Effectiveness of Chronic Wound Debridement with the Use of Larvae of Lucilia Sericata
Abstract
1. Introduction
2. The Mechanism of Action
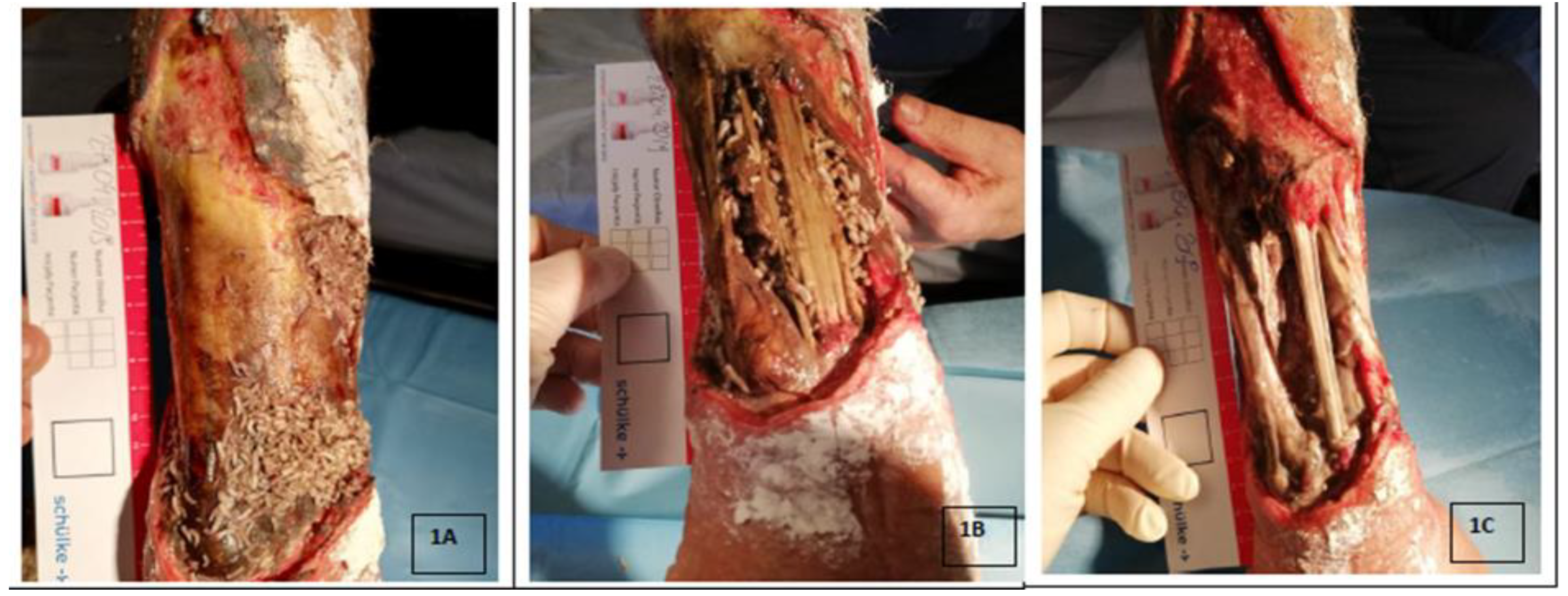
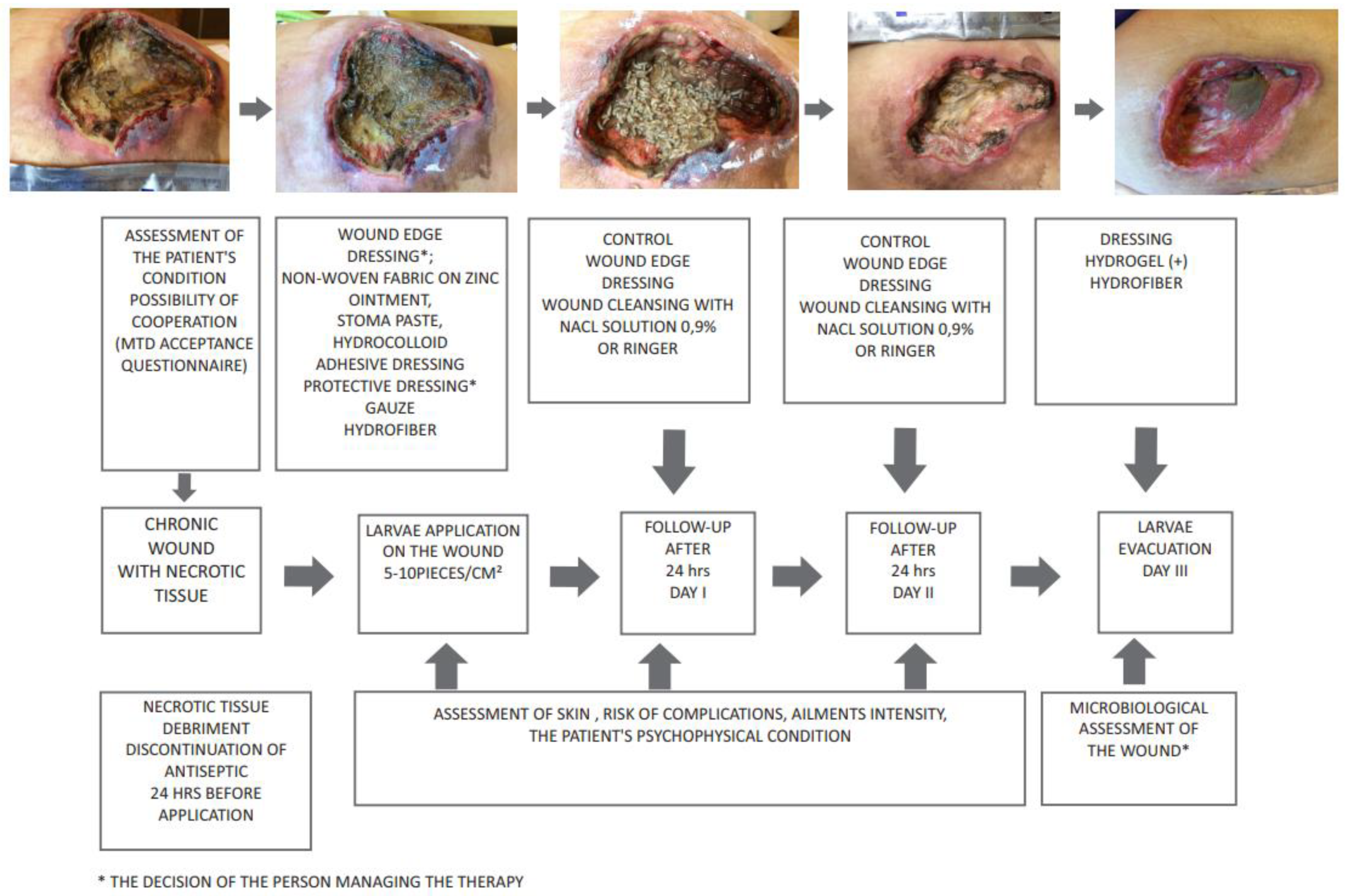
3. Deposition of Larvae on the Wound
4. Use of Lucilia Sericata Larvae in Wound Debridement
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DFU | Diabetic Foot Ulcers |
| ES | Excretions/Secretions |
| EWMA | European Wound Management Association |
| MAMP | Alpha-methoxyphenol |
| MIC | Minimum Inhibitory Concentration |
| MDT | Maggot Debridement Therapy |
| MRSA | methicyllin-resistant Staphylococcus aureus |
| NRS | Numerical Rating Scale |
| NPWT | negative pressure wound therapy |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| TIME | tissue, debridement, infection, moisture, edges |
References
- Wollina, U.; Liebold, K.; Schmidt, W.; Hartmann, M.; Fassler, D. Biosurgery supports granulation and debridement in chronic wounds—Clinical data and remittance spectroscopy measurement. Int. J. Dermatol. 2002, 41, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.; Shimoda, K. Presurgical maggot debridement of soft tissue wounds is associated with decreased rates of postoperative infection. Clin. Infect. Dis. 2004, 39, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Pare, A.; Johnson, T.; Spiegel, A. The Works of That Famous Chirurgeon Ambrose Pare; Mary Clarke: London, UK, 1678. [Google Scholar]
- Pechter, E.A.; Sherman, R.A. Maggot therapy: The surgical metamorphosis. Plast. Reconstr. Surg. 1983, 72, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Baer, W.S. The treatment of chronic osteomyelitis with the maggots (larva of the blowfly). J. Bone Joint Surg. 1931, 13, 438. [Google Scholar]
- Baer, W.S. The Classic the Treatment of Chronic Osteomyelitis With the Maggot (Larva of the Blow Fly). Clin. Orthop. Relat. Res. 2011, 469, 920–944. [Google Scholar] [CrossRef] [PubMed]
- Fine, A.; Alexander, H. Maggot therapy: Technique and clinical application. J. Bone Jt. Surg. 1934, 16, 572–582. [Google Scholar]
- Sherman, R.A.; Pechter, E.A. Maggot therapy: A review of the therapeutic applications of fly larvae in human medicine, especially for treating osteomyelitis. Med. Vet. Entomol. 1988, 2, 225–230. [Google Scholar] [CrossRef]
- Robinson, W. Progress of maggot therapy in the United States and Canada in the treatment of suppurative diseases. Am. J. Surg. 1935, 29, 67–71. [Google Scholar] [CrossRef]
- Stegeman, S.A.; Steenvoorde, P. Maggot debridement therapy. Proc. Neth. Entomol. Soc. Meet. 2011, 22, 61–66. [Google Scholar]
