Abstract
Background/Objectives: Several studies describe the sex-specific differences in cardiovascular diseases. However, there is still limited research reporting the difference between men and women with chronic thromboembolic pulmonary hypertension (CTEPH) treated with balloon pulmonary angioplasty (BPA). The aim of this study was to evaluate sex-specific differences in patients with CTEPH treated with BPA. Methods: This retrospective study included CTEPH patients treated with BPA. The patients’ hemodynamic and clinical parameters were assessed at baseline and 3 months after completion of BPA treatment. Results: This study included 94 patients (44 women, 46.8%). At baseline, women had higher systolic pulmonary arterial pressure (sPAP) (76 ± 18.5 vs. 85 ± 17.6 mmHg; p = 0.03) and pulmonary vascular resistance (8.21 [5.55–10.17] vs. 9.89 [6.31–14.06] Wood Units; p = 0.03) compared to men. There were no differences in clinical characteristics between the sexes. At follow-up, women had lower sPAP (49 [41–54] vs. 43 [37–49] mmHg; p = 0.04) and pulmonary capillary wedge pressure (10 [9–14] vs. 9 [8–11] mmHg; p = 0.03), but a higher cardiac index (2.57 ± 0.53 vs. 2.82 ± 0.50 L/min/m2; p = 0.03), as well as better Dyspnea Borg Scale outcomes, compared to men. Women had a greater reduction in mean pulmonary artery pressure (−43% vs. −37%; p = 0.049) than men. Conclusions: At baseline, women with CTEPH had worse hemodynamic parameters than men despite similar clinical symptoms. However, the hemodynamic status of women was better after BPA therapy. Hence, women seem better adapted to the disease at baseline and respond better to BPA. Further data are needed to investigate whether the management of CTEPH patients should be sex-differentiated.
1. Introduction
Cardiovascular diseases (CVDs) remain the leading cause of death among women and men worldwide [1]. In most clinical trials in cardiology, men dominate, and women are underrepresented. However, in the case of various forms of pulmonary hypertension (PH), the opposite is true: women predominate quantitatively among the participants in clinical trials [2,3,4,5]. This is due to the fact that women are more susceptible than men to several forms of PH [6,7].
Chronic thromboembolic pulmonary hypertension (CTEPH) is a condition defined by elevated pressure in the pulmonary vascular bed caused by partial occlusion of the pulmonary arteries due to organized persistent thrombi, often accompanied by the remodeling of patent resistive pulmonary arterioles [8]. Based on registry data, the prevalence of CTEPH is approximately 25.8–38.4 per million [9,10,11]. Balloon pulmonary angioplasty (BPA) is a minimally invasive procedure that has become an effective treatment option for CTEPH patients who are ineligible for pulmonary endarterectomy (PEA) or present with persistent PH after surgery [8,12,13,14].
Sex-specific differences in CTEPH have been studied, with some evidence suggesting that women may be at higher risk for developing CTEPH [15]. Some studies suggest that there may be differences in the clinical presentation, risk factors, and outcomes of CTEPH treated by PEA between males and females [16]. However, there is limited research on the differences between women and men in CTEPH treated with BPA.
Therefore, the aim of this study was to evaluate sex-specific differences in patients with CTEPH who were treated with BPA procedures.
2. Materials and Methods
2.1. Study Design and Settings
This retrospective study included patients consulted by the CTEPH team in the Department of Pulmonary Circulation, Thromboembolic Diseases, and Cardiology between October 2011 and September 2020. Medical history and clinical data were obtained retrospectively from the patients’ medical records. As this was a retrospective study, patients were routinely diagnosed and treated, and no additional interventions were performed. A positive opinion from the Bioethics Committee was obtained (L.dz.OIL/KBL/27/2018).
Within routine patient management, the CTEPH team provided multi-specialist consultations, established the CTEPH diagnosis based on invasive measurement of hemodynamic parameters during right heart catheterization (RHC) [17] and results of imaging studies, and then referred patients to appropriate treatment methods: PEA, BPA, medical therapy, or combined therapy involving more than one of the mentioned methods according to current guidelines [8,18,19,20].
Data regarding the patients’ characteristics were evaluated including age, anthropometric data, comorbidities, anticoagulant and supporting treatment, and reasons for rejection from PEA. Hemodynamic data were obtained during RHC performed according to the current guidelines [17]. In addition, the functional class defined by the World Health Organization was noted, and results of the 6 min walking test (6-MWT) and Borg Dyspnea Scale ratings after 6-MWT were collected. Laboratory tests were performed including the levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP)—reference value <125 pg/mL, high-sensitive troponin T—reference value <0.014 ng/mL, and creatinine—reference value <0.9 mg/dl for women and <1.20 mg/dl for men.
The patients’ data were analyzed at two points of time: at baseline and at follow-up. “Baseline” was defined as the moment of RHC performed before the first BPA procedure. “Follow-up” tests were performed three to six months after the last BPA procedure.
2.2. Statistical Analysis
Statistical analysis was performed with Statistica PL software (version 13, StatSoft, Tulsa, OK, USA). Categorical variables were presented as numbers and percentages. Continuous variables were presented as mean and standard deviation or median with interquartile range, depending on the distribution of the analyzed variable assessed using the Shapiro–Wilk test. The t-test was performed for data with a normal distribution, while the Mann–Whitney U-test was performed for data that did not follow a normal distribution. These tests were used to compare quantitative variables between groups. The Chi-square test for categorized variables was used to determine the differences between groups. For categorical variables with more than two categories, the Chi-square test was also used. Statistical significance in this study was established at p < 0.05.
3. Results
3.1. Patients
Of the 417 consulted patients, 128 were included in this study (Figure 1). In 21 patients, subsequent BPA sessions were planned; in 11 cases, due to the patient’s mortality, the BPA series was unfinished, and follow-up was not performed. Contact loss occurred in two cases before the treatment with BPA was completed. Hence, the final population included 94 patients who terminated BPA treatment and had follow-up tests performed after the last BPA session. Among them, there were 44 (46.8%) females and 50 (53.2%) males.
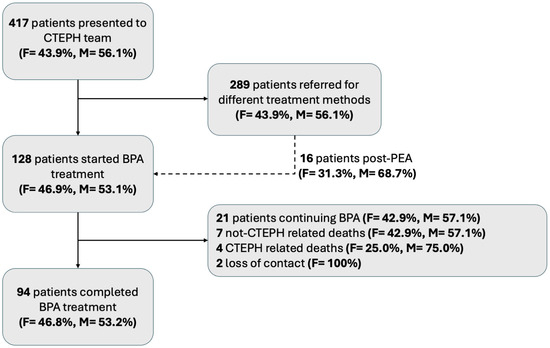
Figure 1.
Flow chart of patient selection for this study. BPA—balloon pulmonary angioplasty; CTEPH—chronic thromboembolic pulmonary hypertension; F—female, M—male.
3.2. Evaluation at Baseline
Table 1 summarizes the baseline characteristics and clinical data stratified by gender. The women were nominally younger than the men (median age 54 (47–70) years vs. 66 (54–73) years), though the difference did not reach statistical significance (p = 0.07). The men were significantly taller, heavier, and had a greater body surface area, consistent with general population trends, while BMI was similar between the genders (p = 0.19).

