The Effects of Eplerenone on the Circadian Blood Pressure Pattern and Left Ventricular Hypertrophy in Patients with Obstructive Sleep Apnea and Resistant Hypertension—A Randomized, Controlled Trial
Abstract
1. Introduction
2. Experimental Section
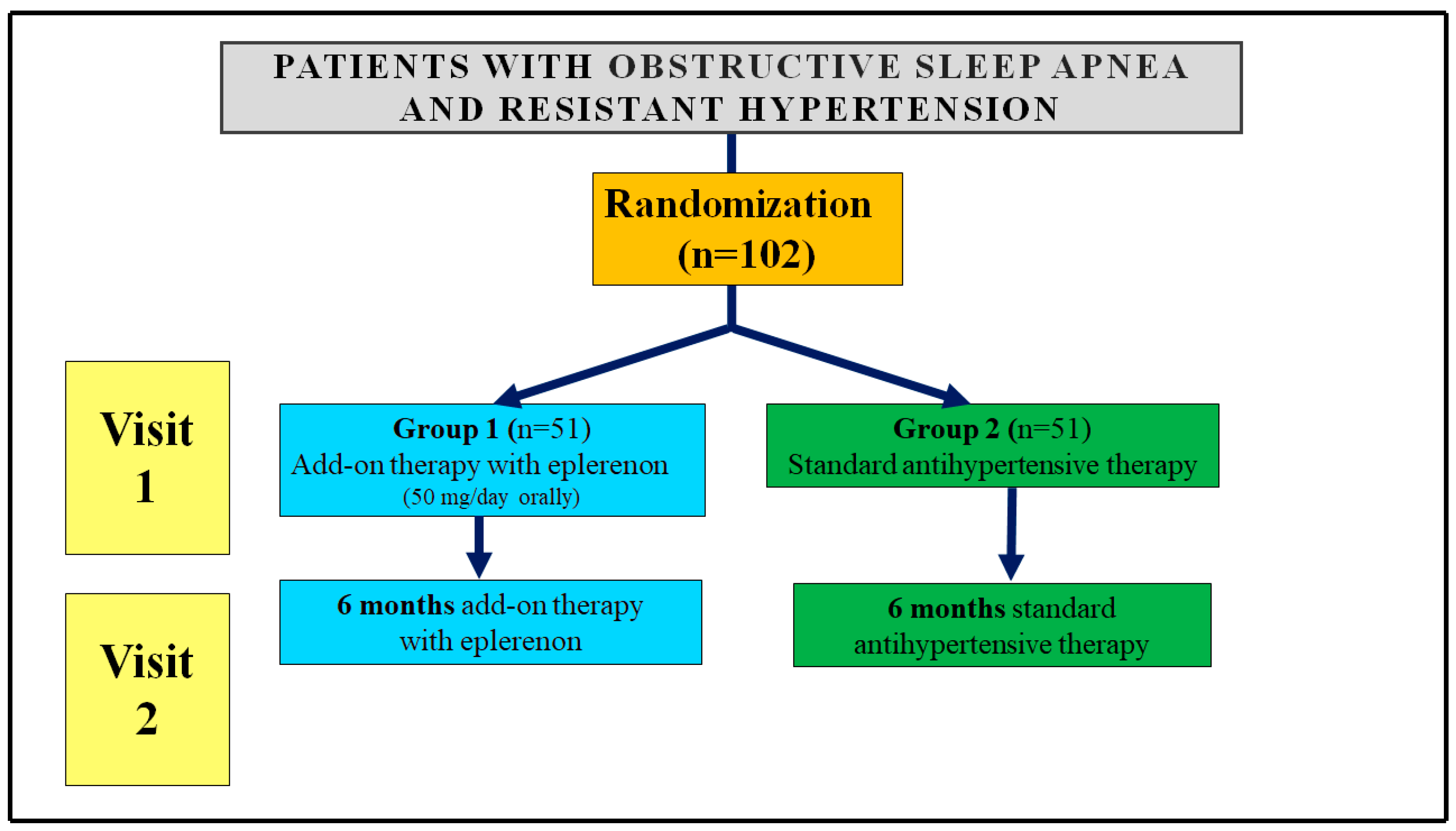
2.1. Study Design
2.2. Eligibility Criteria for Inclusion, Exclusion Criteria, and Recruitment
2.3. Ethical Aspects and Informed Consent
2.4. Measurements
2.4.1. Blood Pressure
2.4.2. Neck Circumference Measurement
2.4.3. Echocardiographic Measurement
2.4.4. Polysomnography (PSG)
2.5. Statistical Analysis
3. Results
3.1. Studied Group
3.2. Body Composition Parameters
3.3. Renal and Biochemical Parameters
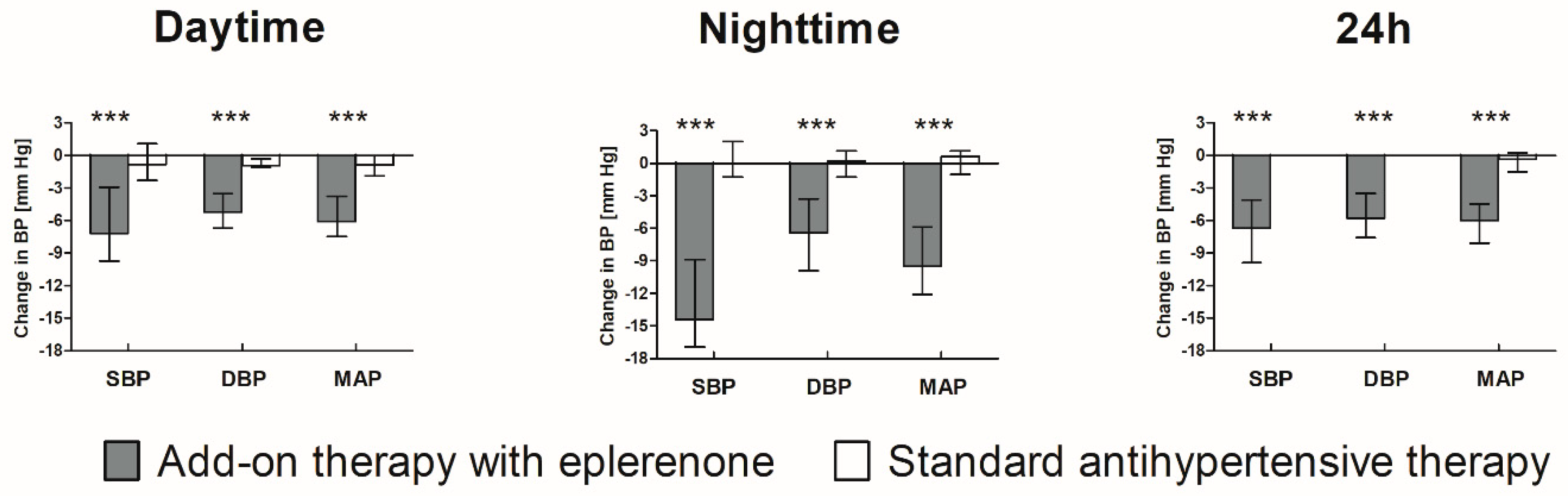
3.4. Blood Pressure Parameters
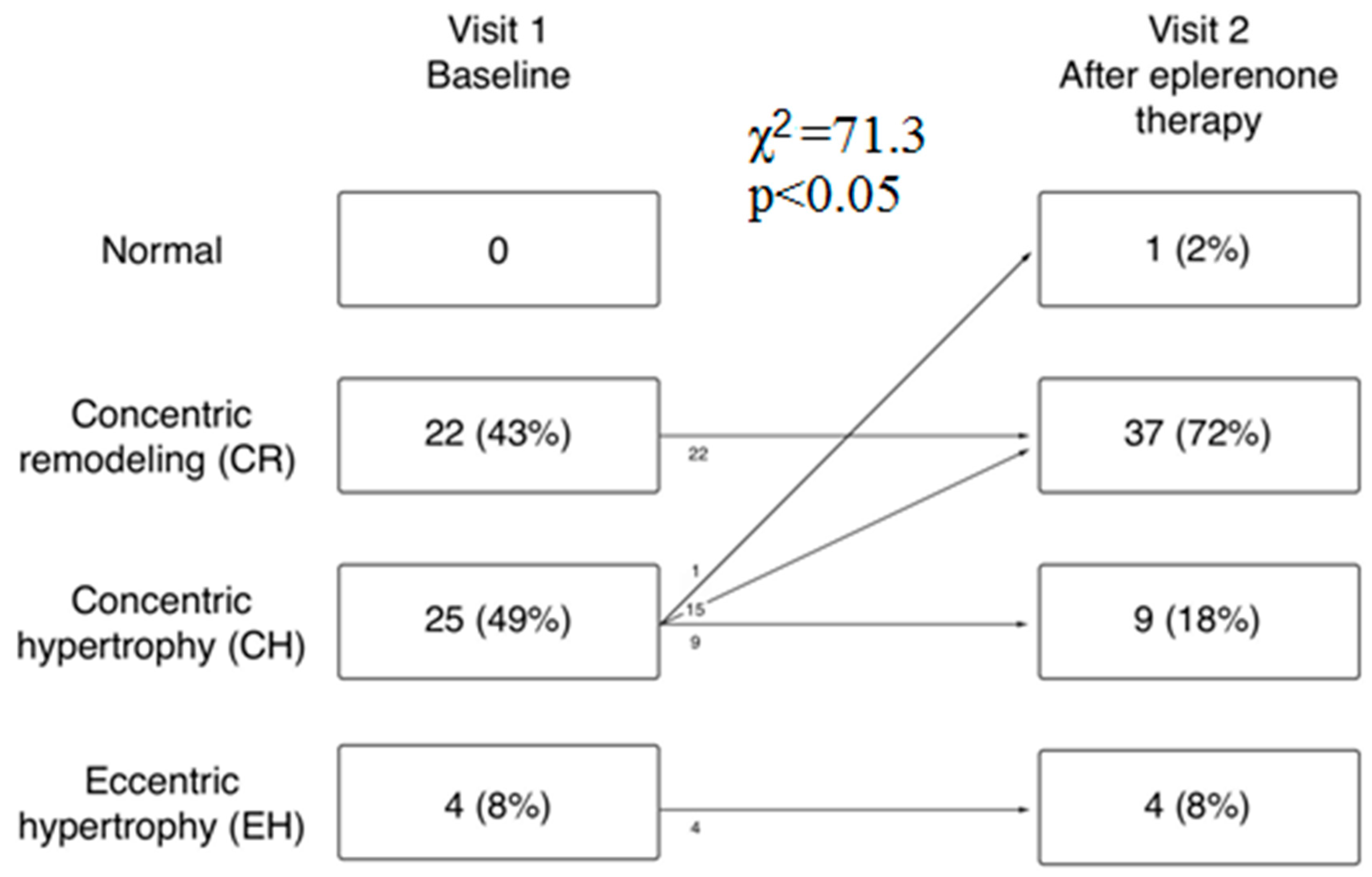
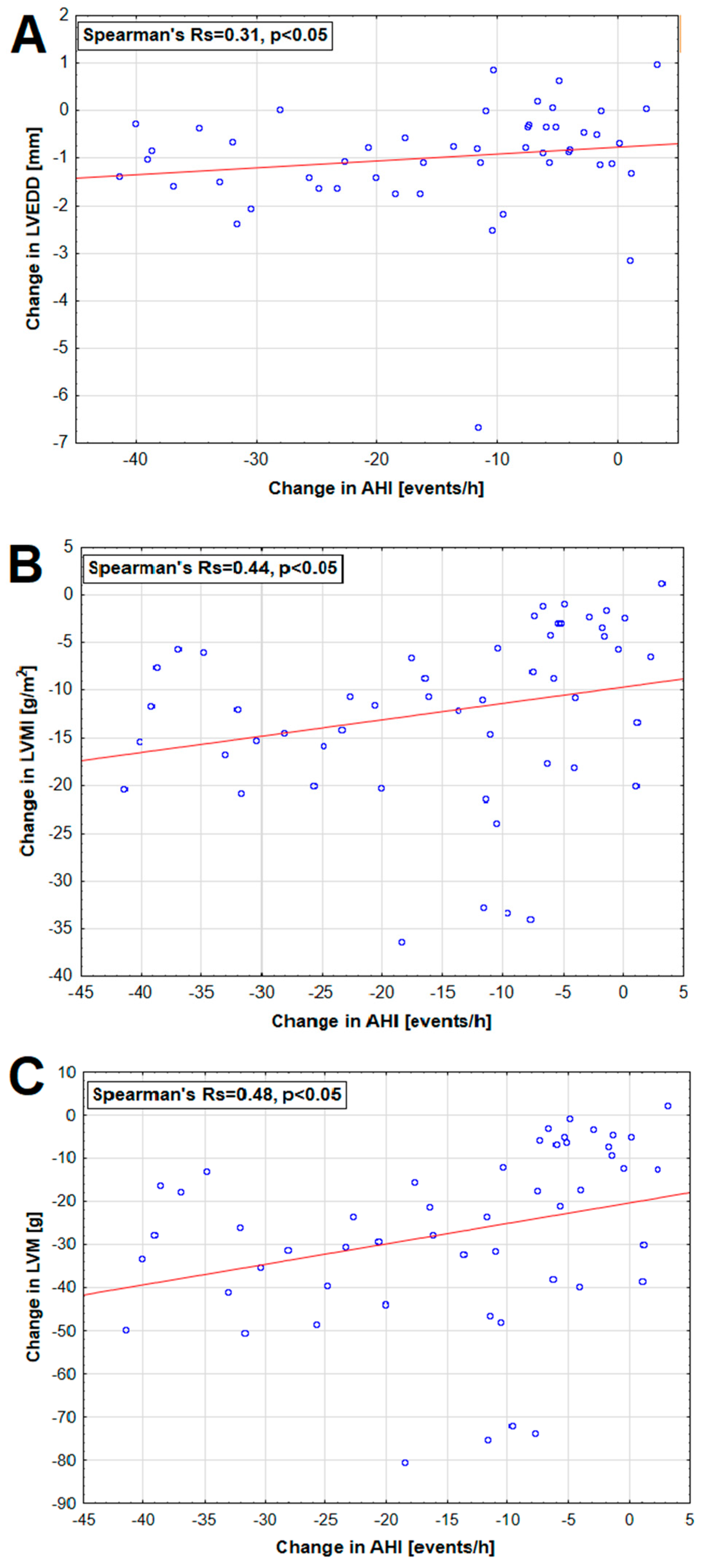
3.5. Polysomnographic and Echocardiographic Parameters
3.6. Side Effects
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Peppard, E.P.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar] [PubMed]
- Logan, A.G.; Perlikowski, S.M.; Mente, A.; Tisler, A.; Tkacova, R.; Niroumand, M.; Leung, R.S.T.; Bradley, T.D. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J. Hypertens. 2001, 19, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Konecny, T.; Kara, T.; Somers, V.K. Obstructive sleep apnea and hypertension: An update. Hypertension 2014, 63, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Florczak, E.; Prejbisz, A.; Szwench-Pietrasz, E.; Śliwiński, P.; Bieleń, P.; Klisiewicz, A.; Michałowska, I.; Warchoł, E.; Januszewicz, M.; Kała, M.; et al. Clinical characteristics of patients with resistant hypertension: The RESIST-POL study. J. Hum. Hypertens. 2013, 27, 678–685. [Google Scholar] [CrossRef]
- Maeder, M.T.; Schoch, O.D.; Rickli, H. A clinical approach to obstructive sleep apnea as a risk factor for cardiovascular disease. Vasc. Health Risk Manag 2016, 12, 85–103. [Google Scholar] [CrossRef]
- Drager, L.F.; Bortolotto, L.A.; Figueiredo, A.C.; Silva, B.C.; Krieger, E.M.; Lorenzi-Filho, G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest 2007, 131, 1379–1386. [Google Scholar] [CrossRef]
- Cioffi, G.; Russo, T.E.; Stefenelli, C.; Selmi, A.; Furlanello, F.; Cramariuc, D.; Gerdts, E.; de Simone, G. Severe obstructive sleep apnea elicits concentric left ventricular geometry. J. Hypertens 2010, 28, 1074–1082. [Google Scholar] [CrossRef]
