Neurorehabilitation of Spatial Memory Using Virtual Environments: A Systematic Review
Abstract
1. Introduction
2. Method
2.1. Search Methodology
2.2. Inclusion Criteria
2.3. Risk of Bias Assessment
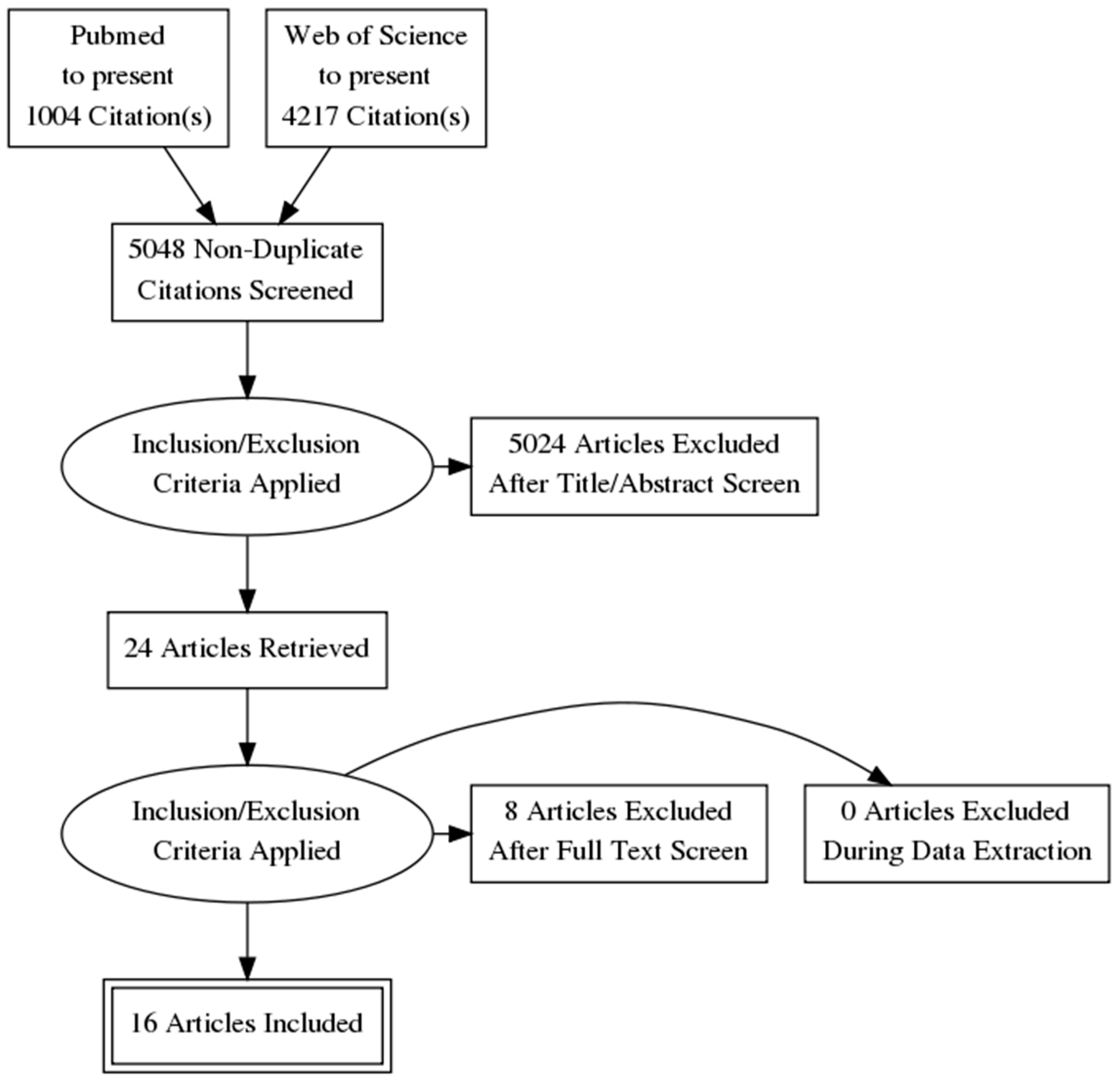
2.4. PRISMA Flow Diagram
3. Results
3.1. Which Virtual Apparatus Is Recommended for Spatial Memory Rehabilitation?
3.1.1. Type of Device and Controllers During Navigation
3.1.2. VR Spatial Navigation
3.2. Which Virtual Training Method Is Suitable for Spatial Memory Rehabilitation?
3.2.1. VR Training Duration
3.2.2. Time Elapsed Since Damage
3.2.3. Training Procedure
3.2.4. Visual Cues
3.3. Which Assessment Method Is Best for Spatial Memory?
3.3.1. Spatial Memory Outcomes
3.3.2. Traditional and Virtual Assessment
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Bohil, C.J.; Alicea, B.; Biocca, F.A. Virtual reality in neuroscience research and therapy. Nat. Rev. Neurosci. 2011, 12, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Steuer, J. Defining Virtual Reality: Characteristics Determining Telepresence. J. Commun. 1992, 42, 73–94. [Google Scholar] [CrossRef]
- Sanchez-Vives, M.V.; Slater, M. Opinion: From presence to consciousness through virtual reality. Nat. Rev. Neurosci. 2005, 6, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Riva, G. From Virtual to Real Body. J. Cyberther. Rehabil. 2008, 1, 7–22. [Google Scholar]
- Riva, G.; Baños, R.M.; Botella, C.; Mantovani, F.; Gaggioli, A. Transforming Experience: The Potential of Augmented Reality and Virtual Reality for Enhancing Personal and Clinical Change. Front. Psychol. 2016, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Slater, M. Place illusion and plausibility can lead to realistic behaviour in immersive virtual environments. Philos. Trans. R. Soc. B Boil. Sci. 2009, 364, 3549–3557. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Waterworth, J.A.; Waterworth, E.L. The Layers of Presence: A Bio-cultural Approach to Understanding Presence in Natural and Mediated Environments. CyberPsychol. Behav. 2004, 7, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Castelnuovo, G.; Mantovani, F. Transformation of flow in rehabilitation: The role of advanced communication technologies. Behav. Res. Methods 2006, 38, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Gaggioli, A.; Villani, D.; Preziosa, A.; Morganti, F.; Corsi, R.; Faletti, G.; Vezzadini, L. NeuroVR: An open source virtual reality platform for clinical psychology and behavioral neurosciences. Stud. Health Technol. Inform. 2007, 125, 394–399. [Google Scholar]
- Witmer, B.G.; Singer, M.J. Measuring Presence in Virtual Environments: A Presence. Presence 1998, 7, 225–241. [Google Scholar] [CrossRef]
- Laviola, J.J. A discussion of cybersickness in virtual environments. ACM SIGCHI Bull. 2000, 32, 47–56. [Google Scholar] [CrossRef]
- Pot-kolder, R.; Veling, W.; Counotte, J.; van der Gaag, M. Anxiety Partially Mediates Cybersickness Symptoms in Immersive Virtual Reality Environments. Cyberpsychol. Behav. Soc. Netw. 2017, 21, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Gatica-Rojas, V.; Méndez-Rebolledo, G. Virtual reality interface devices in the reorganization of neural networks in the brain of patients with neurological diseases. Neural Regen. Res. 2014, 9, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Dakanalis, A.; Gaggioli, A. From body dissatisfaction to obesity: How virtual reality may improve obesity prevention and treatment in adolescents. Stud. Health Technol. Inform. 2011, 184, 356–362. [Google Scholar]
- Slater, M.; Sanchez-Vives, M.V. Enhancing Our Lives with Immersive Virtual Reality. Front. Robot. AI 2016, 3, 1–47. [Google Scholar] [CrossRef]
- Slater, M. Grand Challenges in Virtual Environments. Front. Robot. AI 2014, 1, 1–4. [Google Scholar] [CrossRef]
- Slater, M.; Spanlang, B.; Corominas, D. Simulating virtual environments within virtual environments as the basis for a psychophysics of presence. ACM Trans. Grapghics 2010, 29, 1–9. [Google Scholar] [CrossRef]