Table 1.
Characteristics of patients at baseline.
Regarding comorbidities, chronic obstructive pulmonary disease (COPD) was observed exclusively in the men (26%), a finding that aligns with known gender differences in COPD prevalence and may contribute to differences in disease presentation and response to treatment. Other comorbidities showed no significant differences between the groups.
Anticoagulant treatment patterns were also similar, with the majority of both the women (54.6%) and men (54.0%) using DOACs, reflecting their widespread adoption in CTEPH management and alignment with current treatment guidelines. There were no significant differences in the use of specific pulmonary hypertension (PH) therapies, with sildenafil or riociguat being used by over 78% of both groups. The reasons for rejection from pulmonary endarterectomy (PEA) were comparable, with distal pulmonary vascular obstruction being the most common cause in both the women (63.6%) and men (52.0%).
Most of the women and men presented symptoms of III and IV WHO functional classes at baseline. There was no difference between the women and men regarding the severity of dyspnea assessed with the Borg Scale after 6-MWT. The men presented higher serum levels of creatinine (Table 2).

Table 2.
Patients’ clinical status at baseline.
Hemodynamic measurements at baseline (Table 3) showed that the women had higher values of systolic pulmonary arterial pressure (sPAP) and pulmonary vascular resistance (PVR). In turn, the men had higher stroke volume (SV).