- Chami, H.A.; Devereux, R.B.; Gottdiener, J.S.; Mehra, R.; Roman, M.J.; Benjamin, E.J.; Gottlieb, D.J. Left ventricular morphology and systolic function in sleep-disordered breathing: The Sleep Heart Health Study. Circulation 2008, 117, 2599–2607. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takata, Y.; Usui, Y.; Asanuma, R.; Nishihata, Y.; Kato, K.; Shiina, K.; Yamashina, A. Nocturnal Intermittent Hypoxia Is Associated With Left Ventricular Hypertrophy in Middle-Aged Men With Hypertension and Obstructive Sleep Apnea. Am. J. Hypertens. 2016, 29, 372–378. [Google Scholar] [CrossRef]
- Alchanatis, M.; Paradellis, G.; Pini, H.; Tourkohoriti, G.; Jordanoglou, J. Left ventricular function in patients with obstructive sleep apnoea syndrome before and after treatment with nasal continuous positive airway pressure. Respiration 2000, 67, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, K.; Pimenta, E.; Thomas, S.J.; Cofield, S.S.; Oparil, S.; Harding, S.M.; Calhoun, D.A. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: A preliminary report. J. Hum. Hypertens. 2010, 24, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Hla, K.M.; Young, T.; Finn, L.; Peppard, P.E.; Szklo-Coxe, M.; Stubbs, M. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep 2008, 31, 795–800. [Google Scholar] [CrossRef]
- Loredo, J.S.; Ancoli-Israel, S.; Dimsdale, J.E. Sleep quality and blood pressure dipping in obstructive sleep apnea. Am. J. Hypertens 2001, 14, 887–892. [Google Scholar] [CrossRef]
- Cuspidi, C.; Giudici, V.; Negri, F.; Sala, C. Nocturnal nondipping and left ventricular hypertrophy in hypertension: An updated review. Expert Rev. Cardiovasc. Ther. 2010, 8, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Sala, C.; Valerio, C.; Negri, F.; Mancia, G. Nocturnal hypertension and organ damage in dippers and nondippers. Am. J. Hypertens 2012, 25, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Pickering, T.G. The clinical significance of diurnal blood pressure variations. Dippers and nondippers. Circulation 1990, 81, 700–702. [Google Scholar] [CrossRef]
- Kario, K.; Shimada, K. Risers and extreme-dippers of nocturnal blood pressure in hypertension: Antihypertensive strategy for nocturnal blood pressure. Clin. Exp. Hypertens 2004, 26, 177–189. [Google Scholar] [CrossRef]
- Holt-Lunstad, J.; Jones, B.Q.; Birmingham, W. The influence of close relationships on nocturnal blood pressure dipping. Int. J. Psychophysiol 2009, 71, 211–217. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef]