- Whitaker, I.S.; Twine, C.; Whitaker, M.J.; Welck, M.; Brown, C.S.; Shandall, A. Larval therapy from antiquity to the present day: Mechanism of action, clinical applications and future potential. Postgrad. Med. J. 2007, 83, 409–413. [Google Scholar] [CrossRef]
- Teich, S.; Myers, R.A. Maggot therapy for severe skin infections. South. Med. J. 1986, 79, 1153–1155. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.A.; Wyle, F.; Vulpe, M. Maggot therapy for treating pressure ulcers in spinal cord injury patients. J. Spinal Cord Med. 1995, 18, 71–74. [Google Scholar] [CrossRef]
- Sherman, R.A. Maggot Therapy Takes Us Back to the Future of Wound Care: New and Improved Maggot Therapy for the 21st Century. J. Diabetes Sci. Technol. 2009, 3, 336–344. [Google Scholar] [CrossRef] [PubMed]
- FDA. 510(k) Premarket Notification. Medical Maggots. K033391. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=K033391 (accessed on 2 October 2019).
- Sherman, R.A. Mechanisms of maggot-induced wound healing: What do we know, and where do we go from here? Evid. Based. Complement. Alternat. Med. 2014, 2014, 592419. [Google Scholar] [CrossRef] [PubMed]
- Borkataki, S.; Katoch, R.; Goswami, P.; Bhat, A.; Bhardwaj, H.R.; Chakraborty, D.; Chandrawathani, P. Therapeutic use of Lucilia sericata maggot in controlling bacterial bio-burden in Rat wound model. Trop. Biomed. 2018, 35, 627–638. [Google Scholar]
- Van der Plas, M.J.A.; Baldry, M.; Van Dissel, J.T.; Jukema, G.N.; Nibbering, P.H. Maggot secretions suppress pro-inflammatory responses of human monocytes through elevation of cyclic AMP. Diabetologia 2009, 52, 1962–1970. [Google Scholar] [CrossRef]
- Van der Plas, M.J.A.; Van Dissel, J.T.; Nibbering, P.H. Maggot secretions skewmonocyte-macrophage differentiation away from a pro-inflammatory to a pro-angiogenic type. PLoS ONE 2009, 30, e8071. [Google Scholar] [CrossRef]
- Cazander, G.; Schreurs, M.W.J.; Renwarin, L.; Dorresteijn, C.; Hamann, D.; Jukema, G.N. Maggot excretions affect the human complement system. Wound Repair Regen. 2012, 20, 879–886. [Google Scholar] [CrossRef]
- Horobin, A.J.; Shakesheff, K.M.; Pritchard, D.I. Promotion of human dermal fibroblast migration, matrix remodelling and modification of fibroblast morphology within a novel 3D model by Lucilia sericata larval secretions. J. Investig. Dermatol. 2006, 126, 1410–1418. [Google Scholar] [CrossRef]
