Associations between Prenatal Physical Activity and Neonatal and Obstetric Outcomes—A Secondary Analysis of the Cluster-Randomized GeliS Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. The GeliS Study
2.2. Data Collection and Outcomes
2.3. Statistical Analysis
3. Results
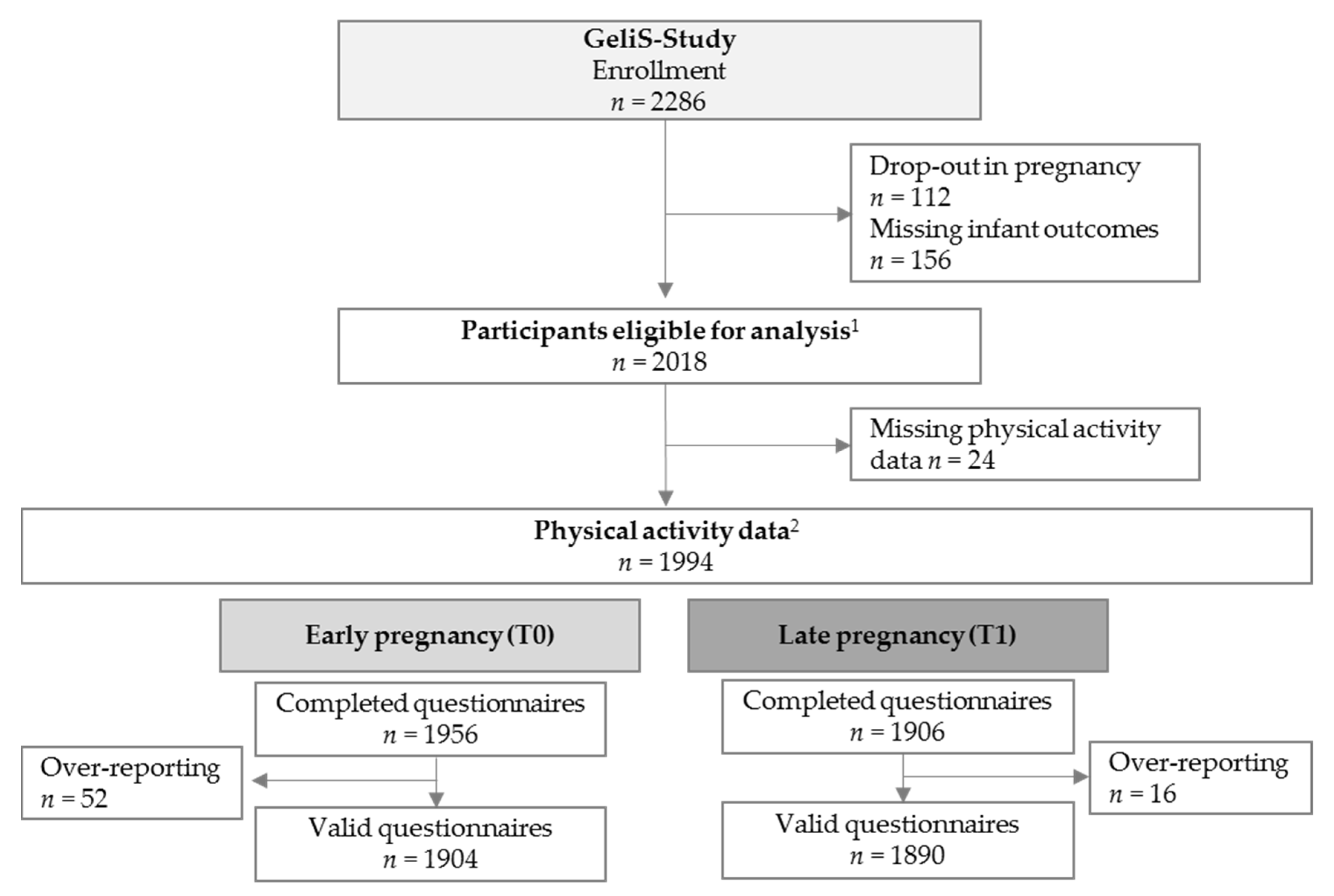
3.1. Participant Flow and Baseline Characteristics
3.2. Associations Between Prenatal Physical Activity and Infant Anthropometrics, Neonatal, and Obstetric Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| ACOG | American College of Obstetrics and Gynecology |
| BMI | Body mass index |
| C | Control group |
| CI | Confidence interval |
| GeliS | “Gesund leben in der Schwangerschaft”/“Healthy living in pregnancy“ |
| IV | Intervention group |
| MET | Metabolic equivalent of task |
| OR | Odds ratio |
| PA | Physical activity |
| PPAQ | Pregnancy Physical Activity Questionnaire |
| RCT | Randomized-controlled trial |
| SD | Standard deviation |
| TALIA | Total physical activity of light intensity and above |
| LGA | Large for gestational age |
| SGA | Small for gestational age |
References
- World Health Organization. Childhood Overweight and Obesity. Available online: https://www.who.int/dietphysicalactivity/childhood/en/ (accessed on 28 January 2019).