- Ganau, A.; Devereux, R.B.; Roman, M.J.; de Simone, G.; Pickering, T.G.; Saba, P.S.; Vargiu, P.; Simongini, I.; Laragh, J.H. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J. Am. Coll Cardiol. 1992, 19, 1550–1558. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.S.; Oei, T.; Douglas, J.A.; Brown, I.; Jorgensen, G.; Andrews, J. Confirmatory factor analysis of the Epworth Sleepiness Scale (ESS) in patients with obstructive sleep apnoea. Sleep Med. 2008, 9, 739–744. [Google Scholar] [CrossRef]
- Park, J.G.; Ramar, K.; Olson, E.J. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin. Proc. 2011, 86, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Ayas, N.T.; Hirsch, A.A.; Laher, I.; Bradley, T.D.; Malhotra, A.; Polotsky, V.Y.; Tasali, E. New frontiers in obstructive sleep apnoea. Clin. Sci. 2014, 127, 209–216. [Google Scholar] [CrossRef]
- Struthers, A.; Krum, H.; Williams, G.H. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin. Cardiol. 2008, 31, 153–158. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Cai, M.; Zou, Y.B.; Jiang, X.J.; Song, L.; Liang, E.; Bian, J.; Wu, H.; Hui, R. Effect of spironolactone on patients with resistant hypertension and obstructive sleep apnea. Clin. Exp. Hypertens 2016, 38, 464–468. [Google Scholar] [CrossRef]
- Kasai, T.; Bradley, T.D.; Friedman, O.; Logan, A.G. Effect of intensified diuretic therapy on overnight rostral fluid shift and obstructive sleep apnoea in patients with uncontrolled hypertension. J. Hypertens 2014, 32, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Krasinska, B.; Miazga, A.; Cofta, S.; Szczepaniak-Chicheł, L.; Trafas, T.; Krasiński, Z.; Pawlaczyk-Gabriel, K.; Tykarski1, A. Effect of eplerenone on the severity of obstructive sleep apnea and arterial stiffness in patients with resistant arterial hypertension. Pol. Arch. Med. Wewn. 2016, 126, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Guo, W.; Peng, H.; Hu, C.H.; Zhang, H.H.; Peng, C.G.; Wang, X.Q. Association of aldosterone excess and apnea-hypopnea index in patients with resistant hypertension. Sci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Garofoli, M.; Angeli, F. Response to morning surge, dipping and sleep-time blood pressure as prognostic markers of cardiovascular risk. Hypertension 2013. [Google Scholar] [CrossRef]