- Slater, M.; Marcos, D.P.; Ehrsson, H.H.; Sanchez-Vives, M.V. Inducing Illusory Ownership of a Virtual Body. Front. Mol. Neurosci. 2009, 3, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Serino, S.; Baglio, F.; Rossetto, F.; Realdon, O.; Cipresso, P.; Parsons, T.D.; Cappellini, G.; Mantovani, F.; De Leo, G.; Nemni, R.; et al. Picture Interpretation Test (PIT) 360°: An Innovative Measure of Executive Functions. Sci. Rep. 2017, 7, 16000. [Google Scholar] [CrossRef]
- Grewe, P.; Kohsik, A.; Flentge, D.; Dyck, E.; Botsch, M.; Winter, Y.; Markowitsch, H.J.; Bien, C.G.; Piefke, M. Learning real-life cognitive abilities in a novel 360 degrees -virtual reality supermarket: A neuropsychological study of healthy participants and patients with epilepsy. J. Neuroeng. Rehabil. 2013, 10, 42. [Google Scholar] [CrossRef]
- Serino, S.; Pedroli, E.; Tuena, C.; De Leo, G.; Stramba-Badiale, M.; Goulene, K.; Mariotti, N.G.; Riva, G. A novel virtual reality-based training protocol for the enhancement of the ‘mental frame syncing’ in individuals with Alzheimer’s disease: A development-of-concept trial. Front. Aging Neurosci. 2017, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mishkind, M.C.; Norr, A.M.; Katz, A.C.; Reger, G.M. Review of Virtual Reality Treatment in Psychiatry: Evidence Versus Current Diffusion and Use. Curr. Psychiatry Rep. 2017, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Pope, Z.; Lee, J.E.; Gao, Z. Virtual Reality Exercise for Anxiety and Depression: A Preliminary Review of Current Research in an Emerging Field. J. Clin. Med. 2018, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-García, M.; Gutiérrez-Maldonado, J.; Pla-Sanjuanelo, J.; Vilalta-Abella, F.; Riva, G.; Clerici, M.; Ribas-Sabaté, J.; Andreu-Gracia, A.; Fernandez-Aranda, F.; Forcano, L.; et al. A Randomised Controlled Comparison of Second-Level Treatment Approaches for Treatment-Resistant Adults with Bulimia Nervosa and Binge Eating Disorder: Assessing the Benefits of Virtual Reality Cue Exposure Therapy. Eur. Eat. Disord. Rev. 2017, 25, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Clus, D.; Larsen, M.E.; Lemey, C.; Berrouiguet, S. The Use of Virtual Reality in Patients with Eating Disorders: Systematic Review. J. Med. Internet Res. 2018, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Spreij, L.A.; Visser-Meily, J.M.A.; Van Heugten, C.M.; Nijboer, T.C.W. Novel insights into the rehabilitation of memory post acquired brain injury: A systematic review. Front. Hum. Neurosci. 2014, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Valladares-Rodriguez, S.; Perez-Rodriguez, R.; Facal, D.; Fernandez-Iglesias, M.J.; Anido-Rifon, L.; Mouriño-Garcia, M. Design process and preliminary psychometric study of a video game to detect cognitive impairment in senior adults. PeerJ 2017, 5, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Schedlbauer, A.M.; Copara, M.S.; Watrous, A.J.; Ekstrom, A.D. Multiple interacting brain areas underlie successful spatiotemporal memory retrieval in humans. Sci. Rep. 2014, 4, 6431. [Google Scholar] [CrossRef]
- Carrieri, M.; Petracca, A.; Lancia, S.; Moro, S.B.; Brigadoi, S.; Spezialetti, M.; Ferrari, M.; Placidi, G.; Quaresima, V. Prefrontal Cortex Activation Upon a Demanding Virtual Hand-Controlled Task: A New Frontier for Neuroergonomics. Front. Hum. Neurosci. 2016, 10, 1–13. [Google Scholar] [CrossRef]
- Zanier, E.R.; Zoerle, T.; Di Lernia, D.; Riva, G. Virtual Reality for Traumatic Brain Injury. Front. Neurol. 2018, 9, 1–4. [Google Scholar] [CrossRef]
- Colombo, D.; Serino, S.; Tuena, C.; Pedroli, E.; Dakanalis, A.; Cipresso, P.; Riva, G. Egocentric and allocentric spatial reference frames in aging: A systematic review. Neurosci. Biobehav. Rev. 2017, 80, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Serino, S.; Riva, G. Getting lost in Alzheimer’s disease: A break in the mental frame syncing. Med. Hypotheses 2013, 80, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.L.; Fagan, A.M.; Morris, J.C.; Head, D. Spatial Navigation in Preclinical Alzheimer’s Disease. J. Alzheimers Dis. 2016, 52, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, E.; Perez-Lopez, M.B.; Diersch, N.; Döhler, J.; Wolbers, T.; Riemer, M. Embodiment in the aging mind. Neurosci. Biobehav. Rev. 2018, 86, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Lester, A.W.; Moffat, S.D.; Wiener, J.M.; Barnes, C.A.; Wolbers, T. The Aging Navigational System. Neuron 2017, 95, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- E Cullen, K.; Taube, J.S. Our sense of direction: Progress, controversies and challenges. Nat. Neurosci. 2017, 20, 1465–1473. [Google Scholar] [CrossRef]