- Singh, A.S.; Mulder, C.; Twisk, J.W.R.; van Mechelen, W.; Chinapaw, M.J.M. Tracking of childhood overweight into adulthood: A systematic review of the literature. Obes. Rev. 2008, 9, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Schellong, K.; Schulz, S.; Harder, T.; Plagemann, A. Birth weight and long-term overweight risk: Systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS ONE 2012, 7, e47776. [Google Scholar] [CrossRef] [PubMed]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef] [PubMed]
- Weight Gain During Pregnancy. Reexamining the Guidelines; Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines: Washington, DC, USA, 2009; ISBN 9780309131131.
- The International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: Meta-analysis of individual participant data from randomised trials. BMJ 2017, 358, j3119. [Google Scholar] [CrossRef]
- Muktabhant, B.; Lawrie, T.A.; Lumbiganon, P.; Laopaiboon, M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst. Rev. 2015, 6, CD007145. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, H.W.; Boulé, N.G.; Chari, R.; Davenport, M.H. The effect of supervised prenatal exercise on fetal growth: A meta-analysis. Obstet. Gynecol. 2015, 125, 1185–1194. [Google Scholar] [CrossRef]
- Pastorino, S.; Bishop, T.; Crozier, S.R.; Granström, C.; Kordas, K.; Küpers, L.K.; O’Brien, E.; Polanska, K.; Sauder, K.A.; Zafarmand, M.H.; et al. Associations between maternal physical activity in early and late pregnancy and offspring birth size: Remote federated individual level meta-analysis from eight cohort studies. BJOG Int. J. Obstet. Gynaecol. 2018. [Google Scholar] [CrossRef]
- Bisson, M.; Lavoie-Guénette, J.; Tremblay, A.; Marc, I. Physical Activity Volumes during Pregnancy: A Systematic Review and Meta-Analysis of Observational Studies Assessing the Association with Infant’s Birth Weight. AJP Rep. 2016, 6, e170–e197. [Google Scholar] [CrossRef]
- Aune, D.; Schlesinger, S.; Henriksen, T.; Saugstad, O.D.; Tonstad, S. Physical activity and the risk of preterm birth: A systematic review and meta-analysis of epidemiological studies. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1816–1826. [Google Scholar] [CrossRef]
- Du, M.-C.; Ouyang, Y.-Q.; Nie, X.-F.; Huang, Y.; Redding, S.R. Effects of physical exercise during pregnancy on maternal and infant outcomes in overweight and obese pregnant women: A meta-analysis. Birth 2018. [Google Scholar] [CrossRef]
- Davenport, M.H.; Ruchat, S.-M.; Sobierajski, F.; Poitras, V.J.; Gray, C.E.; Yoo, C.; Skow, R.J.; Jaramillo Garcia, A.; Barrowman, N.; Meah, V.L.; et al. Impact of prenatal exercise on maternal harms, labour and delivery outcomes: A systematic review and meta-analysis. Br. J. Sports Med. 2019, 53, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Domenjoz, I.; Kayser, B.; Boulvain, M. Effect of physical activity during pregnancy on mode of delivery. Am. J. Obstet. Gynecol. 2014, 211, 401.e1. [Google Scholar] [CrossRef]