- Staessen, J.A.; Thijs, L.; Birkenhager, W.H.; Bulpitt, C.J.; Fagard, R. Update on the systolic hypertension in Europe (Syst-Eur) trial. The Syst-Eur Investigators. Hypertension 1999, 33, 1476–1477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, D.X.; Zhao, W.; Sun, Y.S.; Tian, Q.P.; Chen, Y. [The predictive value of the ambulatory blood pressure monitoring parameters on left ventricular hypertrophy and carotid artery intima-media thickness in hypertensives]. Zhonghua Xin Xue Guan Bing Za Zhi 2005, 33, 243–246. [Google Scholar] [PubMed]
- Che, X.; Mou, S.; Zhang, W.; Zhang, M.; Gu, L.; Yan, Y.C.; Hua, Y.; Hu, C.H.; Qian, J.Q.; Ni, Z.H. The impact of non-dipper circadian rhythm of blood pressure on left ventricular hypertrophy in patients with non-dialysis chronic kidney disease. Acta. Cardiol. 2017, 72, 149–155. [Google Scholar] [CrossRef]
- Karadag, B.; Ozyigit, T.; Serindag, Z.; Ilhan, A.; Ozben, B. Blood pressure profile is associated with microalbuminuria and retinopathy in hypertensive nondiabetic patients. Wien. Klin. Wochenschr. 2018, 130, 204–210. [Google Scholar] [CrossRef]
- Koga, S.; Ikeda, S.; Nakata, T.; Yasunaga, T.; Maemura, K. Effects of nasal continuous positive airway pressure on left ventricular concentric hypertrophy in obstructive sleep apnea syndrome. Intern. Med. 2012, 51, 2863–2868. [Google Scholar] [CrossRef]
- Javaheri, S.; Sharma, R.K.; Bluemke, D.A.; Redline, S. Association between central sleep apnea and left ventricular structure: The Multi-Ethnic Study of Atherosclerosis. J. Sleep. Res. 2017, 26, 477–480. [Google Scholar] [CrossRef]
- Myslinski, W.; Duchna, H.-W.; Rasche, K.; Dichmann, M.; Mosiewicz, J.; Schultze-Werninghaus, G. Left ventricular geometry in patients with obstructive sleep apnea coexisting with treated systemic hypertension. Respiration 2007, 74, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Avelar, E.; Cloward, T.V.; Walker, J.M.; Farney, R.J.; Strong, M.; Pendleton, R.C.; Segerson, N.; Adams, T.A.; Gress, R.C.; Hunt, S.C.; et al. Left ventricular hypertrophy in severe obesity: Interactions among blood pressure, nocturnal hypoxemia, and body mass. Hypertension 2007, 49, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Dursunoglu, D.; Dursunoglu, N.; Evrengül, H.; Özkurt, S.; Kuru, Ö.; Kiliç, M.; Fisekci, F. Impact of obstructive sleep apnoea on left ventricular mass and global function. Eur. Respir. J. 2005, 26, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Altintas, N.; Aslan, E.; Helvaci, A.; Malhotra, A. Relationship between obstructive sleep apnea severity index and left ventricular function and volume. Ann. Saudi. Med. 2012, 32, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Sekizuka, H.; Kida, K.; Akashi, Y.J.; Yoneyama, K.; Osada, N.; Miyake, F. Relationship between sleep apnea syndrome and sleep blood pressure in patients without hypertension. J. Cardiol. 