- Bird, C.M.; Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 2008, 9, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.C.; Sholl, M.J. Allocentric Coding of Object-to-Object Relations in Overlearned and Novel Environments. J. Exp. Psychol. Learn. Mem. Cogn. 2005, 31, 1069–1087. [Google Scholar] [CrossRef]
- Hartley, T.; Lever, C.; Burgess, N.; O’Keefe, J. Space in the brain: How the hippocampal formation supports spatial cognition. Philos. Trans. R. Soc. B Biol. Sci. 2013, 369, 20120510. [Google Scholar] [CrossRef]
- Burgess, N. Spatial cognition and the brain. Ann. N. Y. Acad. Sci. 2008, 1124, 77–97. [Google Scholar] [CrossRef]
- D’Esposito, M.; Aguirre, G.K. Topographical disorientation: A synthesis and taxonomy. Brain 1999, 122, 1613–1628. [Google Scholar]
- Vann, S.D.; Aggleton, J.P.; Maguire, E.A. What does the retrosplenial cortex do? Nat. Rev. Neurosci. 2009, 10, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Doeller, C.F.; King, J.A.; Burgess, N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc. Natl. Acad. Sci. USA 2008, 105, 5915–5920. [Google Scholar] [CrossRef] [PubMed]
- Borrego, A.; Latorre, J.; Llorens, R.; Alcañiz, M.; Noé, E. Feasibility of a walking virtual reality system for rehabilitation: Objective and subjective parameters. J. Neuroeng. Rehabil. 2016, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Grp, P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement (Reprinted from Annals of Internal Medicine). Phys. Ther. 2009, 89, 873–880. [Google Scholar] [PubMed]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Version 5; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Pugnetti, L.; Mendozzi, L.; Brooks, B.; Attree, E.; Barbieri, E.; Alpini, D.; Motta, A.; Rose, D. Active versus passive exploration of virtual environments modulates spatial memory in MS patients: A yoked control study. Ital. J. Neurol. Sci. 1998, 19, S424–S430. [Google Scholar] [CrossRef]
- Akhutina, T.; Foreman, N.; Krichevets, A.; Matikka, L.; Närhi, V.; Pylaeva, N.; Vahakuopus, J. Improving spatial functioning in children with cerebral palsy using computerized and traditional game tasks. Disabil. Rehabil. 2003, 25, 1361–1371. [Google Scholar] [CrossRef]
- Caglio, M.; Latini-Corazzini, L.; D’Agata, F.; Cauda, F.; Sacco, K.; Monteverdi, S.; Zettin, M.; Duca, S.; Geminiani, G. Virtual navigation for memory rehabilitation in a traumatic brain injured patient. Neurocase (Psychology Press) 2012, 18, 123–131. [Google Scholar] [CrossRef]
- Kober, S.E.; Wood, G.; Hofer, D.; Kreuzig, W.; Kiefer, M.; Neuper, C. Virtual reality in neurologic rehabilitation of spatial disorientation. J. Neuroeng. Rehabil. 2013, 10, 1–13. [Google Scholar] [CrossRef]
- Grewe, P.; Lahr, D.; Kohsik, A.; Dyck, E.; Markowitsch, H.; Bien, C.; Botsch, M.; Piefke, M. Real-life memory and spatial navigation in patients with focal epilepsy: Ecological validity of a virtual reality supermarket task. Epilepsy Behav. 2014, 31, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Claessen, M.H.G.; van der Ham, I.J.M.; Jagersma, E.; Visser-Meily, J.M.A. Navigation strategy training using virtual reality in six chronic stroke patients: A novel and explorative approach to the rehabilitation of navigation impairment. Neuropsychol. Rehabil. 2016, 26, 822–846. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.L.; Andrade, A.; Soares, L.; I Badia, S.B. Benefits of virtual reality based cognitive rehabilitation through simulated activities of daily living: A randomized controlled trial with stroke patients. J. Neuroeng. Rehabil. 2016, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Moussavi, Z. Neuro-Cognitive Treatment for an Alzheimer’s Patient using a Virtual Reality Navigational Environment. J. Exp. Neurosci. 2016, 10, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Bate, S.; Adams, A.; Bennetts, R.; Line, H. Developmental prosopagnosia with concurrent topographical difficulties: A case report and virtual reality training programme. Neuropsychol. Rehabil. 2017, 29, 1–23. [Google Scholar] [CrossRef]