- Poyatos-León, R.; García-Hermoso, A.; Sanabria-Martínez, G.; Álvarez-Bueno, C.; Sánchez-López, M.; Martínez-Vizcaíno, V. Effects of exercise during pregnancy on mode of delivery: A meta-analysis. Acta Obstet. Gynecol. Scand. 2015, 94, 1039–1047. [Google Scholar] [CrossRef]
- The American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 650 (Reaffirmed 2019): Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet. Gynecol. 2015, 126, e135–e142. [Google Scholar] [CrossRef]
- Koletzko, B.; Bauer, C.-P.; Bung, P.; Cremer, M.; Flothkötter, M.; Hellmers, C.; Kersting, M.; Krawinkel, M.; Przyrembel, H.; Rasenack, R.; et al. Practice recommendations of the Network “Healthy Start—Young Family Network”. Dtsch. Med. Wochenschr. 2012, 137, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Rauh, K.; Kunath, J.; Rosenfeld, E.; Kick, L.; Ulm, K.; Hauner, H. Healthy living in pregnancy: A cluster-randomized controlled trial to prevent excessive gestational weight gain—Rationale and design of the GeliS study. BMC Pregnancy Childbirth 2014, 14, 119. [Google Scholar] [CrossRef]
- Kunath, J.; Günther, J.; Rauh, K.; Hoffmann, J.; Stecher, L.; Rosenfeld, E.; Kick, L.; Ulm, K.; Hauner, H. Effects of a lifestyle intervention during pregnancy to prevent excessive gestational weight gain in routine care - the cluster-randomised GeliS trial. BMC Med. 2019, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Günther, J.; Hoffmann, J.; Kunath, J.; Spies, M.; Meyer, D.; Stecher, L.; Rosenfeld, E.; Kick, L.; Rauh, K.; Hauner, H. Effects of a Lifestyle Intervention in Routine Care on Prenatal Dietary Behavior-Findings from the Cluster-Randomized GeliS Trial. J. Clin. Med. 2019, 8, 960. [Google Scholar] [CrossRef]
- Hoffmann, J.; Günther, J.; Geyer, K.; Stecher, L.; Rauh, K.; Kunath, J.; Meyer, D.; Sitzberger, C.; Spies, M.; Rosenfeld, E.; et al. Effects of a lifestyle intervention in routine care on prenatal physical activity—findings from the cluster-randomised GeliS trial. BMC Pregnancy Childbirth 2019, in press. [Google Scholar]
- Hoffmann, J.; Günther, J.; Stecher, L.; Spies, M.; Meyer, D.; Kunath, J.; Raab, R.; Rauh, K.; Hauner, H. Effects of a Lifestyle Intervention in Routine Care on Short- and Long-Term Maternal Weight Retention and Breastfeeding Behavior-12 Months Follow-up of the Cluster-Randomized GeliS Trial. J. Clin. Med. 2019, 8, 876. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine—ClinicalTrials.gov. Healthy Living in Pregnancy—NCT01958307. Available online: https://clinicaltrials.gov/ct2/show/NCT01958307 (accessed on 16 April 2019).
