Mitchell, L.J.; Davidson, Z.E.; Bonham, M.; O’Driscoll, D.M.; Hamilton, G.S.; Truby, H. Weight loss from lifestyle interventions and severity of sleep apnoea: A systematic review and meta-analysis. Sleep Med. 2014, 15, 1173–1183. [Google Scholar] [CrossRef]
- Joosten, S.A.; Hamilton, G.S.; Naughton, M.T. Impact of Weight Loss Management in OSA. Chest 2017, 152, 194–203. [Google Scholar] [CrossRef]
- Tuomilehto, H.; Seppa, J.; Uusitupa, M.; Tuomilehto, J.; Gylling, H. Weight reduction and increased physical activity to prevent the progression of obstructive sleep apnea: A 4-year observational postintervention follow-up of a randomized clinical trial. JAMA Intern. Med. 2013, 173, 929–930. [Google Scholar] [CrossRef]
- Yee, B.J.; Phillips, C.L.; Banerjee, D.; Caterson, I.; Hedner, J.A.; Grunstein, R.R. The effect of sibutramine-assisted weight loss in men with obstructive sleep apnoea. Int. J. Obes. (Lond.) 2007, 31, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ferland, A.; Poirier, P.; Sériès, F. Sibutramine versus continuous positive airway pressure in obese obstructive sleep apnoea patients. Eur. Respir. J. 2009, 34, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Blackman, A.; Foster, G.D.; Zammit, G.; Rosenberg, R.; Aronne, L.; Wadden, T.; Claudius, B.; Jensen, C.B.; Mignot, E. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: The SCALE Sleep Apnea randomized clinical trial. Int. J. Obes. 2016, 40, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Redolfi, S.; Bettinzoli, M.; Venturoli, N.; Ravanelli, M.; Pedroni, L.; Taranto-Montemurro, L.; Arnulf, I.; Similowski, T.; Tantucci, C. Attenuation of Obstructive Sleep Apnea and Overnight Rostral Fluid Shift by Physical Activity. Am. J. Respir. Crit. Care Med. 2015, 191, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, R.P.; Drager, L.F.; Gonzaga, C.C.; Sousa, M.G.; de Paula, L.K.; Amaro, A.C.; Amodeo, C.; Bortolotto, L.A.; Krieger, E.M.; Bradley, T.D.; et al. Obstructive sleep apnea: The most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011, 58, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Yumino, D.; Redolfi, S.; Ruttanaumpawan, P.; Su, M.C.; Smith, S.; Newton, G.E.; Mak, S.; Bradley, T.D. Nocturnal rostral fluid shift: A unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 2010, 121, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Lyons, O.D.; Inami, T.; Perger, E.; Yadollahi, A.; Chan, C.T.; Bradley, T.D. The effect of fluid overload on sleep apnoea severity in haemodialysis patients. Eur. Respir. J. 2017, 49, 1601789. [Google Scholar] [CrossRef]
- Redolfi, S.; Yumino, D.; Ruttanaumpawan, P.; Yau, B.; Su, M.-C.; Lam, J.; Bradley, T.D. Relationship between Overnight Rostral Fluid Shift and Obstructive Sleep Apnea in Nonobese Men. Am. J. Respir. Crit. Care Med. 2009, 179, 241–246. [Google Scholar] [CrossRef]
- Gaddam, K.; Pimenta, E.; Thomas, S.J.; Cofield, S.S.; Oparil, S.; Harding, S.M.; Calhoun, D.A. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: A preliminary report. J. Hum. Hypertens. 2010, 24, 532–537. [Google Scholar] [CrossRef]
- Kasai, T.; Bradley, T.D.; Friedman, O.; Logan, A.G. Effect of intensified diuretic therapy on overnight rostral fluid shift and obstructive sleep apnoea in patients with uncontrolled hypertension. J. Hypertens. 2014, 32, 673–680. [Google Scholar] [CrossRef]
- Fiori, C.Z.; Martinez, D.; Montanari, C.C.; Lopez, P.; Camargo, R.; Sezerá, L.; Gonçalves, S.C.; Fuchs, F.D. Diuretic or sodium-restricted diet for obstructive sleep apnea—A randomized trial. Sleep 2018, 41. [Google Scholar] [CrossRef] [PubMed]
- Acar, M.; Cingi, C.; Sakallioglu, O.; San, T.; Yimenicioglu, M.F.; Bal, C. The Effects of Mometasone Furoate and Desloratadine in Obstructive Sleep Apnea Syndrome Patients with Allergic Rhinitis. Am. J. Rhinol. Allergy 2013, 27, e113–e116. [Google Scholar] [CrossRef] [PubMed]
- Kiely, J.L.; Nolan, P.; McNicholas, W.T. Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with co-existing rhinitis. Thorax 2004, 59, 50–55. [Google Scholar] [PubMed]
- Smith, D.F.; Sarber, K.M.; Spiceland, C.P.; Ishman, S.L.; Augelli, D.M.; Romaker, A.M. Effects of Medical Therapy on Mild Obstructive Sleep Apnea in Adult Patients. J. Clin. Sleep Med. 2019, 15, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Koutsourelakis, I.; Minaritzoglou, A.; Zakynthinos, G.; Vagiakis, E.; Zakynthinos, S. The effect of nasal tramazoline with dexamethasone in obstructive sleep apnoea patients. Eur. Respir. J. 2013, 42, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Meurice, J.C.; Marc, I.; Carrier, G.; Series, F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am. J. Respir. Crit. Care Med. 1996, 153, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Clarenbach, C.F.; Kohler, M.; Senn, O.; Thurnheer, R.; Bloch, K.E. Does nasal decongestion improve obstructive sleep apnea? J. Sleep Res. 2008, 17, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, T. Effect of Dupilumab on Sleep Apnea in Patients with Rhinosinusitis. NCT03675022; 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03675022?term=dupilumab&draw=2&rank=1 (accessed on 1 November 2019).