- De La Torre-Luque, A.; Valero-Aguayo, L.; De La Rubia-Cuestas, E.J. Visuospatial Orientation Learning through Virtual Reality for People with Severe Disability. Int. J. Disabil. Dev. Educ. 2017, 64, 420–435. [Google Scholar] [CrossRef]
- De Luca, R.; Buono, V.L.; Leo, A.; Russo, M.; Aragona, B.; Leonardi, S.; Buda, A.; Naro, A.; Calabrò, R.S. Use of virtual reality in improving poststroke neglect: Promising neuropsychological and neurophysiological findings from a case study. Appl. Neuropsychol. 2017, 26, 1–5. [Google Scholar] [CrossRef]
- De Luca, R.; Russo, M.; Naro, A.; Tomasello, P.; Leonardi, S.; Santamaria, F.; Desireè, L.; Bramanti, A.; Silvestri, G.; Bramanti, P.; et al. Effects of virtual reality-based training with BTs-Nirvana on functional recovery in stroke patients: Preliminary considerations. Int. J. Neurosci. 2018, 7454, 1–6. [Google Scholar] [CrossRef]
- Maresca, G.; Maggio, M.G.; Buda, A.; la Rosa, G. A novel use of virtual reality in the treatment of cognitive and motor de fi cit in spinal cord injury. Medicine (Baltimore) 2018, 97, e13559. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Di Santo, S.G.; Franchini, F.; Arlati, S.; Zangiacomi, A.; Greci, L.; Moretti, S.; Jesuthasan, N.; Marzorati, M.; Rizzo, G.; et al. Effects of Combined Physical and Cognitive Virtual Reality-Based Training on Cognitive Impairment and Oxidative Stress in MCI Patients: A Pilot Study. Front. Aging Neurosci. 2018, 10, 1–11. [Google Scholar] [CrossRef]
- Cipresso, P.; Serino, S.; Riva, G. Psychometric assessment and behavioral experiments using a free virtual reality platform and computational science. BMC Med. Inform. Decis. Mak. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Bockholt, U.; Fellner, D. Instantreality—A Framework for Industrial Augmented and Virtual Reality Applications. In Virtual Reality & Augmented Reality in Industry; Ma, D., Fan, X., Gausemeier, J., Grafe, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Brotons-Mas, J.R.; O’Mara, S.; Sanchez-Vives, M.V. Neural processing of spatial information: What we know about place cells and what know about place cells and what they can tell us about presence. Presence Teleoperators Virtual Environ. 2006, 15, 485–499. [Google Scholar] [CrossRef]
- De Luca, R.; Portaro, S.; Le Cause, M.; De Domenico, C.; Maggio, M.G.; Ferrera, M.C.; Giuffrè, G.; Bramanti, A.; Calabrò, R.S. Cognitive rehabilitation using immersive virtual reality at young age: A case report on traumatic brain injury. Appl. Neuropsychol. Child 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Darken, R.P.; Sibert, J.L. Navigating Large Virtual Spaces. Int. J. Hum-Comput. Interact. 1996, 8, 49–72. [Google Scholar] [CrossRef]
- Serino, S.; Mestre, D.; Mallet, P.; Pergandi, J.; Cipresso, P.; Riva, G. Don’t Get Lost in Translation: The Role of Egocentric Heading in Spatial Orientation; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Cogné, M.; Auriacombe, S.; Vasa, L.; Tison, F.; Klinger, É.; Sauzeon, H.; Joseph, P.-A.; N′kaoua, B. Are visual cues helpful for virtual spatial navigation and spatial memory in patients with mild cognitive impairment or Alzheimer’s disease? Neuropsychology 2018, 32, 385–400. [Google Scholar]
- Flores-Mateo, G.; Argimon, J.M. Evidence based practice in postgraduate healthcare education: A systematic review. BMC Health Serv. Res. 2007, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chrastil, E.R.; Warren, W.H. Active and passive contributions to spatial learning. Psychon. Bull. Rev. 2012, 19, 1–23. [Google Scholar] [CrossRef]
- Serino, S.; Riva, G. What is the role of spatial processing in the decline of episodic memory in Alzheimer’s disease? The ‘mental frame syncing’ hypothesis. Front. Aging Neurosci. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Senn, S. Cross-over Trials in Clinical Research; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2002. [Google Scholar]
- Loomis, J.M.; Blascovich, J.J.; Beall, A.C. Immersive virtual environment technology as a basic research tool in psychology. Behav. Res. Methods Instrum. Comput. 1999, 31, 557–564. [Google Scholar] [CrossRef]