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr. Kinderheilkunde 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Chasan-Taber, L.; Schmidt, M.D.; Roberts, D.E.; Hosmer, D.; Markenson, G.; Freedson, P.S. Development and validation of a Pregnancy Physical Activity Questionnaire. Med. Sci. Sports Exerc. 2004, 36, 1750–1760. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef]
- Byrne, N.M.; Hills, A.P.; Hunter, G.R.; Weinsier, R.L.; Schutz, Y. Metabolic equivalent: One size does not fit all. J. Appl. Physiol. 2005, 99, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Chasan-Taber, L.; Schmidt, M.D. Pregnancy Physical Activity Questionnaire. Can. J. Public Health 2016, 106, e563. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.L.; Pham, N.M.; Lee, A.H.; Nguyen, P.T.H.; Chu, T.K.; Ha, A.V.V.; Duong, D.V.; Duong, T.H.; Binns, C.W. Physical activity during pregnancy is associated with a lower prevalence of gestational diabetes mellitus in Vietnam. Acta Diabetol. 2018. [Google Scholar] [CrossRef]
- Koushkie Jahromi, M.; Namavar Jahromi, B.; Hojjati, S. Relationship between Daily Physical Activity During Last Month of Pregnancy and Pregnancy Outcome. Iran. Red Crescent Med. J. 2011, 13, 15–20. [Google Scholar] [PubMed]
- Badon, S.E.; Littman, A.J.; Chan, K.C.G.; Williams, M.A.; Enquobahrie, D.A. Maternal sedentary behavior during pre-pregnancy and early pregnancy and mean offspring birth size: A cohort study. BMC Pregnancy Childbirth 2018, 18, 267. [Google Scholar] [CrossRef]
- Bisson, M.; Croteau, J.; Guinhouya, B.C.; Bujold, E.; Audibert, F.; Fraser, W.D.; Marc, I. Physical activity during pregnancy and infant’s birth weight: Results from the 3D Birth Cohort. BMJ Open Sport Exerc. Med. 2017, 3, e000242. [Google Scholar] [CrossRef] [PubMed]
- Hegaard, H.K.; Petersson, K.; Hedegaard, M.; Ottesen, B.; Dykes, A.K.; Henriksen, T.B.; Damm, P. Sports and leisure-time physical activity in pregnancy and birth weight: A population-based study. Scand. J. Med. Sci. Sports 2010, 20, e96–e102. [Google Scholar] [CrossRef]
- Harrod, C.S.; Chasan-Taber, L.; Reynolds, R.M.; Fingerlin, T.E.; Glueck, D.H.; Brinton, J.T.; Dabelea, D. Physical activity in pregnancy and neonatal body composition: The Healthy Start study. Obstet. Gynecol. 2014, 124, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Ming, W.-K.; Ding, W.; Zhang, C.J.P.; Zhong, L.; Long, Y.; Li, Z.; Sun, C.; Wu, Y.; Chen, H.; Chen, H.; et al. The effect of exercise during pregnancy on gestational diabetes mellitus in normal-weight women: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2018, 18, 440. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijsen, M.J.; Northstone, K.; Golding, J. Swimming and birth weight. Epidemiology 2002, 13, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Juhl, M.; Olsen, J.; Andersen, P.K.; Nøhr, E.A.; Andersen, A.-M.N. Physical exercise during pregnancy and fetal growth measures: A study within the Danish National Birth Cohort. Am. J. Obstet. Gynecol. 2010, 202, 63-e1. [Google Scholar] [CrossRef]