- Horner, R.L.; Hughes, S.W.; Malhotra, A. State-dependent and reflex drives to the upper airway: Basic physiology with clinical implications. J. Appl. Physiol. 2014, 116, 325–336. [Google Scholar] [CrossRef]
- Nicholas, C.L.; Jordan, A.S.; Heckel, L.; Worsnop, C.; Bei, B.; Saboisky, J.P.; Eckert, D.J.; White, D.P.; Malhotra, A.; Trinder, J. Discharge Patterns of Human Tensor Palatini Motor Units during Sleep Onset. Sleep 2012, 35, 699–707. [Google Scholar] [CrossRef]
- Wilkinson, V.; Malhotra, A.; Nicholas, C.L.; Worsnop, C.; Jordan, A.S.; Butler, J.E.; Saboisky, J.P.; Gandevia, S.C.; White, D.P.; Trinder, J. Discharge Patterns of Human Genioglossus Motor Units during Sleep Onset. Sleep 2008, 31, 525–533. [Google Scholar] [CrossRef]
- Basner, R.C.; Ringler, J.; Schwartzstein, R.M.; Weinberger, S.E.; Weiss, J.W. Phasic electromyographic activity of the genioglossus increases in normals during slow-wave sleep. Respir. Physiol. 1991, 83, 189–200. [Google Scholar] [CrossRef]
- Sands, S.A.; Eckert, D.J.; Jordan, A.S.; Edwards, B.A.; Owens, R.L.; Butler, J.P.; Schwab, R.J.; Loring, S.H.; Malhotra, A.; White, D.P.; et al. Enhanced Upper-Airway Muscle Responsiveness Is a Distinct Feature of Overweight/Obese Individuals without Sleep Apnea. Am. J. Respir. Crit. Care Med. 2014, 190, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Taranto-Montemurro, L.; Sands, S.A.; Edwards, B.A.; Azarbarzin, A.; Marques, M.; De Melo, C.; Eckert, D.J.; White, D.P.; Wellman, A. Desipramine improves upper airway collapsibility and reduces OSA severity in patients with minimal muscle compensation. Eur. Respir. J. 2016, 48, 1340–1350. [Google Scholar] [CrossRef]
- Kubin, L. Neural Control of the Upper Airway: Respiratory and State-Dependent Mechanisms. Compr. Physiol. 2016, 6, 1801–1850. [Google Scholar] [PubMed]
- Veasey, S.C. Serotonin agonists and antagonists in obstructive sleep apnea: Therapeutic potential. Am. J. Respir. Med. 2003, 2, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Shaheen, F.; Mackiewicz, M.; Fenik, P.; Veasey, S.C. Single cell laser dissection with molecular beacon polymerase chain reaction identifies 2A as the predominant serotonin receptor subtype in hypoglossal motoneurons. Neuroscience 2002, 113, 145–154. [Google Scholar] [CrossRef]
- Okabe, S.; Mackiewicz, M.; Kubin, L. Serotonin receptor mRNA expression in the hypoglossal motor nucleus. Respir. Physiol. 1997, 110, 151–160. [Google Scholar] [CrossRef]
- Veasey, S.C.; Chachkes, J.; Fenik, P.; Hendricks, J.C. The effects of ondansetron on sleep-disordered breathing in the English bulldog. Sleep 2001, 24, 155–160. [Google Scholar] [CrossRef][Green Version]
- Stradling, J.; Smith, D.; Radulovacki, M.; Carley, D. Effect of ondansetron on moderate obstructive sleep apnoea, a single night, placebo-controlled trial. J. Sleep Res. 2003, 12, 169–170. [Google Scholar] [CrossRef]
- Mendelson, W.B.; Maczaj, M.; Holt, J. Buspirone Administration to Sleep Apnea Patients. J. Clin. Psychopharmacol. 1991, 11, 71. [Google Scholar] [CrossRef]
- Carley, D.W.; Olopade, C.; Ruigt, G.S.; Radulovacki, M. Efficacy of mirtazapine in obstructive sleep apnea syndrome. Sleep 2007, 30, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.S.; Yee, B.J.; Desai, A.V.; Buchanan, P.R.; Wong, K.K.H.; Crompton, R.; Melehan, K.L.; Zack, N.; Rao, S.G.; Gendreau, R.M.; et al. Two Randomized Placebo-Controlled Trials to Evaluate the Efficacy and Tolerability of Mirtazapine for the Treatment of Obstructive Sleep Apnea. Sleep 2008, 31, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Yamaura, E.M.; Gill, K.; Reist, C. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep 1999, 22, 1087–1092. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hanzel, D.A.; Proia, N.G.; Hudgel, D.W. Response of Obstructive Sleep Apnea to Fluoxetine and Protriptyline. Chest 1991, 100, 416–421. [Google Scholar] [CrossRef]
- Prasad, B.; Radulovacki, M.; Olopade, C.; Herdegen, J.J.; Logan, T.; Carley, D.W. Prospective Trial of Efficacy and Safety of Ondansetron and Fluoxetine in Patients with Obstructive Sleep Apnea Syndrome. Sleep 2010, 33, 982–989. [Google Scholar] [CrossRef][Green Version]