- Bazaliński, D.; Karnas, M.; Wołkowicz, M.; Kózka, M.; Więch, P. The use of Lucilia sericata larvae in the treatment of chronic wounds—A study of three cases. Leczenie Ran 2018, 15, 105–111. (In Polish) [Google Scholar] [CrossRef]
- Soares, M.O.; Iglesias, C.P.; Bland, J.M.; Cullum, N.; Dumville, J.C.; Nelson, E.A.; Torgerson, D.J.; Worthy, G. Cost effectiveness analysis of larval therapy for leg ulcers. BMJ 2009, 338, b825. [Google Scholar] [CrossRef] [PubMed]
- Wayman, J.; Nirojogi, V.; Walker, A.; Sowinski, A.; Walker, M.A. The cost effectiveness of larval therapy in venous ulcers. J. Tissue Viability 2000, 10, 91–94. [Google Scholar] [CrossRef]
- Nigam, Y.; Bexfield, A.; Thomas, S.; Ratcliffe, N. Maggot therapy: The science and implication for CAM part I—History and bacterial resistance. Evid. Based Complement. Alternat. Med. 2006, 3, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jiang, K.; Chen, J.; Wu, L.; Lu, H.; Wanga, A.; Wang, J. A systematic review of maggot debridement therapy for chronically infected wounds and ulcers. Int. J. Infect. Dis. 2014, 25, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Wolff, H.; Hansson, C. Rearing larvae of Lucilia sericata fo chronic ulcer treatment—An improved method. Acta Derm. Venereol. 2005, 85, 126–131. [Google Scholar] [CrossRef]
- Mumcuoglu, K.Y. Clinical applications for maggots in wound care. Am. J. Clin. Dermatol. 2001, 2, 219–227. [Google Scholar] [CrossRef]
- Limsopatham, K.; Khamnoi, P.; Sukontason, K.L.; Boonyawan, D.; Chaiwong, T.; Sukontason, K. Sterilization of blow fly eggs, Chrysomya megacephala and Lucilia cuprina, (Diptera: Calliphoridae) for maggot debridement therapy application. Parasitol. Res. 2017, 116, 1581–1589. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Tian, X.; Zhao, Z.; Zhang, J.; Lv, D. A new effective scaffold to facilitate peripheral nerve regeneration: Chitosan tube coated with maggot homogenate product. Med. Hypotheses 2010, 74, 12–14. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Zhang, B.; Liu, H.; Song, W.; He, J.; Lv, D.; Wang, S.; Xu, X. Activity of antibacterial protein from maggots against staphylococcus aureus in vitro and in vivo. Int. J. Mol. Med. 2013, 31, 1159–1165. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Horobin, A.J.; Shakesheff, K.M.; Pritchard, D.I. Maggots and wound healing: An investigation of the effects of secretions from Lucilia sericata larvae upon the migration of human dermal fibroblastsover a fibronectin-coated surface. Wound Repair Regen. 2005, 13, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Nigam, Y.; Morgan, C. Does maggot therapy promote wound healing? The clinical and cellular evidence. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Bexfield, A.; Bond, A.E.; Morgan, C.; Wagstaff, J.; Newton, R.P.; Ratcliffe, N.A.; Dudley, E.; Nigam, Y. Amino acid derivatives from Lucilia sericata excretions/secretions may contribute to the beneficial effects ofmaggot therapy via increased angiogenesis. Br. J. Dermatol. 2010, 162, 554–562. [Google Scholar] [CrossRef]
- Cazander, G.; Pritchard, D.I.; Nigam, Y.; Jung, W.; Nibbering, P.H. Multiple actions of Lucilia sericata larvae in hard-to-heal wounds: Larval secretions contain molecules that accelerate wound healing, reduce chronic inflammation and inhibit bacterial infection. Bioessays 2013, 35, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Watts, R. Evidence summary: Wound management: Larval therapy. Wound Practice Res. 2016, 24, 180–182. [Google Scholar]
- Van der Plas, M.J.; van der Does, A.M.; Baldry, M.; Dogterom-Ballering, H.C.; van Gulpen, C.; van Dissel, J.T.; Nibbering, P.H.; Jukema, G.N. Maggot excretions/se-cretions inhibit multiple neutrophil pro-inflammatory responses. Microbes Infect. 2007, 9, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Pecivova, J.; Macickova, T.; Takac, P.; Kovacsova, M.; Cupanikova, D.; Kozanek, M. Effect of the extract from salivary glands of Lucilia sericata on human neutrophils. Neuro Endocrinol. Lett. 2008, 29, 794–797. [Google Scholar]
- Opletalova, K.; Blaizot, X.; Mourgeon, B.; Chene, Y.; Creveuil, C.; Combemale, P.; Laplaud, A.L.; Sohyer-Lebreuilly, I.; Dompmartin, A. Maggot therapy for wound debridement. Arch. Dermatol. 2012, 148, 432–438. [Google Scholar] [CrossRef]
- Apelqvist, J.; Armstrong, D.G.; Lavery, L.A.; Boulton, A.J. Resource utilization and economic costs of care based on a randomized trial of vacuum-assisted closure therapy in the treatment of diabetic foot wounds. Am. J. Surg. 2008, 195, 782–788. [Google Scholar] [CrossRef]
- Monsen, C.; Acosta, S.; Kumlien, C. Patients experiences of negative pressure wound therapy at home for the treatment of deep perivascular groin infection after vascular surgery. J. Clin. Nurs. 2017, 26, 1405–1413. [Google Scholar] [CrossRef]
- Banasiewicz, T. NPWT Sentenced to Success. Negat. Press. Wound Ther. 2014, 1, 1–4. [Google Scholar]