2010, 55, 92–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fagard, R.; Staessen, J.A.; Thijs, L. The relationships between left ventricular mass and daytime and night-time blood pressures: A meta-analysis of comparative studies. J. Hypertens 1995, 13, 823–829. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Add-on Therapy—with Eplerenone | Standard Antihypertensive Therapy | ||
|---|---|---|---|---|
| Gender (n/%) | V1 | p > 0.05 | ||
| M | 29 | 30 | ||
| F | 22 | 21 | ||
| Age (years) | V1 | 59.0 (52.0–62.0) | 59.0 (52.0–62.0) | p > 0.05 |
| Height (cm) | V1 | 1.7 (1.64–1.75) | 1.7 (1.65–1.76) | p > 0.05 |
| Weight (kg) | V1 | 102.3 (88.7–112.4) | 102.3 (90.3–111.2) | p > 0.05 |
| V2 | 101.2 (87.7–111.9) | 101.3 (83.4–116.9) | p > 0.05 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| Body mass index (kg/m2) | V1 | 36.1 (33.0–37.6) | 35.4 (32.1–37.4) | p > 0.05 |
| V2 | 35.6 (32.3–37.2) | 35.3 (30.3–39.1) | p > 0.05 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| Neck (cm) | V1 | 43.0 (40.0–47.0) | 44.0 (39.0–46.0) | p > 0.05 |
| V2 | 42.0 (39.0–44.0) | 44.0 (34.0–47.0) | p > 0.05 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| Waist (cm) | V1 | 105.0 (98.0–118.0) | 104.0 (98.0–116.0) | p > 0.05 |
| V2 | 104.0 (99.0–116.0) | 103.0 (87.0–121.0) | p > 0.05 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| Serum potassium (mmol/L) | V1 | 4.1 (3.9–4.3) | 4.1 (3.8–4.6) | p > 0.05 |
| V2 | 4.4 (4.2–4.6) | 4.1 (3.5–4.6) | p > 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| Serum creatinine (µmol/L) | V1 | 82.4 (73.4–90.9) | 80.1 (74.6–89.3) | p > 0.05 |
| V2 | 85.7 (77.5–92.4) | 80.9 (74.3–91.3) | p > 0.05 | |
| WLCXN | p < 0.01 | p < 0.05 | ||
| Glomerular filtration rate (mL/min) | V1 | 85.0 (62.0–99.0) | 81.0 (63.0–96.0) | p > 0.05 |
| V2 | 80.0 (62.0–96.0) | 80.0 (63.0–96.0) | p > 0.05 | |
| WLCXN | p < 0.01 | p < 0.05 | ||
| Plasma renin activity (ng/mL/h) | V1 | 0.2 (0.2–1.3) | 1.0 (0.2–1.2) | p < 0.01 |
| V2 | 1.3 (1.1–2.1) | 1.2 (0.2–1.8) | p < 0.001 | |
| WLCXN | p < 0.001 | p < 0.05 | ||
| Plasma aldosterone (ng/dL) | V1 | 10.1 (6.5–12.9) | 10.3 (7.4–11.3) | p > 0.05 |
| V2 | 5.5 (3.5–7.3) | 10.2 (5.7–13.5) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| Aldosterone–renin ratio | V1 | 4.8 (0.4–8.6) | 1.0 (0.7–5.3) | p > 0.05 |