- Schmidt, H.S. L-tryptophan in the treatment of impaired respiration in sleep. Bull. Eur. Physiopathol. Respir. 1983, 19, 625–629. [Google Scholar]
- Veasey, S.C.; Fenik, P.; Panckeri, K.; Pack, A.I.; Hendricks, J.C. The effects of trazodone with L-tryptophan on sleep-disordered breathing in the English bulldog. Am. J. Respir. Crit. Care Med. 1999, 160, 1659–1667. [Google Scholar] [CrossRef]
- Strumpf, I.J.; Drucker, R.D.; Anders, K.H.; Cohen, S.; Fajolu, O. Acute Eosinophilic Pulmonary Disease Associated with the Ingestion of L-Tryptophan-Containing Products. Chest 1991, 99, 8–13. [Google Scholar] [CrossRef][Green Version]
- Sood, S.; Morrison, J.L.; Liu, H.; Horner, R.L. Role of Endogenous Serotonin in Modulating Genioglossus Muscle Activity in Awake and Sleeping Rats. Am. J. Respir. Crit. Care Med. 2005, 172, 1338–1347. [Google Scholar] [CrossRef]
- Sood, S.; Raddatz, E.; Liu, X.; Liu, H.; Horner, R.L. Inhibition of serotonergic medullary raphe obscurus neurons suppresses genioglossus and diaphragm activities in anesthetized but not conscious rats. J. Appl. Physiol. 2006, 100, 1807–1821. [Google Scholar] [CrossRef]
- Fenik, V.B.; Davies, R.O.; Kubin, L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am. J. Respir. Crit. Care Med. 2005, 172, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Kraiczi, H.; Hedner, J.; Dahlöf, P.; Ejnell, H.; Carlson, J. Effect of serotonin uptake inhibition on breathing during sleep and daytime symptoms in obstructive sleep apnea. Sleep 1999, 22, 61–67. [Google Scholar] [PubMed]
- Chan, E.; Steenland, H.W.; Liu, H.; Horner, R.L. Endogenous Excitatory Drive Modulating Respiratory Muscle Activity across Sleep–Wake States. Am. J. Respir. Crit. Care Med. 2006, 174, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Taranto-Montemurro, L.; Edwards, B.A.; Sands, S.A.; Marques, M.; Eckert, D.J.; White, D.P.; Wellman, A. Desipramine Increases Genioglossus Activity and Reduces Upper Airway Collapsibility during Non-REM Sleep in Healthy Subjects. Am. J. Respir. Crit. Care Med. 2016, 194, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Brownell, L.G.; West, P.; Sweatman, P.; Acres, J.C.; Kryger, M.H. Protriptyline in obstructive sleep apnea: A double-blind trial. N. Engl. J. Med. 1982, 307, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.L.; Haponik, E.F.; Allen, R.P.; Bleecker, E.R. The Effects of Protriptyline in Sleep-Disordered Breathing 1,2. Am. Rev. Respir. Dis. 1983, 127, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Bart Sangal, R.; Sangal, J.M.; Thorp, K. Atomoxetine improves sleepiness and global severity of illness but not the respiratory disturbance index in mild to moderate obstructive sleep apnea with sleepiness. Sleep Med. 2008, 9, 506–510. [Google Scholar] [CrossRef]
- Gould, G.A.; Airlie, G.A.; Shapiro, C.M.; Whyte, K.F.; Douglas, N.J. Role of Protriptyline and Acetazolamide in the Sleep Apnea/Hypopnea Syndrome. Sleep 1988, 11, 463–472. [Google Scholar]
- Conway, W.A.; Zorick, F.; Piccione, P.; Roth, T. Protriptyline in the treatment of sleep apnoea. Thorax 1982, 37, 49–53. [Google Scholar] [CrossRef]
- Clark, R.W.; Schmidt, H.S.; Schaal, S.F.; Boudoulas, H.; Schuller, D.E. Sleep apnea: Treatment with protriptyline. Neurology 1979, 29, 1287. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Messineo, L.; Sands, S.A.; Azarbarzin, A.; Marques, M.; Edwards, B.A.; Eckert, D.J.; White, D.P.; Wellman, A. The Combination of Atomoxetine and Oxybutynin Greatly Reduces Obstructive Sleep Apnea Severity. A Randomized, Placebo-controlled, Double-Blind Crossover Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Carberry, J.C.; Wellman, A.; Grunstein, R.; Eckert, D.J. Reboxetine and hyoscine butylbromide improve upper airway function during nonrapid eye movement and suppress rapid eye movement sleep in healthy individuals. Sleep 2019, 42, zsy261. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Messineo, L.; Grunstein, R.; Carberry, J.; Eckert, D.J. Reboxetine and Hyoscine Butylbromide Reduce Obstructive Sleep Apnoea Severity. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/jsr.66_12912 (accessed on 1 November 2019).