- Singh, B.; Wells, J.D. Entomological Society of America. J. Med. Entomol. 2013, 50, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Nigam, Y.; Bexfield, A.; Thomas, S.; Ratcliffe, N.A. Maggot therapy: The science and implication for CAM part II-Maggots Combat Infection. Evid. Based Complement. Alternat. Med. 2006, 3, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Horobin, A.; Blount, D.G.; Hill, P.J.; English, J.; Rich, A.; Wiliams, P.M.; Pritchard, D.I. Blow fly Lucilia sericata nuclease digests DNA associated with wound slough/eschar and with Pseudomonas aeruginosa biofilm. Med. Vet. Entomol. 2012, 26, 432–439. [Google Scholar] [CrossRef]
- Teh, C.H.; Nazni, W.A.; Nurulhusna, A.H.; Norazah, A.; Lee, H.L. Determination of antibacterial activity andminimum inhibitory concentration of larva extract of fly via resazurin-based turbidometric assay. BMC Microbiol. 2017, 17, 36. [Google Scholar] [CrossRef]
- Davydov, L.; Pharm, B.S. Maggot Therapy in Wound Management in Modern Era and a Review of Published Literature. J. Pharm. Pract. 2011, 24, 89–93. [Google Scholar] [CrossRef]
- Van der Plas, M.J.; Jukema, G.N.; Wai, S.W.; Dogterom-Ballering, H.C.; Lagendijk, E.L.; van Gulpen, C.; van Dissel, J.T.; Bloemberg, G.V.; Nibbering, P.H. Maggot excretions/secretions are differentially effective against biofilms of Staphylococcus aureus and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008, 61, 117–122. [Google Scholar] [CrossRef]
- Margolin, L.; Gialanella, P. Assessment of the antimicrobial properties of maggots. Int. Wound J. 2010, 7, 202–204. [Google Scholar] [CrossRef]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef]
- Bexfield, A.; Bond, A.E.; Roberts, E.C.; Dudley, E.; Nigam, Y.; Thomas, S.; Newton, R.P.; Ratcliffe, N.A. The antibacterial activity against MRSA strains and other bacteria of a <500 Da fraction from maggot excretions/secretions of Lucilia sericata (Diptera: Calliphoridae). Microbes Infect. 2008, 10, 325–333. [Google Scholar] [CrossRef]
- Bowling, F.L.; Salgami, E.V.; Boulton, A.J. Larval therapy: A novel treatment in eliminating methicillin-resistant Staphylococcus aureus from diabetic foot ulcers. Diabet. Care 2007, 30, 370–371. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Jensen, P.Ø.; Madsen, K.G.; Phipps, R.; Krogfelt, K.; Høiby, N.; Givskov, M. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen. 2008, 16, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Hurlow, J.J.; Humphreys, G.J.; Bowling, F.L.; McBain, A.J. Diabetic foot infection: A critical complication. Int. Wound J. 2018, 15, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.; Dryburgh, N.; Donaldson, J.; Mitchell, M. Debridement for surgical wounds. Cochrane Database Syst. Rev. 2011, 11, CD006214. [Google Scholar] [CrossRef]