- Gibson, J.J. The Theory of Affordances. In The Ecological Approach to Visual Perception; Routledge: Abingdon, UK, 1979. [Google Scholar]
- Meade, M.E.; Meade, J.G.; Fernandes, M.A. Active Navigation in Virtual Environments Benefits Spatial Memory in Older Adults. Brain Sci. 2019, 9, 47. [Google Scholar] [CrossRef]
- Maggio, M.G.; Maresca, G.; De Luca, R.; Stagnitti, M.C.; Porcari, B.; Ferrera, M.C.; Galletti, F.; Casella, C.; Manuli, A.; Calabrò, R.S. The Growing Use of Virtual Reality in Cognitive Rehabilitation: Fact, Fake or Vision? A Scoping Review. J. Natl. Med. Assoc. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Lan, X.; Zhou, Y.; Li, G.; Hsu, S.-H.; Jung, T.-P. The Study of Evaluation and Rehabilitation of Patients with Different Cognitive Impairment Phases Based on Virtual Reality and EEG. Front. Aging Neurosci. 2018, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | Sample (N) | Sample Characteristics | Mean Age (SD or Range) | VR Task | VR Apparatus | Pre- and Post- Assessment | Primary Outcomes | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pugnetti et al. [48] | 1998 | 30 | Experimental Group (EG) 15 MS patients. Control Group (CG) 15 healthy controls. | EG active condition mean age = 39.1; Standard Deviation (SD) =11.1/passive condition mean age = 37.7; SD = 8.1. CG active condition mean age = 35.8; SD = 9.41/passive condition mean age = 35.4; SD = 12.2 | The aim was to explore the VE of a house, composed of four rooms and corridors, in search of an object. | Nonimmersive Virtual Reality (Superscape Software, version 4). | ROF, CBTT, Raven’s matrices IQ. | Spatial memory improved in the active subject (MS and healthy) suggesting that direct interaction with the environment can enhance navigation ability. |
| 2 | Akhutina et al. [49] | 2003 | EXP 1. 21 EXP 2. 45 | EXP 1. EG/CG 21 patients with a diagnosis of cerebral palsy. EXP 2. EG/CG 45 patients with a diagnosis of cerebral palsy. | EXP 1. EG 12 (range 7–14) CG 9 (range 7–14) EXP 2. EG 23 (range 7–14) CG 22 (range 7–14) | The aim in each version of the task (drawn, real or virtual) was to move through a maze to reach a tree. | Non immersive environments IBM-PC and a mouse (Super Scape VRT 3-D Software) displayed on a 40,630 cm monitor. | EXP1. computer versions of the Koos Block Design Test, and a Clown Assembly Test. Decentration of Viewpoint Test, and Directional Pointing to a Hidden Object Test. EXP 2. Additional measures: Raven Progressive Matrices; The Benton Judgment of Line Orientation Test; The arrows subtest of the Nepsy; The Roads Test. | The studies have demonstrated that the general spatial abilities of a group of children with motor disabilities can be enhanced using a battery of training tasks that demand the use of various spatial skills. The battery included VEs that provided the children with navigational spatial experience, of a kind that most would rarely (if ever) experience in the course of their daily lives. |
| 3 | Caglio et al. [50] | 2012 | 1 | TBI patient with hemorrhagic contusions in the bilateral frontal, temporal and parietal lobes. | 24 (male) | The aim was to explore part of a virtual town (London) from a ground-level perspective. | Nonimmersive Virtual Reality (Midtown Madness 2 videogame). | Corsi Block-Tapping Test, Corsi Supra-Span Test, Backward digit span, RAVLT, TMT A-B, Phonemic fluency, ADAS, RBMT. | Improvement in immediate verbal learning, immediate and delayed spatial learning and everyday-spatial memory persisted at follow-ups. |
| 4 | Grewe et al. [20] | 2013 | 24 | EG 5 patients with focal epilepsy (2 right temporo-parietal; 1 right hippocampal; 1 bilateral temporal; 1 bilateral occipital periventricular). CG 19 healthy participants | EG mean age = 35.04; SD = 8.08; CG mean age = 23; SD = 3.45 | The aim was to navigate into a virtual medium-sized supermarket, modeled according to a real standard supermarket, in search of a specific list of objects. | OctaVis, semi-immersive Virtual Reality device. | ROF | The supermarket training provided preliminary evidence of effectiveness, but significant improvement was not found. A strong limitation was due to the small sample size. |