- Grace, K.P.; Hughes, S.W.; Horner, R.L. Identification of the Mechanism Mediating Genioglossus Muscle Suppression in REM Sleep. Am. J. Respir. Crit. Care Med. 2013, 187, 311–319. [Google Scholar] [CrossRef] [PubMed]
- White, R.P.; Daigneault, E.A. The antagonisms of atropine to the EEG effects of adrenergic drugs. J. Pharmacol. Exp. Ther. 1959, 125, 339–346. [Google Scholar] [PubMed]
- Grace, K.P.; Hughes, S.W.; Shahabi, S.; Horner, R.L. K+ Channel modulation causes genioglossus inhibition in REM sleep and is a strategy for reactivation. Respir. Physiol. Neurobiol. 2013, 188, 277–288. [Google Scholar] [CrossRef]
- Suratt, P.M.; Wilhoit, S.C.; Brown, E.D.; Findley, L.J. Effect of doxapram on obstructive sleep apnea. Bull. Eur. Physiopathol. Respir. 1986, 22, 127–131. [Google Scholar]
- Taranto-Montemurro, L.; Sands, S.A.; Azarbarzin, A.; Marques, M.; De Melo, C.M.; Edwards, B.A.; Eckert, D.J.; Messineo, L.; White, D.P.; Wellman, A. Effect of 4-Aminopyridine on Genioglossus Muscle Activity during Sleep in Healthy Adults. Ann. Am. Thorac. Soc. 2017, 14, 1177–1183. [Google Scholar] [CrossRef]
- Obstructive Sleep Apnea (OSA) Treated with a Potassium Channel Inhibitor (SANDMAN). 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03603678?term=sandman&draw=2&rank=2 (accessed on 1 November 2019).
- Grace, K.P.; Hughes, S.W.; Horner, R.L. Identification of a Pharmacological Target for Genioglossus Reactivation throughout Sleep. Sleep 2014, 37, 41–50. [Google Scholar] [CrossRef]
- Guo, J.; Ikeda, S.R. Endocannabinoids Modulate N-Type Calcium Channels and G-Protein-Coupled Inwardly Rectifying Potassium Channels via CB1 Cannabinoid Receptors Heterologously Expressed in Mammalian Neurons. Mol. Pharm. 2004, 65, 665–674. [Google Scholar] [CrossRef]
- Prasad, B.; Radulovacki, M.G.M.; Carley, D.W. Proof of Concept Trial of Dronabinol in Obstructive Sleep Apnea. Front. Psychol. 2013, 4, 1. [Google Scholar] [CrossRef]
- Carley, D.W.; Prasad, B.; Reid, K.J.; Malkani, R.; Attarian, H.; Abbott, S.M.; Vern, B.; Xie, H.; Yuan, C.; Zee, P.C. Pharmacotherapy of Apnea by Cannabimimetic Enhancement, the PACE Clinical Trial: Effects of Dronabinol in Obstructive Sleep Apnea. Sleep 2018, 41. [Google Scholar] [CrossRef] [PubMed]
- Haxhiu, M.A.; Van Lunteren, E.; Van De Graaff, W.B.; Strohl, K.P.; Bruce, E.N.; Mitra, J.; Cherniack, N.S. Action of nicotine on the respiratory activity of the diaphragm and genioglossus muscles and the nerves that innervate them. Respir. Physiol. 1984, 57, 153–169. [Google Scholar] [CrossRef]
- Gothe, B.; Strohl, K.P.; Levin, S.; Cherniack, N.S. Nicotine: A Different Approach to Treatment of Obstructive Sleep Apnea. Chest 1985, 87, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Davila, D.G.; Hurt, R.D.; Offord, K.P.; Harris, C.D.; Shepard, J.W. Acute effects of transdermal nicotine on sleep architecture, snoring, and sleep-disordered breathing in nonsmokers. Am. J. Respir. Crit. Care Med. 1994, 150, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Zevin, S.; Swed, E.; Cahan, C. Clinical effects of locally delivered nicotine in obstructive sleep apnea syndrome. Am. J. Ther. 2003, 10, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Marshall, N.S.; Duffin, J.; Yee, B.J.; Wong, K.K.H.; Noori, N.; Ng, S.S.W.; Grunstein, R.R. Phenotyping interindividual variability in obstructive sleep apnoea response to temazepam using ventilatory chemoreflexes during wakefulness. J. Sleep Res. 2011, 20, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Höijer, U.; Hedner, J.; Ejnell, H.; Grunstein, R.; Odelberg, E.; Elam, M. Nitrazepam in patients with sleep apnoea: A double-blind placebo-controlled study. Eur. Respir. J. 1994, 7, 2011–2015. [Google Scholar]
- Park, E.; Younes, M.; Liu, H.; Horner, R.L. Systemic vs. Central Administration of Common Hypnotics Reveals Opposing Effects on Genioglossus Muscle Activity in Rats. Sleep 2008, 31, 355–365. [Google Scholar] [CrossRef]
- Younes, M.; Park, E.; Horner, R.L. Pentobarbital sedation increases genioglossus respiratory activity in sleeping rats. Sleep 2007, 30, 478–488. [Google Scholar] [CrossRef]
- Eikermann, M.; Eckert, D.J.; Chamberlin, N.L.; Jordan, A.S.; Zaremba, S.; Smith, S.; Rosow, C.; Malhotra, A. Effects of pentobarbital on upper airway patency during sleep. Eur. Respir. J. 2010, 36, 569–576. [Google Scholar] [CrossRef]