| V2 | 0.4 (0.2–0.6) | 0.9 (0.5–5.2) | p < 0.001 | |
| p < 0.001 | p < 0.05 |
| Add-On Therapy—with Eplerenone | Standard Antihypertensive Therapy | |||
|---|---|---|---|---|
| cSBP | V1 | 150.0 (144.0–155.0) | 148.0 (145.0–155.0) | p > 0.05 |
| V2 | 142.0 (138.0–145.0) | 149 (144.0–153.0) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| cDBP | V1 | 92.0 (89.0–99.0) | 91.0 (90.0–98.0) | p > 0.05 |
| V2 | 90.0 (84.0–93.0) | 91.0 (88.0–98.0) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| SBPd | V1 | 147.7 (141.2–155.2) | 147.6 (142.3–154.3) | p > 0.05 |
| V2 | 140.3 (137.7–147.5) | 146.6 (141.3–152.3) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| DBPd | V1 | 96.5 (90.2–101.0) | 96.4 (92.3–99.8) | p > 0.05 |
| V2 | 90.3 (86.2–94.7) | 95.5 (91.8–99.5) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| MAPd | V1 | 112.8 (109.2–117.6) | 112.9 (110.7–116.6) | p > 0.05 |
| V2 | 107.5 (103.7–110.3) | 112.8 (109.7–116.1) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| SBPn | V1 | 141.0 (132.1–146.8) | 136.7 (132.0–140.3) | p > 0.05 |
| V2 | 125.4 (121.0–131.6) | 136.5 (133.3–140.4) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| DBPn | V1 | 91.2 (86.7–97.8) | 90.1 (85.8–93.2) | p > 0.05 |
| V2 | 84.4 (80.2–89.8) | 90.3 (86.6–92.3) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| MAPn | V1 | 108.3 (103.1–114.6) | 104.5 (102.0–107.6) | p > 0.05 |
| V2 | 98.2 (94.6–103.1) | 104.9 (102.0–107.4) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| SBP-24 | V1 | 142.9 (139.4–151.2) | 143.3 (140.4–150.3) | p > 0.05 |
| V2 | 138.5 (133.3–142.2) | 142.8 (139.4–149.0) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| DBP-24 | V1 | 94.3 (88.8–99.9) | 93.5 (90.8–97.8) | p > 0.05 |
| V2 | 88.7 (82.6–92.5) | 94.0 (91.1–97.8) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| MAP-24 | V1 | 109.9 (106.9–116.6) | 111.0 (109.1–114.3) | p > 0.05 |
| V2 | 104.8 (101.3–108.0) | 111.0 (107.6–113.2) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| NBPF (%) | V1 | 4.6 (2.9–6.2) | 7.7 (6.7–10.0) | p < 0.01 |
| V2 | 8.9 (6.7–10.0) | 7.4 (4.1–10.1) | p > 0.05 | |
| WLCXN | p < 0.001 | p > 0.05 |
| V1 | V2 | |||
|---|---|---|---|---|
| Add-On Therapy—with Eplerenone | Standard Antihypertensive Therapy | Add-On Therapy—with Eplerenone | Standard Antihypertensive Therapy | |
| Non-Dippers | 41/80.4 | 38/74.5 | 18/35.3 | 39/76.5 |