| 5 | Kober et al. [51] | 2013 | 23 | EG 23 patients: 3 right and 1 arteria cerebri media stroke, 1 basal ganglia and thalamus stroke, 1 right arteria cerebri media, 1 basal ganglia stroke, 1 right fronto-parietal stroke, 2 right aneurysm and subsequent infarct (arteria cerebri posterior and arteria communicans with parietal infarct), 1 arteria cerebri media hemorrhage, 1 TBI (left hippocampus and pons). CG 11 healthy participants | EG mean age = 66.09; SD = 3.30 CG mean age = 66.18; SD = 2.97 | The aim was a route-finding in a district of the real-world town of Graz, Austria. | Nonimmersive Virtual Reality. | Four spatial tests before and after the five VR training sessions: the Benton Test, the LPS 50+, the LVT, and the CBTT. | Route finding ability in the VR task increased over the five training sessions. Moreover, both groups improved different aspects of spatial abilities after VR training in comparison to the spatial performance before VR training. |
| 6 | Grewe et al. [52] | 2014 | 33 | EG 14 patients with focal epilepsy (frontal = 3, temporal = 8, central = 2, parietal = 1). CG 19 healthy participants | EG mean age = 31.29; SD = 9.44; 8 males. CG mean age = 31.21; SD = 14.26; 4 males | The aim was to navigate into a virtual medium-sized supermarket, modeled according to a real standard supermarket, in search of a specific list of objects. | OctaVis, semi-immersive Virtual Reality device. | BRLD-A, BRLD-B; ROCF copy, ROCF immediate and delayed recall; RWT Total Score; Digit Span Forward and Backward; VLMT immediate recallB, VLMT total learningB Trials, VLMT loss after InterferenceB, VLMT loss after delayB. | Spatial navigation and memory performance (n° of correct products, movements trajectories, time) significantly increased in the course of the 8-day training. Due to the small sample sizes in the subgroups, it could not be established the effects of different sites of epileptic foci. |
| 7 | Claessen et al. [53] | 2015 | 6 | 6 stroke patients with left (N = 3), right (N = 2) and bilateral (N = 1) supratentorial stroke. No control group. | mean age = 57; SD = 8.9; 2 males | The aim was a route-finding in the Virtual Tubingen town. | Nonimmersive Virtual Reality with a joystick (Virtual Tübingen). | CBTT, TMT A-B, WAIS-III, DART, Virtual Tübingen Test (Scene recognition, Route continuation/sequence/order/progression/distance, Pointing to start/to end, Map drawing/recognition). | Navigation abilities clearly improved in one patient, partially in four cases. For other cases, were successful in adopting an alternative navigation strategy and improved on most of the trained abilities. VR was judged as highly feasible by the patients. |
| 8 | Faria et al. [54] | 2016 | 18 | EG 9 stroke patients. CG 9 stroke patients | EG mean age = 58 – 71; male = 44%. CG mean age = 53; male = 44% | The aim was to navigate in order to accomplish some common ADL’s (in a supermarket, a post office, a bank, and a pharmacy) in a virtual city with streets, sidewalks, commercial buildings, parks and moving cars. | Nonimmersive Virtual Reality with a joystick (Reh@City). | ACE, TMT A-B, Picture Arrangement Test, SIS 3.0. | VR group improved in attention, visuospatial abilities, memory, executive functions, emotion, global cognition, and overall recovery. Between comparisons showed training effect on global cognition, executive functions and attention for VR group. |
| 9 | White & Moussavi [55] | 2016 | 1 | MCI patient with probable development of AD | 74 (male) | The aim was to navigate into a virtual building in search of specific targets. | Immersive Virtual Reality system with Head-mounted Display and joypad. | MoCA, VRN task (Byagowi & Moussavi, 2012), navigation diary. | The patient improved navigation during the sessions assessed with the VRN task and as reported with the wife’s diary. |