- Perkins, C.C.D.; Pivarnik, J.M.; Paneth, N.; Stein, A.D.; Stein, A.D. Physical activity and fetal growth during pregnancy. Obstet. Gynecol. 2007, 109, 81–87. [Google Scholar] [CrossRef]
- Ferraro, Z.M.; Gaudet, L.; Adamo, K.B. The potential impact of physical activity during pregnancy on maternal and neonatal outcomes. Obstet. Gynecol. Surv. 2012, 67, 99–110. [Google Scholar] [CrossRef]
- Clapp, J.F. Influence of endurance exercise and diet on human placental development and fetal growth. Placenta 2006, 27, 527–534. [Google Scholar] [CrossRef]
- Ruchat, S.-M.; Davenport, M.H.; Giroux, I.; Hillier, M.; Batada, A.; Sopper, M.M.; McManus, R.; Hammond, J.-A.; Mottola, M.F. Effect of exercise intensity and duration on capillary glucose responses in pregnant women at low and high risk for gestational diabetes. Diabetes Metab. Res. Rev. 2012, 28, 669–678. [Google Scholar] [CrossRef]
- Salvesen, K.Å.; Hem, E.; Sundgot-Borgen, J. Fetal wellbeing may be compromised during strenuous exercise among pregnant elite athletes. Br. J. Sports Med. 2012, 46, 279–283. [Google Scholar] [CrossRef]
- Morison, P.N.; Bacardi-Gascon, M.; Lopez-Corrales, M.; Jimenez-Cruz, A. Combined dietary-exercise intervention for gestational weight gain and birthweight: A meta-analysis. Asia Pac. J. Clin. Nutr. 2018, 27, 860–868. [Google Scholar] [CrossRef]
- Juhl, M.; Andersen, P.K.; Olsen, J.; Madsen, M.; Jørgensen, T.; Nøhr, E.A.; Andersen, A.-M.N. Physical exercise during pregnancy and the risk of preterm birth: A study within the Danish National Birth Cohort. Am. J. Epidemiol. 2008, 167, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, D.; Magro-Malosso, E.R.; Saccone, G.; Marhefka, G.D.; Berghella, V. Exercise during pregnancy in normal-weight women and risk of preterm birth: A systematic review and meta-analysis of randomised controlled trials. Am. J. Obstet. Gynecol. 2016, 215, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Magro-Malosso, E.R.; Saccone, G.; Di Mascio, D.; Di Tommaso, M.; Berghella, V. Exercise during pregnancy and risk of preterm birth in overweight and obese women: A systematic review and meta-analysis of randomised controlled trials. Acta Obstet. Gynecol. Scand. 2017, 96, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Jukic, A.M.Z.; Evenson, K.R.; Daniels, J.L.; Herring, A.H.; Wilcox, A.J.; Hartmann, K.E. A prospective study of the association between vigorous physical activity during pregnancy and length of gestation and birthweight. Matern. Child Health J. 2012, 16, 1031–1044. [Google Scholar] [CrossRef][Green Version]
- Kahn, M.; Robien, K.; DiPietro, L. Maternal Leisure-time Physical Activity and Risk of Preterm Birth: A Systematic Review of the Literature. J. Phys. Act. Health 2016, 13, 796–807. [Google Scholar] [CrossRef]
- Baena-García, L.; Ocón-Hernández, O.; Acosta-Manzano, P.; Coll-Risco, I.; Borges-Cosic, M.; Romero-Gallardo, L.; de La Flor-Alemany, M.; Aparicio, V.A. Association of sedentary time and physical activity during pregnancy with maternal and neonatal birth outcomes. The GESTAFIT Project. Scand. J. Med. Sci. Sports 2018. [Google Scholar] [CrossRef]