- Jordan, A.S.; Wellman, A.; Eckert, D.J.; Yim-Yeh, S.; Smith, S.A.; Stevenson, K.E.; Malhotra, A.; White, D.P.; Lo, Y.-L.; Eikermann, M. Airway Dilator Muscle Activity and Lung Volume During Stable Breathing in Obstructive Sleep Apnea. Sleep 2009, 32, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Loewen, A.H.; Ostrowski, M.; Laprairie, J.; Maturino, F.; Hanly, P.J. Genioglossus activity available via non-arousal mechanisms vs. that required for opening the airway in obstructive apnea patients. J. Appl. Physiol. 2012, 112, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; Younes, M.K. Arousal from sleep: Implications for obstructive sleep apnea pathogenesis and treatment. J. Appl. Physiol. 2014, 116, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Ostrowski, M.; Atkar, R.; Laprairie, J.; Siemens, A.; Hanly, P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J. Appl. Physiol. 2007, 103, 1929–1941. [Google Scholar] [CrossRef] [PubMed]
- Longobardo, G.; Gothe, B.; Goldman, M.; Cherniack, N. Sleep apnea considered as a control system instability. Respir. Physiol. 1982, 50, 311–333. [Google Scholar] [CrossRef]
- Edwards, B.A.; Connolly, J.G.; Campana, L.M.; Sands, S.A.; Trinder, J.A.; White, D.P.; Wellman, A.; Malhotra, A. Acetazolamide Attenuates the Ventilatory Response to Arousal in Patients with Obstructive Sleep Apnea. Sleep 2013, 36, 281–285. [Google Scholar] [CrossRef]
- Khoo, M.C.; Kronauer, R.E.; Strohl, K.P.; Slutsky, A.S. Factors inducing periodic breathing in humans: A general model. J. Appl. Physiol. 1982, 53, 644–659. [Google Scholar] [CrossRef]
- Ratnavadivel, R.; Stadler, D.; Windler, S.; Bradley, J.; Paul, D.; McEvoy, R.D.; Catcheside, P.G. Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnoea. Thorax 2010, 65, 107–112. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Sands, S.A.; Grace, K.P.; Azarbarzin, A.; Messineo, L.; Salant, R.; White, D.P.; Wellman, D.A. Neural memory of the genioglossus muscle during sleep is stage-dependent in healthy subjects and obstructive sleep apnoea patients. J. Physiol. 2018, 596, 5163–5173. [Google Scholar] [CrossRef]
- Ratnavadivel, R.; Chau, N.; Stadler, D.; Yeo, A.; McEvoy, R.D.; Catcheside, P.G. Marked Reduction in Obstructive Sleep Apnea Severity in Slow Wave Sleep. J. Clin. Sleep Med. 2009, 5, 519–524. [Google Scholar]
- Taranto-Montemurro, L.; Sands, S.A.; Edwards, B.A.; Azarbarzin, A.; Marques, M.; De Melo, C.; Eckert, D.J.; White, D.P.; Wellman, A. Effects of Tiagabine on Slow Wave Sleep and Arousal Threshold in Patients with Obstructive Sleep Apnea. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed]
- Cirignotta, F.; Mondini, S.; Zucconi, M.; Gerardi, R.; Farolfi, A.; Lugaresi, E. Zolpidem-polysomnographic study of the effect of a new hypnotic drug in sleep apnea syndrome. Pharm. Biochem. Behav. 1988, 29, 807–809. [Google Scholar] [CrossRef]
- Cirignotta, F.; Mondini, S.; Gerardi, R.; Zucconi, M. Effect of brotizolam on sleep-disordered breathing in heavy snorers with obstructive apnea. Curr. Ther. Res. 1992, 51, 360–366. [Google Scholar]
- Berry, R.B.; Kouchi, K.; Bower, J.; Prosise, G.; Light, R.W. Triazolam in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1995, 151, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.E.; Morin, C.M. The Effect of Temazepam on Respiration in Elderly Insomniacs with Mild Sleep Apnea. Sleep 1995, 18, 644–645. [Google Scholar] [CrossRef] [PubMed]
- Carberry, J.C.; Fisher, L.P.; Grunstein, R.R.; Gandevia, S.C.; McKenzie, D.K.; Butler, J.E.; Eckert, D.J. Role of common hypnotics on the phenotypic causes of obstructive sleep apnoea: Paradoxical effects of zolpidem. Eur. Respir. J. 2017, 50, 1701344. [Google Scholar] [CrossRef]
- George, C.F.; Feldman, N.; Inhaber, N.; Steininger, T.L.; Grzeschik, S.M.; Lai, C.; Zheng, Y. A safety trial of sodium oxybate in patients with obstructive sleep apnea: Acute effects on sleep-disordered breathing. Sleep Med. 2010, 11, 38–42. [Google Scholar] [CrossRef]
- Carberry, J.C.; Grunstein, R.R.; Eckert, D.J. The effects of zolpidem in obstructive sleep apnea—An open-label pilot study. J. Sleep Res. 2019, e12853. [Google Scholar] [CrossRef]
- Rosenberg, R.; Roach, J.M.; Scharf, M.; Amato, D.A. A pilot study evaluating acute use of eszopiclone in patients with mild to moderate obstructive sleep apnea syndrome. Sleep Med. 2007, 8, 464–470. [Google Scholar] [CrossRef]
- Eckert, D.J.; Owens, R.L.; Kehlmann, G.B.; Wellman, A.; Rahangdale, S.; Yim-Yeh, S.; White, D.P.; Malhotra, A. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin. Sci. 2011, 120, 505–514. [Google Scholar] [CrossRef]