| 10 | Bate et al. [56] | 2017 | 1 | Patient with developmental prosopagnosia with concurrent topographical disorientation | 58 (female) | The aim was to navigate in a virtual city, (containing six landmarks such as cinema, restaurant, pub, hotel, pharmacy, and florist) and recall the position of each landmark on a top-view map of the city. | Nonimmersive Virtual Reality with the keypad. | WAIS-III, WMS-IV, Wisconsin Card Sorting Test, CBTT, Rey’s complex figures, Picture Naming, WTAR, VOSP. Face processing tasks: CFMT, famous faces, CFPT, Ekman 60, navigational assessment: Benton, Santa Barbara Sense of direction Scale, Memory of building, ‘O clock task, route map. | Following the last session of treatment, the patient was able to form a cognitive map faster than the first one and the performance in the retrieval task was improved. A similar performance was observed at the one-week follow-up session. |
| 11 | De La Torre - Luque et al. [57] | 2017 | 20 | 20 patients with a neurological diagnosis included cerebral palsy (20%), intellectual development disorder (20%) and both disorders (55%); TBI (5%). | mean age= 34.35, SD= 10.2; 13 males and 7 females. | The aim was to move through the virtual environment, and then through the equivalent real-life one and to find the same two rooms for both environments. | Semi-immersive Virtual Reality with a joystick and a mouse. A Mitsubishi® projector (model XL8U), projecting onto a × 1.5-m screen. | For the assessment of cognitive visuospatial planning and orientation, 2 tests: Porteus Maze Test; Mindscape’s Brain Trainer® 2 Maze Stair Test. | Both groups improved in a similar way, though we can say that the best. results in the virtual and the real building and generalization goals were due to virtual training. Firstly, a reduction in errors and time needed to locate the objectives in the virtual building was found after the training, so as to point out that the active navigational training showed changes. In addition, the participants had better scores in the posttest and generalization tasks in the real environment and when using maps of the building, and these tasks were not directly trained. |
| 12 | De Luca et al. [58] | 2017 | 1 | Neglect patient (subarachnoid hemorrhage, right fronto-temporal-parietal region). | 57 | The aim was to move in the virtual environment and manipulate specific objects, in order to realize specific associations. | Semi-immersive VR (BTs Nirvana PC System connected to a projector or a big screen). | MMSE, BIT; line crossing and bisection, letter and star cancellation, map navigation, card, and coin sorting, drawing and copying tests, phone dialing, menu and article reading, telling and setting the time. | The training enhanced spatial cognition, visual search, and attention. In addition, with standard cognitive treatment was observed a nearly complete recovery of Unilateral Spatial Neglect. |
| 13 | Serino et al. [21] | 2017 | 28 | EG 10 patients with AD. 8 healthy participants. CG 10 patients with AD | EG patients mean age = 86.60; SD = 6.13; 1male. healthy mean age = 86.62; SD = 6.19; 4 males. CG patients mean age = 88.7; SD = 3.59; 2 males | The aim was to navigate inside the virtual environment, to discover one, two or three hidden objects (i.e., a bottle of milk, a plant in a vase and a trunk) to retrieve their positions in the last phase. | Nonimmersive VR (NeuroVR software). | MMSE, Phonemic fluency, Categorical fluency, FAB, Attentional Matrices Test, Digit span test, Corsi Block-Tapping Test, Corsi Supra-Span Test. | The training enhanced spatial learning in the VR group-AD compared to control group-AD and VR healthy group improved executive functions compared to VR group-AD. |
| 14 | De Luca et al. [59] | 2018 | 12 | EG 6 post-stroke patients. CG 6 post-stroke patients | EG/CG mean age = 40; SD = 14 | The aim was to move in the virtual environment and manipulate specific objects, in order to realize specific associations. | Semi-immersive VR (BTs Nirvana PC System connected to a projector or a big screen). | MoCA, FIM, FAB, AM, TMT A, TMT B, TMT A/B. | VR can be useful in potentiating the cognitive recovery in post-stroke chronic phase. It improved visuospatial and attention in the experimental group. |