- Günther, J.; Hoffmann, J.; Spies, M.; Meyer, D.; Kunath, J.; Stecher, L.; Rosenfeld, E.; Kick, L.; Rauh, K.; Hauner, H. Associations between the Prenatal Diet and Neonatal Outcomes-A Secondary Analysis of the Cluster-Randomised GeliS Trial. Nutrients 2019, 11, 1889. [Google Scholar] [CrossRef]
- Prince, S.A.; Adamo, K.B.; Hamel, M.E.; Hardt, J.; Connor Gorber, S.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56. [Google Scholar] [CrossRef]
- Mensink, G.B.M.; Schienkiewitz, A.; Haftenberger, M.; Lampert, T.; Ziese, T.; Scheidt-Nave, C. Übergewicht und Adipositas in Deutschland. Gesundheitsschutz 2013, 56, 786–794. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 2018) |
|---|---|
| Maternal Characteristics | |
| Pre-pregnancy age, years | 30.3 ± 4.4 |
| Pre-pregnancy weight, kg | 68.2 ± 13.4 |
| Pre-pregnancy BMI, kg/m2 | 24.4 ± 4.5 |
| Pre-Pregnancy BMI Category | |
| BMI 18.5–24.9 kg/m2 | 1311/2018 (65.0%) |
| BMI 25.0–29.9 kg/m2 | 464/2018 (23.0%) |
| BMI 30.0–40.0 kg/m2 | 243/2018 (12.0%) |
| Educational Level | |
| General secondary school | 320/2014 (15.9%) |
| Intermediate secondary school | 856/2014 (42.5%) |
| (Technical) High school | 838/2014 (41.6%) |
| Country of Birth | |
| Germany | 1790/2014 (88.9%) |
| Others | 224/2014 (11.1%) |
| Nulliparous | 1162/2018 (57.6%) |
| Living with a partner | 1939/2011 (96.4%) |
| Full-time employed | 1056/1996 (52.9%) |
| Neonatal and Obstetric Characteristics | |
| Birth weight, g | 3337.6 ± 517.8 |
| Birth length, cm | 51.3 ± 2.6 |
| Head circumference, cm | 34.7 ± 1.6 |
| BMI, kg/m2 | 12.7 ± 1.3 |
| BMI-z-Score a | 0.04 ± 1.02 |
| LGA | 148/2016 (7.3%) |
| SGA | 172/2016 (8.5%) |
| Low birth weight | 101/2018 (5.0%) |
| High birth weight | 169/2018 (8.4%) |
| Macrosomia | 19/2018 (0.9%) |
| Preterm birth | 132/2016 (6.5%) |
| Caesarean section | 582/2017 (28.9%) |
| Time Point | Active | Inactive | |||||
|---|---|---|---|---|---|---|---|
| Anthropometrics | na | Mean±SD | na | Mean±SD | Adjusted Effect Size b (95% CI) | Adjusted p Value b | |
| Birth weight, g | T0 | n = 893 | 3338.0 ± 527.9 | n = 1008 | 3337.4 ± 508.9 | 11.44 (−35.02, 57.91) | 0.629 |
| T1 | n = 1061 | 3364.5 ± 481.0 | n = 827 | 3341.4 ± 492.5 | 49.74 (4.94, 94.53) | 0.030 | |
| Birth length, cm | T0 | n = 885 | 51.3 ± 2.6 | n = 1000 | 51.4 ± 2.6 | −0.00 (−0.24, 0.23) | 0.980 |
| T1 | n = 1056 | 51.4 ± 2.4 | n = 824 | 51.3 ± 2.6 | 0.23 (−0.00, 0.46) | 0.054 | |
| Head circum-ference, cm | T0 | n = 875 | 34.7 ± 1.6 | n = 989 | 34.7 ± 1.6 | −0.02 (−0.17, 0.12) | 0.762 |
| T1 | n = 1047 | 34.8 ± 1.5 | n = 814 | 34.7 ± 1.6 | 0.11 (−0.04, 0.25) | 0.148 | |
| BMI, kg/m2 | T0 | n = 885 | 12.7 ± 1.3 | n = 1000 | 12.6 ± 1.2 | 0.04 (−0.08, 0.15) | 0.503 |
| T1 | n = 1056 | 12.7 ± 1.3 | n = 824 | 12.7 ± 1.2 | 0.09 (−0.03, 0.20) | 0.140 | |
| BMI-z-scorec | T0 | n = 884 | 0.05 ± 1.07 | n = 1000 | 0.03 ± 0.98 | 0.04 (−0.06, 0.13) | 0.463 |
| T1 | n = 1055 | 0.07 ± 1.04 | n = 824 | 0.04 ± 0.96 | 0.07 (−0.02, 0.16) | 0.134 | |