| Dippers | 10/19.6 | 10/19.6 | 31/60.8 | 9/17.6 |
| Extreme Dippers | 0/0.0 | 3/5.9 | 2 (3.9) | 3/5.9 |
| χ2 = 3.2, p > 0.05 | χ2 = 20.0, p < 0.001 | |||
| Parameter | Add-On Therapy—with Eplerenone | Standard Antihypertensive Therapy | MW-U test | |
|---|---|---|---|---|
| AHI (/h) | V1 | 44.0 (25.6–61.4) | 45.9 (28.4–61.3) | p > 0.05 |
| V2 | 28.8 (22.3–38.5) | 44.0 (29.0–60.0) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| AI (/h) | V1 | 33.9 (14.4–46.7) | 33.9 (14.4–46.7) | p > 0.05 |
| V2 | 33.5 (17.6–45.8) | 37.0 (20.9–54.6) | p > 0.05 | |
| WLCXN | p>0.05 | p > 0.05 | ||
| ODI (/h) | V1 | 23.0 (14.2–54.3) | 22.4 (13.3–49.6) | p > 0.05 |
| V2 | 28.5 (15.2–55.7) | 34.0 (15.0–57.0) | p > 0.05 | |
| WLCXN | p < 0.001 | p < 0.05 | ||
| Mean saturation (%) | V1 | 89.2 (86.0–92.4) | 90.3 (88.8–91.3) | p > 0.05 |
| V2 | 92.2 (90.2–93.5) | 89.8 (87.6–91.3) | p < 0.001 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| Lowest saturation (%) | V1 | 72.4 (59.0–82.3) | 69.3 (61.3–80.3) | p > 0.05 |
| V2 | 75.4 (66.6–79.7) | 66.8 (62.4–80.3) | p > 0.05 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| LVEDD (cm) | V1 | 49.9 (45.7–55.7) | 49.9 (45.6–53.3) | p > 0.05 |
| V2 | 48.7 (45.2–54.4) | 49.9 (45.3–53.2) | p > 0.05 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| IVS (cm) | V1 | 13.2 (11.4–14.3) | 13.1 (11.3–13.9) | p > 0.05 |
| V2 | 12.3 (11.1–13.3) | 13.2 (11.4–13.9) | p < 0.05 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| PWd (cm) | V1 | 12.9 (11.5–13.5) | 13.0 (11.1–13.5) | p > 0.05 |
| V2 | 12.1 (11.1–13.0) | 13.0 (1.5–13.7) | p < 0.05 | |
| WLCXN | p < 0.001 | p < 0.05 | ||
| LVMI (g/m2) | V1 | 116.9 (93.8–153.9) | 118.7 (87.6–150.9) | p > 0.05 |
| V2 | 105.9 (87.2–141.0) | 118.4 (90.8–144.4) | p > 0.05 | |
| WLCXN | p < 0.001 | p > 0.05 | ||
| RWT | V1 | 0.51 (0.47–0.53) | 0.50 (0.47–0.54) | p > 0.05 |
| V2 | 0.49 (0.46–0.52) | 0.52 (0.48–0.55) | p < 0.05 | |
| WLCXN | p < 0.001 | p < 0.01 |
| Group | LVEDD | IVSd | PWd | LVMI | LVM | RWT | |
|---|---|---|---|---|---|---|---|
| Day-TimeSBP | EPL | 0.38 | 0.14 | 0.05 | 0.29 | 0.32 | −0.17 |
| SAT | −0.04 | −0.01 | −0.06 | −0.02 | −0.01 | 0.00 | |
| Day-Time DBP | EPL | 0.02 | 0.08 | −0.12 | 0.02 | 0.05 | −0.12 |
| SAT | 0.26 | 0.02 | 0.13 | 0.18 | 0.21 | −0.06 | |
| Day-Time MAP | EPL | 0.15 | 0.11 | −0.11 | 0.10 | 0.13 | −0.20 |
| SAT | 0.21 | 0.02 | 0.02 | 0.11 | 0.14 | −0.09 | |
| Night-time SBP | EPL | 0.17 | 0.12 | 0.12 | 0.23 | 0.24 | 0.02 |