- Carter, S.G.; Berger, M.S.; Carberry, J.C.; Bilston, L.E.; Butler, J.E.; Tong, B.K.; Martins, R.T.; Fisher, L.P.; McKenzie, D.K.; Grunstein, R.R.; et al. Zopiclone Increases the Arousal Threshold without Impairing Genioglossus Activity in Obstructive Sleep Apnea. Sleep 2016, 39, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.G.; Carberry, J.C.; Cho, G.; Fisher, L.P.; Rollo, C.M.; Stevens, D.J.; D’Rozario, A.L.; McKenzie, D.K.; Grunstein, R.R.; Eckert, D.J. Effect of 1 month of zopiclone on obstructive sleep apnoea severity and symptoms: A randomised controlled trial. Eur. Respir. J. 2018, 52, 1800149. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Carberry, J.; Grunstein, R.; Eckert, D. High dose zopiclone does not change osa severity, the respiratory arousal threshold, genioglossus muscle responsiveness or next-day sleepiness and alertness in selected people with OSA. In Proceedings of the World Sleep 2019, Vancouver, BC, Canada, 20–25 September 2019. [Google Scholar]
- Kryger, M.; Wang-Weigand, S.; Roth, T. Safety of ramelteon in individuals with mild to moderate obstructive sleep apnea. Sleep Breath. 2007, 11, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Deacon, N.L.; Smales, E.; Jen, R.; Jaiswal, S.; Malhotra, A. Effects of Melatonin Supplementation in Obstructive Sleep Apnea Patients. In Proceedings of the American Thoracic Society 2016 International Conference, San Francisco, CA, USA, 13–18 May 2016. [Google Scholar]
- Piovezan, R.D.; Kase, C.; Moizinho, R.; Tufik, S.; Poyares, D. Gabapentin acutely increases the apnea-hypopnea index in older men: Data from a randomized, double-blind, placebo-controlled study. J. Sleep Res. 2017, 26, 166–170. [Google Scholar] [CrossRef]
- George, C.F.; Feldman, N.; Zheng, Y.; Steininger, T.L.; Grzeschik, S.M.; Lai, C.; Inhaber, N. A 2-week, polysomnographic, safety study of sodium oxybate in obstructive sleep apnea syndrome. Sleep Breath 2011, 15, 13–20. [Google Scholar] [CrossRef]
- Schutte-Rodin, S.; Broch, L.; Buysse, D.; Dorsey, C.; Sateia, M. Clinical Guideline for the Evaluation and Management of Chronic Insomnia in Adults. J. Clin. Sleep Med. 2008, 4, 487–504. [Google Scholar]
- Heinzer, R.C.; White, D.P.; Jordan, A.S.; Lo, Y.L.; Dover, L.; Stevenson, K.; Malhotra, A. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur. Respir. J. 2008, 31, 1308–1312. [Google Scholar] [CrossRef]
- Eckert, D.J.; Malhotra, A.; Wellman, A.; White, D.P. Trazodone increases the arousal threshold in obstructive sleep apnea patients with a low arousal threshold. Sleep 2014, 37, 811–819. [Google Scholar] [CrossRef]
- Smales, E.T.; Edwards, B.A.; Deyoung, P.N.; McSharry, D.G.; Wellman, A.; Velasquez, A.; Owens, R.; Orr, J.E.; Malhotra, A. Trazodone Effects on Obstructive Sleep Apnea and Non-REM Arousal Threshold. Ann. Am. Thorac. Soc. 2015, 12, 758–764. [Google Scholar] [CrossRef]
- Messineo, L.; Magri, R.; Corda, L.; Pini, L.; Taranto-Montemurro, L.; Tantucci, C. Phenotyping-based treatment improves obstructive sleep apnea symptoms and severity: A pilot study. Sleep Breath. 2017, 21, 861–868. [Google Scholar] [CrossRef]
- Messineo, L.; Taranto-Montemurro, L.; Azarbarzin, A.; Marques, M.D.O.; Calianese, N.; White, D.P.; Wellman, A.; Sands, S.A. Breath-holding as a means to estimate the loop gain contribution to obstructive sleep apnoea. J. Physiol. 2018, 596, 4043–4056. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.A.; Sands, S.A.; Eckert, D.J.; White, D.P.; Butler, J.P.; Owens, R.L.; Malhotra, A.; Wellman, A. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J. Physiol. 2012, 590, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Wellman, A.; Malhotra, A.; Jordan, A.S.; Stevenson, K.E.; Gautam, S.; White, D.P. Effect of oxygen in obstructive sleep apnea: Role of loop gain. Respir. Physiol. Neurobiol. 2008, 162, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Tojima, H.; Kunitomo, F.; Kimura, H.; Tatsumi, K.; Kuriyama, T.; Honda, Y. Effects of acetazolamide in patients with the sleep apnoea syndrome. Thorax 1988, 43, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, D.; Zou, D.; Grote, L.; Hoff, E.; Hedner, J. Acetazolamide Reduces Blood Pressure and Sleep-Disordered Breathing in Patients with Hypertension and Obstructive Sleep Apnea: A Randomized Controlled Trial. J. Clin. Sleep Med. 2018, 14, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Kearley, R.; Wynne, J.W.; Block, A.; Boysen, P.G.; Lindsey, S.; Martin, C. The Effect of Low Flow Oxygen on Sleep-Disordered Breathing and Oxygen Desaturation. Chest 1980, 78, 682–685. [Google Scholar] [CrossRef]