| Neonatal and Obstetric Outcomes | n (%) | n (%) | Adjusted OR b (95% CI) | Adjusted p Value b | |||
| LGA | T0 | n = 893 | 74 (8.3) | n = 1006 | 66 (6.6) | 1.37 (0.96, 1.94) | 0.079 |
| T1 | n = 1061 | 87 (8.2) | n = 826 | 54 (6.5) | 1.39 (0.97, 2.00) | 0.075 | |
| SGA | T0 | n = 893 | 72 (8.1) | n = 1006 | 92 (9.1) | 0.84 (0.61, 1.16) | 0.293 |
| T1 | n = 1061 | 100 (9.4) | n = 826 | 61 (7.4) | 1.16 (0.82, 1.63) | 0.408 | |
| Low birth weight | T0 | n = 893 | 49 (5.5) | n = 1008 | 46 (4.6) | 1.16 (0.77, 1.76) | 0.485 |
| T1 | n = 1061 | 46 (4.3) | n = 827 | 34 (4.1) | 0.95 (0.60, 1.51) | 0.835 | |
| High birth weight | T0 | n = 893 | 81 (9.1) | n = 1008 | 80 (7.9) | 1.20 (0.86, 1.66) | 0.282 |
| T1 | n = 1061 | 92 (8.7) | n = 827 | 66 (8.0) | 1.16 (0.82, 1.62) | 0.402 | |
| Macrosomia | T0 | n = 893 | 10 (1.1) | n = 1008 | 8 (0.8) | 1.37 (0.54, 3.53) | 0.509 |
| T1 | n = 1061 | 11 (1.0) | n = 827 | 7 (0.8) | 1.18 (0.44, 3.14) | 0.746 | |
| Preterm birth | T0 | n = 893 | 61 (6.8) | n = 1006 | 62 (6.2) | 1.09 (0.75, 1.57) | 0.649 |
| T1 | n = 1061 | 52 (4.9) | n = 826 | 57 (6.9) | 0.66 (0.44, 0.98) | 0.038 | |
| Caesarean section | T0 | n = 893 | 264 (29.6) | n = 1008 | 278 (27.6) | 1.11 (0.90, 1.36) | 0.323 |
| T1 | n = 1060 | 301 (28.4) | n = 827 | 232 (28.1) | 0.98 (0.80, 1.21) | 0.868 | |
| Birth Weight | BMI | Preterm Birth | Caesarean Section | |||||
|---|---|---|---|---|---|---|---|---|
| Adjusted Effect Size a (95% CI) | Adjusted p Value a | Adjusted Effect Size a (95% CI) | Adjusted p Value a | Adjusted OR a (95% CI) | Adjusted p Value a | Adjusted OR a (95% CI) | Adjusted p Value a | |
| TALIA | ||||||||
| T0 | 2.81 (−0.68, 6.30) | 0.115 | 0.01 (−0.00, 0.02) | 0.099 | 0.98 (0.95, 1.01) | 0.276 | 1.02 (1.00, 1.03) | 0.040 |
| T1 | 1.05 (−2.44, 4.55) | 0.555 | 0.00 (−0.01, 0.01) | 0.357 | 0.98 (0.95, 1.02) | 0.325 | 1.00 (0.98, 1.01) | 0.789 |
| Sedentary-Intensity | ||||||||
| T0 | −15.92 (−37.41, 5.6) | 0.146 | 0.00 (−0.05, 0.05) | 0.998 | 1.16 (0.99, 1.35) | 0.051 | 1.02 (0.93, 1.12) | 0.661 |
| T1 | −20.62 (−38.79, −2.45) | 0.026 | 0.00 (−0.05, 0.04) | 0.883 | 1.14 (0.99, 1.32) | 0.070 | 0.98 (0.90, 1.07) | 0.717 |
| Light-Intensity | ||||||||
| T0 | 1.17 (−4.72, 7.06) | 0.697 | 0.00 (−0.01, 0.02) | 0.589 | 0.98 (0.93, 1.03) | 0.430 | 1.03 (1.00, 1.06) | 0.032 |
| T1 | 0.77 (−4.55, 6.08) | 0.777 | 0.00 (−0.01, 0.02) | 0.732 | 0.97 (0.92, 1.02) | 0.267 | 1.00 (0.98, 1.03) | 0.757 |
| Moderate-Intensity | ||||||||
| T0 | 3.98 (−0.77, 8.73) | 0.100 | 0.01 (−0.00, 0.02) | 0.087 | 0.98 (0.94, 1.02) | 0.330 | 1.01 (0.99, 1.03) | 0.262 |
| T1 | 3.32 (−2.87, 9.52) | 0.293 | 0.01 (−0.01, 0.03) | 0.228 | 0.98 (0.92, 1.04) | 0.533 | 0.99 (0.96, 1.02) | 0.540 |
| Vigorous-Intensity | ||||||||
| T0 | 6.00 (−56.81, 68.81) | 0.852 | 0.02 (−0.14, 0.17) | 0.852 | 1.06 (0.65, 1.71) | 0.822 | 0.91 (0.68, 1.21) | 0.518 |
| T1 | −38.10 (−131.22, 55.03) | 0.423 | −0.11 (−0.35, 0.13) | 0.350 | 1.71 (0.94, 3.11) | 0.081 | 0.79 (0.49, 1.28) | 0.333 |
| Low Birth Weight | High Birth Weight | LGA | SGA | |||||