- Wolcott, R. Disrupting the biofilm matrix improves wound healing outcomes. J. Wound Care 2015, 24, 366–371. [Google Scholar] [CrossRef]
- Seth, A.K.; Geringer, M.R.; Gurjala, A.N.; Hong, S.J.; Galiano, R.D.; Leung, K.P.; Mustoe, T.A. Treatment of Pseudomonas aeruginosa biofilm-infected wounds with clinical wound care strategies: A quantitative study using an in vivo rabbit ear model. Plast. Reconstr. Surg. 2012, 129, 262e–274e. [Google Scholar] [CrossRef]
- Chambers, L.; Woodrow, S.; Brown, A.; Harris, P.D.; Phillips, D.; Hall, M.; Church, J.C.; Pritchard, D.I. Degradation of extracellular matrix components by defined proteinases from the green bottle larva Lucilia sericata used for the clinical debridement of non-healing wounds. Br. J. Dermatol. 2003, 148, 14–23. [Google Scholar] [CrossRef]
- Dumville, J.C.; Worthy, G.; Bland, J.M.; Cullum, N.; Dowson, C.; Iglesias, C.; Mitchell, J.L.; Nelson, E.A.; Soares, M.O.; Torgerson, D.J.; et al. Larval therapy for leg ulcers (VenUS II): Randomised controlled trial. BMJ 2009, 338, 773. [Google Scholar] [CrossRef]
- Yan, L.; Chu, J.; Li, M.; Wang, X.; Zong, J.; Zhang, X.; Song, M.; Wang, S. Pharmacological Properties of the Medical Maggot: A Novel Therapy Overview. Evid. Based Complement. Alternat. Med. 2018, 3, 4934890. [Google Scholar] [CrossRef]
- Nasoori, A.; Hoomand, R. Maggot debridement therapy for an electrical burn injury with instructions for the use of Lucilia sericata larvae. J. Wound Care 2017, 26, 734–741. [Google Scholar] [CrossRef]
- Lin, Y.; Amin, M.; Donnelly, A.; Amar, S. Maggot Debridement Therapy of a Leg Wound from Kaposi’s Sarcoma: A Case Report. J. Glob. Oncol. 2015, 1, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Bugaj, M.; Strużyna, J.; Mądry, R.; Korzeniowski, T.; Antonov, S. The use of Lucilia sericata larvae in the treatment of burns. Chir. Plast. Oparz. 2014, 2, 91–96. (In Polish) [Google Scholar] [CrossRef]
- Mudge, E.; Price, P.; Walkley, N.; Harding, K.G. A randomized controlled trial of larval therapy for the debridement of leg ulcers: Results of a multicenter, randomized, controlled, open, observer blind, parallel group study. Wound Repair Regen. 2014, 22, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.A. Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy. Diabet. Care 2003, 26, 446–451. [Google Scholar] [CrossRef]
- Elraiyah, T.; Domecq, J.P.; Prutsky, G.; Tsapas, A.; Nabhan, M.; Frykberg, R.G.; Hasan, R.; Firwana, B.; Prokop, L.J.; Murad, M.H. A systematic review and meta-analysis of débridement methods for chronic diabetic foot ulcers. J. Vasc. Surg. 2016, 63, 37S–45S. [Google Scholar] [CrossRef]