| SAT | −0.32 | 0.07 | 0.03 | −0.29 | −0.30 | 0.17 | |
| Night-Time DBP | EPL | 0.15 | 0.22 | 0.09 | 0.21 | 0.24 | 0.02 |
| SAT | 0.18 | −0.04 | 0.39 | 0.31 | 0.29 | 0.28 | |
| Night-Time MAP | EPL | 0.19 | 0.18 | 0.12 | 0.23 | 0.26 | 0.01 |
| SAT | −0.05 | 0.01 | 0.36 | 0.12 | 0.11 | 0.37 | |
| 24-h SBP | EPL | 0.34 | 0.03 | −0.10 | 0.18 | 0.20 | −0.28 |
| SAT | −0.02 | −0.15 | −0.07 | −0.02 | −0.03 | −0.10 | |
| 24-h DBP | EPL | −0.08 | −0.07 | −0.13 | −0.10 | −0.07 | −0.09 |
| SAT | 0.12 | −0.10 | 0.03 | −0.05 | −0.03 | 0.02 | |
| 24-h MAP | EPL | 0.04 | −0.04 | −0.15 | −0.02 | −0.01 | −0.17 |
| SAT | 0.06 | −0.18 | −0.16 | −0.13 | −0.11 | −0.17 | |
| NBPF | EPL | −0.12 | −0.15 | −0.17 | −0.19 | −0.21 | −0.12 |
| SAT | 0.10 | −0.16 | −0.03 | −0.03 | −0.02 | −0.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasińska, B.; Cofta, S.; Szczepaniak-Chicheł, L.; Rzymski, P.; Trafas, T.; Paluszkiewicz, L.; Tykarski, A.; Krasiński, Z. The Effects of Eplerenone on the Circadian Blood Pressure Pattern and Left Ventricular Hypertrophy in Patients with Obstructive Sleep Apnea and Resistant Hypertension—A Randomized, Controlled Trial. J. Clin. Med. 2019, 8, 1671. https://doi.org/10.3390/jcm8101671
Krasińska B, Cofta S, Szczepaniak-Chicheł L, Rzymski P, Trafas T, Paluszkiewicz L, Tykarski A, Krasiński Z. The Effects of Eplerenone on the Circadian Blood Pressure Pattern and Left Ventricular Hypertrophy in Patients with Obstructive Sleep Apnea and Resistant Hypertension—A Randomized, Controlled Trial. Journal of Clinical Medicine. 2019; 8(10):1671. https://doi.org/10.3390/jcm8101671
Chicago/Turabian StyleKrasińska, Beata, Szczepan Cofta, Ludwina Szczepaniak-Chicheł, Piotr Rzymski, Tomasz Trafas, Lech Paluszkiewicz, Andrzej Tykarski, and Zbigniew Krasiński. 2019. "The Effects of Eplerenone on the Circadian Blood Pressure Pattern and Left Ventricular Hypertrophy in Patients with Obstructive Sleep Apnea and Resistant Hypertension—A Randomized, Controlled Trial" Journal of Clinical Medicine 8, no. 10: 1671. https://doi.org/10.3390/jcm8101671
APA StyleKrasińska, B., Cofta, S., Szczepaniak-Chicheł, L., Rzymski, P., Trafas, T., Paluszkiewicz, L., Tykarski, A., & Krasiński, Z. (2019). The Effects of Eplerenone on the Circadian Blood Pressure Pattern and Left Ventricular Hypertrophy in Patients with Obstructive Sleep Apnea and Resistant Hypertension—A Randomized, Controlled Trial. Journal of Clinical Medicine, 8(10), 1671. https://doi.org/10.3390/jcm8101671