|---|---|---|---|---|---|---|---|---|
| Adjusted OR a (95% CI) | Adjusted p Value a | Adjusted OR a (95% CI) | Adjusted p Value a | Adjusted OR a (95% CI) | Adjusted p Value a | Adjusted OR a (95% CI) | Adjusted p Value a | |
| TALIA | ||||||||
| T0 | 0.98 (0.94, 1.01) | 0.181 | 1.02 (0.99, 1.04) | 0.194 | 1.02 (0.99, 1.04) | 0.202 | 0.98 (0.96, 1.01) | 0.148 |
| T1 | 1.00 (0.96, 1.04) | 0.969 | 1.01 (0.98, 1.03) | 0.516 | 1.01 (0.98, 1.03) | 0.740 | 1.00 (0.97, 1.03) | 0.937 |
| Sedentary-Intensity | ||||||||
| T0 | 1.27 (1.08, 1.48) | 0.004 | 0.93 (0.79, 1.10) | 0.387 | 0.92 (0.77, 1.09) | 0.330 | 0.90 (0.76, 1.06) | 0.198 |
| T1 | 1.25 (1.07, 1.46) | 0.005 | 0.91 (0.78, 1.05) | 0.194 | 0.88 (0.75, 1.03) | 0.121 | 1.00 (0.88, 1.15) | 0.979 |
| Light-Intensity | ||||||||
| T0 | 0.97 (0.92, 1.03) | 0.298 | 1.00 (0.96, 1.04) | 0.895 | 1.00 (0.96, 1.05) | 0.995 | 0.96 (0.92, 1.00) | 0.053 |
| T1 | 0.99 (0.93, 1.05) | 0.711 | 0.99 (0.95, 1.03) | 0.681 | 0.99 (0.95, 1.04) | 0.722 | 0.98 (0.94, 1.02) | 0.404 |
| Moderate-Intensity | ||||||||
| T0 | 0.98 (0.93, 1.03) | 0.403 | 1.03 (1.00, 1.06) | 0.080 | 1.02 (0.99, 1.06) | 0.135 | 1.00 (0.96, 1.03) | 0.860 |
| T1 | 1.01 (0.94, 1.07) | 0.842 | 1.04 (1.00, 1.08) | 0.050 | 1.03 (0.99, 1.07) | 0.185 | 1.02 (0.98, 1.07) | 0.383 |
| Vigorous-Intensity | ||||||||
| T0 | 0.84 (0.45, 1.57) | 0.579 | 1.08 (0.70, 1.66) | 0.743 | 1.14 (0.72, 1.80) | 0.587 | 1.08 (0.72, 1.63) | 0.703 |
| T1 | 0.97 (0.36, 2.57) | 0.946 | 1.38 (0.77, 2.49) | 0.278 | 1.24 (0.64, 2.40) | 0.533 | 1.48 (0.86, 2.55) | 0.160 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, J.; Günther, J.; Geyer, K.; Stecher, L.; Kunath, J.; Meyer, D.; Spies, M.; Rosenfeld, E.; Kick, L.; Rauh, K.; et al. Associations between Prenatal Physical Activity and Neonatal and Obstetric Outcomes—A Secondary Analysis of the Cluster-Randomized GeliS Trial. J. Clin. Med. 2019, 8, 1735. https://doi.org/10.3390/jcm8101735
Hoffmann J, Günther J, Geyer K, Stecher L, Kunath J, Meyer D, Spies M, Rosenfeld E, Kick L, Rauh K, et al. Associations between Prenatal Physical Activity and Neonatal and Obstetric Outcomes—A Secondary Analysis of the Cluster-Randomized GeliS Trial. Journal of Clinical Medicine. 2019; 8(10):1735. https://doi.org/10.3390/jcm8101735
Chicago/Turabian StyleHoffmann, Julia, Julia Günther, Kristina Geyer, Lynne Stecher, Julia Kunath, Dorothy Meyer, Monika Spies, Eva Rosenfeld, Luzia Kick, Kathrin Rauh, and et al. 2019. "Associations between Prenatal Physical Activity and Neonatal and Obstetric Outcomes—A Secondary Analysis of the Cluster-Randomized GeliS Trial" Journal of Clinical Medicine 8, no. 10: 1735. https://doi.org/10.3390/jcm8101735
APA StyleHoffmann, J., Günther, J., Geyer, K., Stecher, L., Kunath, J., Meyer, D., Spies, M., Rosenfeld, E., Kick, L., Rauh, K., & Hauner, H. (2019). Associations between Prenatal Physical Activity and Neonatal and Obstetric Outcomes—A Secondary Analysis of the Cluster-Randomized GeliS Trial. Journal of Clinical Medicine, 8(10), 1735. https://doi.org/10.3390/jcm8101735