- Edwards, J.; Stapley, S. Debridement of diabetic foot ulcers. Cochrane Database Syst. Rev. 2010, 20, CD003556. [Google Scholar] [CrossRef]
- Tian, X.; Liang, X.; Song, G.; Zhao, Y.; Yang, X. Maggot debridement therapy for the treatment of diabetic foot ulcers: A meta-analysis. J. Wound Care 2013, 22, 462–469. [Google Scholar] [CrossRef]
- Summers, J.B.; Kaminski, J. Maggot debridement therapy for diabetic necrotic foot. Am. Fam. Physician 2003, 68, 2327–2330. [Google Scholar]
- McCaughan, D.; Cullum, N.; Dumville, J. Patients’ perceptions and experiences of venous leg ulceration and their attitudes to larval therapy: An in-depth qualitative study. Health Expect. 2015, 18, 527–541. [Google Scholar] [CrossRef]
- Steenvoorde, P.; Buddingh, T.J.; van Engeland, A.; Oskam, J. Maggot therapy and the ‘yuk’ factor: An issue for the patient? Wound Repair Regen. 2005, 13, 350–351. [Google Scholar] [CrossRef]
- Kitching, M. Patients’ perceptions and experiences of larval therapy. J. Wound Care 2004, 13, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Bazaliński, D. Efficacy of Biological Therapy using Lucilia sericata Larvae in the Treatment of Chronic Wounds in Patients in Long-Term and Palliative Care. Uniwersytet Rzeszowski: Rzeszów, Poland, 2019. Available online: http://wydawnictwo.univ.rzeszow.pl/product_info.php?products_id=2753 (accessed on 7 October 2019). (In Polish).
- Daeschlein, G.; Napp, M.; Assadian, O.; von Podewils, S.; Reese, K.; Hinz, P.; Matiasek, J.; Spitzmueller, R.; Humphreys, P.; Jünger, M.; et al. Viability of Lucilia sericata maggots after exposure to wound antiseptics. Int. Wound J. 2017, 14, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.I.; Nigam, Y. Maximising the secondary beneficial effects of larval debridement therapy. J. Wound Care 2013, 22, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Blake, F.A.S.; Abromeit, N.; Bubenheim, M.; Li, L.; Schmelzle, R. The biosurgical wound debridement: Experimental investigation of effciency and practicability. Wound Repair Regen. 2007, 15, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Jawień, A.; Szewczyk, M.T.; Kaszuba, A.; Gaciong, Z.; Krasińska, Z.; Wroński, J.; Grzela, T.; Koblik, T. Expert Group guidelines on the healing of venous leg ulcers. Leczenie Ran 2011, 8, 59–80. Available online: https://evereth.pl/wytyczne-grupy-ekspertow-w-sprawie-gojenia-owrzodzen-zylnych-goleni/ (accessed on 7 October 2019). (In Polish).