| 15 | Maresca et al. [60] | 2018 | 1 | A right-handed patient affected by incomplete cervical vertebro-spinal trauma, presented with a moderate tetraparesis, mainly involving the left side. | 60 (male) | The aim was to move in the virtual environment and manipulate specific objects, and to realize specific associations. | A nonimmersive virtual reality rehabilitation system (VRRS) by Khymeia, interacting with a touch screen or a magnetic tracking sensor. | MoCA, AM, TMT, digit span, RAVLI, RAVLR, Wigl’s sorting test, Raven’s colored matrices, VFT, SFT, HRS-D, HRS-A. | The combined approach using VRRS demonstrated a significant improvement in different cognitive domains as spatial abilities, executive functions, selective attention, and memory abilities. |
| 16 | Mrakic-Sposta et al. [61] | 2018 | 10 | EG 5 MCI patients with compromise visuospatial abilities. CG 5 MCI patients with compromise visuospatial abilities | EG/CG aged > 65 years; 4 males and 6 females | The aim was to navigate and to orientate inside three virtual environments (ride a bike in a park, crossroads in a city and shopping in a supermarket). | Semi-immersive scenarios with a finger touch projector and a PlayStation controller and cycle-ergometer, a Wearable smart garment (heart rate) | MMSE; RAVLT-I and RAVLT-D; ROCFT; AM; TMT-A and TMT-B; FAB; VFT. | The presented results suggest that the adopted training protocol was able to affect MMSE tasks and to increase the global cognition levels of MCI patients. |
| Authors | Type of Training | Single Session Duration (min) | Repetitions | Frequency/Period | Total Hours | |
|---|---|---|---|---|---|---|
| 1 | Pugnetti et al. (1998) | Navigational training with active and passive conditions + recall landmarks | 30 | 1 | 30 min | |
| 2 | Akhutina et al. (2003) | Navigational task | 30–60 | 6–8 | within a month | 3–8 h |
| 3 | Caglio et al. (2012) | Navigational training | 90 | 15 | 3 times a week for 5 weeks | 22.5 h |
| 4 | Grewe et al. (2013) | Navigational training + free recall of objects list and positions (at last session) | 20 | 8 | daily | 2.6 h |
| 5 | Kober et al. (2013) | Navigational training + recall up to maximal three different routes | 20 | 6 | - | 2 h |
| 6 | Grewe et al. | Navigational training + free recall of objects list and positions (at last session) + real-life performance | 30 | 8 | Every 1–3 days within 2 weeks | 4 h |
| 7 | Claessen et al. (2015) | Navigational training | 60 | 4 | - | 4 h |
| 8 | Faria et al. (2016) | Navigational training | 20 | 12 | 4–6 weeks | 4 h |
| 9 | White & Moussavi (2016) | Navigational training | 45 | 21 | 3 times a week for 7 weeks | 15.75 h |
| 10 | Bate et al. (2017) | Navigational training + recall landmarks | 60-70 | 7 | Every 3–4 days | 7–8 h |
| 11 | De La Torre Luque et al. (2017) | Navigational training | 20 | 15 | daily | 5 h |
| 12 | De Luca et al. (2017) | Navigational training + association of object position | 45 | 20 | 5 times a week for 1 month | 15 h |
| 13 | Serino et al. (2017) | Navigational training + recall object positions | 30 | 10 | 3 times a week for 3–4 weeks | 5 h |
| 14 | De Luca et al. (2018) | Navigational training + association of object position | 45 | 24 | 3 times a week for 8 weeks | 18 h |
| 15 | Maresca et al. (2018) | Navigational training | 60 | 36 | 3 times a week for 12 weeks | 36 h |
| 16 | Mrakic et al (2018) | Navigational training | 45 | 18 | 3 times a week for 6 weeks | 13.5 h |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montana, J.I.; Tuena, C.; Serino, S.; Cipresso, P.; Riva, G. Neurorehabilitation of Spatial Memory Using Virtual Environments: A Systematic Review. J. Clin. Med. 2019, 8, 1516. https://doi.org/10.3390/jcm8101516
Montana JI, Tuena C, Serino S, Cipresso P, Riva G. Neurorehabilitation of Spatial Memory Using Virtual Environments: A Systematic Review. Journal of Clinical Medicine. 2019; 8(10):1516. https://doi.org/10.3390/jcm8101516
Chicago/Turabian StyleMontana, Jessica Isbely, Cosimo Tuena, Silvia Serino, Pietro Cipresso, and Giuseppe Riva. 2019. "Neurorehabilitation of Spatial Memory Using Virtual Environments: A Systematic Review" Journal of Clinical Medicine 8, no. 10: 1516. https://doi.org/10.3390/jcm8101516
APA StyleMontana, J. I., Tuena, C., Serino, S., Cipresso, P., & Riva, G. (2019). Neurorehabilitation of Spatial Memory Using Virtual Environments: A Systematic Review. Journal of Clinical Medicine, 8(10), 1516. https://doi.org/10.3390/jcm8101516