- Smith, P.L.; Haponik, E.F.; Bleecker, E.R. The effects of oxygen in patients with sleep apnea. Am. Rev. Respir. Dis. 1984, 130, 958–963. [Google Scholar]
- Pokorski, M.; Jernajczyk, U. Nocturnal oxygen enrichment in sleep apnoea. J. Int. Med. Res. 2000, 28, 1–8. [Google Scholar] [CrossRef]
- Friedman, M.; Landsberg, R.; Ascher-Landsberg, J. Treatment of hypoxemia in obstructive sleep apnea. Am. J. Rhinol. 2001, 15, 311–313. [Google Scholar] [CrossRef]
- Phillips, B.A.; Schmitt, F.A.; Berry, D.T.; Lamb, D.G.; Amin, M.; Cook, Y.R. Treatment of obstructive sleep apnea. A preliminary report comparing nasal CPAP to nasal oxygen in patients with mild OSA. Chest 1990, 98, 325–330. [Google Scholar] [CrossRef]
- Loredo, J.S.; Ancoli-Israel, S.; Kim, E.-J.; Lim, W.J.; Dimsdale, J.E. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: A placebo-CPAP-controlled study. Sleep 2006, 29, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Norman, D.; Loredo, J.S.; Nelesen, R.A.; Ancoli-Israel, S.; Mills, P.J.; Ziegler, M.G.; Dimsdale, J.E. Effects of continuous positive airway pressure versus supplemental oxygen on 24-h ambulatory blood pressure. Hypertension 2006, 47, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.J.; Kennedy, B.P.; Loredo, J.S.; Dimsdale, J.E.; Ziegler, M.G. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J. Appl. Physiol. 2006, 100, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Bardwell, W.A.; Norman, D.; Ancoli-Israel, S.; Loredo, J.S.; Lowery, A.; Lim, W.; Dimsdale, J.E. Effects of 2-Week Nocturnal Oxygen Supplementation and Continuous Positive Airway Pressure Treatment on Psychological Symptoms in Patients with Obstructive Sleep Apnea: A Randomized Placebo-Controlled Study. Behav. Sleep Med. 2007, 5, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Block, A.J.; Hellard, D.; Cicale, M.J. Snoring, Nocturnal Hypoxemia, and the Effect of Oxygen Inhalation. Chest 1987, 92, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Duffin, J.; Mohan, R.M.; Vasiliou, P.; Stephenson, R.; Mahamed, S. A model of the chemoreflex control of breathing in humans: Model parameters measurement. Respir. Physiol. 2000, 120, 13–26. [Google Scholar] [CrossRef]
- Sands, S.A.; Edwards, B.A.; Terrill, P.I.; Butler, J.P.; Owens, R.L.; Taranto-Montemurro, L.; Azarbarzin, A.; Marques, M.; Hess, L.B.; Smales, E.T.; et al. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur. Respir. J. 2018, 52, 1800674. [Google Scholar] [CrossRef]
- Edwards, B.A.; Sands, S.A.; Owens, R.L.; Eckert, D.J.; Landry, S.; White, D.P.; Malhotra, A.; Wellman, A. The Combination of Supplemental Oxygen and a Hypnotic Markedly Improves Obstructive Sleep Apnea in Patients with a Mild to Moderate Upper Airway Collapsibility. Sleep 2016, 39, 1973–1983. [Google Scholar] [CrossRef]
- Berssenbrugge, A.; Dempsey, J.; Iber, C.; Skatrud, J.; Wilson, P. Mechanisms of hypoxia-induced periodic breathing during sleep in humans. J. Physiol. 1983, 343, 507–526. [Google Scholar] [CrossRef]
- Lorenzi-Filho, G.; Rankin, F.; Bies, I.; Bradley, T.D. Effects of Inhaled Carbon Dioxide and Oxygen on Cheyne-Stokes Respiration in Patients with Heart Failure. Am. J. Respir. Crit. Care Med. 1999, 159, 1490–1498. [Google Scholar] [CrossRef]
- Xie, A.; Rankin, F.; Rutherford, R.; Bradley, T.D. Effects of inhaled CO2 and added dead space on idiopathic central sleep apnea. J. Appl. Physiol. 1997, 82, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Smith, C.A.; Przybylowski, T.; Chenuel, B.; Xie, A.; Nakayama, H.; Skatrud, J.B. The ventilatory responsiveness to CO2 below eupnoea as a determinant of ventilatory stability in sleep. J. Physiol. 2004, 560, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Teodorescu, M.; Pegelow, D.F.; Teodorescu, M.C.; Gong, Y.; Fedie, J.E.; Dempsey, J.A. Effects of stabilizing or increasing respiratory motor outputs on obstructive sleep apnea. J. Appl. Physiol. 2013, 115, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Messineo, L.; Taranto-Montemurro, L.; Azarbarzin, A.; Marques, M.; Calianese, N.; White, D.P.; Wellman, A.; Sands, S.A. Loop gain in REM versus non-REM sleep using CPAP manipulation: A pilot study. Respirology 2019, 24, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Lagercrantz, H.; Yamamoto, Y.; Fredholm, B.B.; Prabhakar, N.R.; von Euler, C. Adenosine analogues depress ventilation in rabbit neonates. Theophylline stimulation of respiration via adenosine receptors? Pediatr. Res. 1984, 18, 387–390. [Google Scholar] [CrossRef]