- Gottrup, F.; Apelqvist, J.; Price, P. Outcomes in controlled comparative studies on non-healing wounds: Recommendations to improve the quality of evidence in wound management. J. Wound Care 2010, 19, 237–268. [Google Scholar] [CrossRef]
- Apleqvist, J.; Willy, C.; Fagerdahl, A.M.; Fraccalveri, M.; Malmsjo, M.; Piaggesi, A.; Probst, A. EWMA Document: Negative Pressure Wound Therapy. J. Wound Care 2017, 26, S1–S154. [Google Scholar] [CrossRef]
- Gray, M. Is larval (maggot) debridement effective for removal of necrotic tissue from chronic wounds? J. Wound Ostomy Cont. Nurs. 2008, 35, 378–384. [Google Scholar] [CrossRef]
- Paul, A.G.; Ahmad, N.W.; Lee, H.L.; Ariff, A.M.; Saranum, M.; Naicker, A.S.; Osman, Z. Maggot debridement therapy with Lucilia cuprina: A comparison with conventional debridement in diabetic foot ulcers. Int. Wound J. 2009, 6, 39–46. [Google Scholar] [CrossRef]
- Der Simonian, R. Meta-analysis in the design and monitoring of clinical trials. Stat. Med. 1996, 15, 1237–1248. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.G.; Naik, G. Maggot debridement therapy: The current perspectives. Chronic Wound Care Manag. Res. 2017, 4, 121–128. [Google Scholar] [CrossRef]
- Sun, X.; Chen, J.; Zhang, J.; Wang, W.; Sun, J.; Wang, A. Maggot debridement therapy promotes diabetic foot wound healing by up-regulating endothelial cell activity. J. Diabetes Complicat. 2016, 30, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Poppel, A.K.; Kahl, M.; Baumann, A.; Wiesner, J.; Gokçen, A.; Beckert, A.; Preissner, K.T.; Vilcinskas, A.; Franta, Z. A Jonah-like chymotrypsin from the therapeutic maggot Lucilia sericata plays a role in wound debridement and coagulation. Insect Biochem. Mol. Biol. 2016, 70, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Cazander, G.; Rooijakkers, S.H.M.; Trouw, L.A.; Nibbering, P.H. Excretions/secretions from medicinal larvae (Lucilia sericata) inhibit complement activation by two mechanisms. Wound Repair Regen. 2017, 25, 41–50. [Google Scholar] [CrossRef]
- Linger, R.J.; Belikoff, E.J.; Yan, Y.; Li, F.; Wantuch, H.A.; Fitzsimons, H.L.; Scott, M.J. Towards next generation maggot debridement therapy: Transgenic Lucilia sericata larvae that produce and secrete a human growth factor. BMC Biotechnol. 2016, 22, 30. [Google Scholar] [CrossRef]
- Turkman, A.; Graham, K.; McGrouther, D. Therapeutic applications of the larvae for wound debridement. J. Plast Reconstr. Aesthet. Surg. 2010, 63, 184–188. [Google Scholar] [CrossRef]
- Sherman, R. Maggot versus conservative debridement therapy for the treatment of pressure ulcers. Wound Rep. Regen. 2002, 10, 208–214. [Google Scholar] [CrossRef]
- Spilsbury, K.; Cullum, N.; Dumville, J.; O’Meara, S.; Petherick, E.; Thompson, C. Exploring patient perceptions of larval therapy as a potential treatment for venous leg ulceration. Health Expect. 2008, 11, 148–159. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazaliński, D.; Kózka, M.; Karnas, M.; Więch, P. Effectiveness of Chronic Wound Debridement with the Use of Larvae of Lucilia Sericata. J. Clin. Med. 2019, 8, 1845. https://doi.org/10.3390/jcm8111845
Bazaliński D, Kózka M, Karnas M, Więch P. Effectiveness of Chronic Wound Debridement with the Use of Larvae of Lucilia Sericata. Journal of Clinical Medicine. 2019; 8(11):1845. https://doi.org/10.3390/jcm8111845
Chicago/Turabian StyleBazaliński, Dariusz, Maria Kózka, Magdalena Karnas, and Paweł Więch. 2019. "Effectiveness of Chronic Wound Debridement with the Use of Larvae of Lucilia Sericata" Journal of Clinical Medicine 8, no. 11: 1845. https://doi.org/10.3390/jcm8111845
APA StyleBazaliński, D., Kózka, M., Karnas, M., & Więch, P. (2019). Effectiveness of Chronic Wound Debridement with the Use of Larvae of Lucilia Sericata. Journal of Clinical Medicine, 8(11), 1845. https://doi.org/10.3390/jcm8111845