- Kassim, Z.; Greenough, A.; Rafferty, G.F. Effect of caffeine on respiratory muscle strength and lung function in prematurely born, ventilated infants. Eur. J. Nucl. Med. Mol. Imaging 2009, 168, 1491–1495. [Google Scholar] [CrossRef]
- Javaheri, S.; Parker, T.; Wexler, L.; Liming, J.; Lindower, P.; Roselle, G. Effect of Theophylline on Sleep-Disordered Breathing in Heart Failure. N. Engl. J. Med. 1996, 335, 562–567. [Google Scholar] [CrossRef]
- Mulloy, E.; McNicholas, W.T. Theophylline in Obstructive Sleep Apnea. Chest 1992, 101, 753–757. [Google Scholar] [CrossRef][Green Version]
- Hein, H.; Behnke, G.; A Jörres, R.; Magnussen, H. The therapeutic effect of theophylline in mild obstructive sleep Apnea/Hypopnea syndrome: Results of repeated measurements with portable recording devices at home. Eur. J. Med. Res. 2000, 5, 391–399. [Google Scholar]
- Espinoza, H.; Antic, R.; Thornton, A.T.; McEvoy, R.D. The Effects of Aminophylline on Sleep and Sleep-Disordered Breathing in Patients with Obstructive Sleep Apnea Syndrome. Am. Rev. Respir. Dis. 1987, 136, 80–84. [Google Scholar] [CrossRef]
- Guilleminault, C.; Hayes, B. Naloxone, theophylline, bromocriptine, and obstructive sleep apnea. Negative results. Bull. Eur. Physiopathol. Respir. 1983, 19, 632–634. [Google Scholar] [PubMed]
- Atkinson, R.L.; Suratt, P.M.; Wilhoit, S.C.; Recant, L. Naloxone improves sleep apnea in obese humans. Int. J. Obes. 1985, 9, 233–239. [Google Scholar] [PubMed]
- Diamond, E.; Druz, W.; D’Sousa, V.; Sharp, J.T. Effect of naloxone on obstructive sleep apnoea. Am. Rev. Respir. Dis. 1982, 125 (Suppl. 4), 235. [Google Scholar]
- Ferber, C.; Duclaux, R.; Mouret, J. Naltrexone improves blood gas patterns in obstructive sleep apnoea syndrome through its influence on sleep. J. Sleep Res. 1993, 2, 149–155. [Google Scholar] [CrossRef]
- Moraes, W.; Poyares, D.; Sukys-Claudino, L.; Guilleminault, C.; Tufik, S. Donepezil improves obstructive sleep apnea in Alzheimer disease: A double-blind, placebo-controlled study. Chest 2008, 133, 677–683. [Google Scholar] [CrossRef]
- Sukys-Claudino, L.; Moraes, W.; Guilleminault, C.; Tufik, S.; Poyares, D. Beneficial effect of donepezil on obstructive sleep apnea: A double-blind, placebo-controlled clinical trial. Sleep Med. 2012, 13, 290–296. [Google Scholar] [CrossRef]
- Hunchaisri, N.; Chalermsuwiwattanakan, W. Efficacy of Donepezil in the Treatment of Obstructive Sleep Apnea: A Placebo-Controlled Trial. J. Med. Assoc. Thai 2016, 99 (Suppl. 8), S31–S35. [Google Scholar]
- Li, Y.; Owens, R.L.; Sands, S.; Orr, J.; Moraes, W.; Deyoung, P.; Smales, E.; Jen, R.; Malhotra, A. The Effect of Donepezil on Arousal Threshold and Apnea–Hypopnea Index. A Randomized, Double-Blind, Cross-Over Study. Ann. Am. Thorac. Soc. 2016, 13, 2012–2018. [Google Scholar] [CrossRef]
- Hedner, J.; Kraiczi, H.; Peker, Y.; Murphy, P. Reduction of Sleep-disordered Breathing after Physostigmine. Am. J. Respir. Crit. Care Med. 2003, 168, 1246–1251. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taranto-Montemurro, L.; Messineo, L.; Wellman, A. Targeting Endotypic Traits with Medications for the Pharmacological Treatment of Obstructive Sleep Apnea. A Review of the Current Literature. J. Clin. Med. 2019, 8, 1846. https://doi.org/10.3390/jcm8111846
Taranto-Montemurro L, Messineo L, Wellman A. Targeting Endotypic Traits with Medications for the Pharmacological Treatment of Obstructive Sleep Apnea. A Review of the Current Literature. Journal of Clinical Medicine. 2019; 8(11):1846. https://doi.org/10.3390/jcm8111846
Chicago/Turabian StyleTaranto-Montemurro, Luigi, Ludovico Messineo, and Andrew Wellman. 2019. "Targeting Endotypic Traits with Medications for the Pharmacological Treatment of Obstructive Sleep Apnea. A Review of the Current Literature" Journal of Clinical Medicine 8, no. 11: 1846. https://doi.org/10.3390/jcm8111846
APA StyleTaranto-Montemurro, L., Messineo, L., & Wellman, A. (2019). Targeting Endotypic Traits with Medications for the Pharmacological Treatment of Obstructive Sleep Apnea. A Review of the Current Literature. Journal of Clinical Medicine, 8(11), 1846. https://doi.org/10.3390/jcm8111846