Table 3.
Patients’ hemodynamic status at baseline.
3.3. Evaluation at Follow-Up
After BPA procedures, there were no differences in the number of sessions (median: 5; IQR: 4–7), the number of treated vessels during one session (mean: 7, SD 2.5), or the required amount of contrast (mean: 253 mL, SD 42.8) and radiation (median: 146 mGy; IQR: 90–248) between the men and women. There were no differences in WHO functional classes of CTEPH regarding gender with most females and males presenting WHO class I or II after the BPA treatment was finished. Serum levels of troponin and creatinine were significantly higher in the men than in the women. Evaluation with the Dyspnea Borg Scale after 6-MWT revealed some differences between the sexes—94.9% of the women reported none or mild dyspnea while 20.4% of the men reported moderate to rather/very intense dyspnea (Table 4).

Table 4.
Patients’ clinical outcomes at follow-up.
The post-treatment RHC data are presented in Table 5. The men had higher sPAP compared to the women, as well as pulmonary arterial wedge pressure. In turn, the women had a higher mean value of the cardiac index.

Table 5.
Patients’ hemodynamic outcomes at follow-up and changes in some variables from baseline to follow-up.
3.4. Treatment Outcomes
Analyzing the results of the CTEPH treatment with BPA, the classification of the patients according to WHO class improved. The proportions of both the women and men being classes I-II and III-IV were reversed at follow-up when compared with the baseline outcomes (Figure 2).
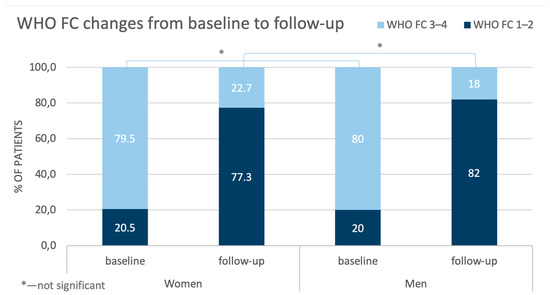
Figure 2.
Comparison of changes in WHO FC in women and men from baseline to follow-up. WHO FC—World Health Organization functional class.
Also, the outcomes of the Borg Dyspnea Scale improved, but the benefits were more pronounced for the women than for the men (Figure 3).
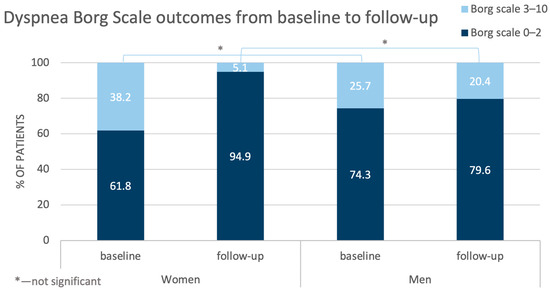
Figure 3.
Comparison of changes in the outcomes of the Dyspnea Borg Scale in the women and men from baseline to follow-up.
Additionally, a detailed analysis of RHC results at baseline and at follow-up was performed. The percentage changes in the hemodynamic variables are graphically presented in Figure 4.
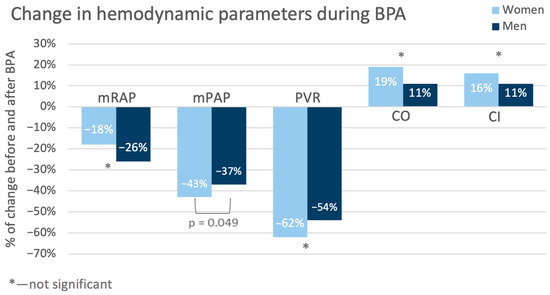
Figure 4.
Changes in the values of hemodynamic variables during treatment (from baseline to follow-up). CI—cardiac index; CO—cardiac output; mPAP—mean pulmonary artery pressure; mRAP—mean right atrial pressure; PVR—pulmonary vascular resistance.
The values (both nominal and percentage) of mean pulmonary arterial pressure (mPAP) in the women decreased more than in the men (−20.9 mmHg vs. −15.8 mmHg, p = 0.04; −43% vs. −37%, p = 0.049). Also, pulmonary vascular resistance (PVR) in the women was nominally lower than in the men. But this observation pertained only to the nominal values (−6.64 j.W. vs. −3.85 j.W. p = 0.048; −62% vs. −54%, p = 0.12). Detailed hemodynamic variable changes are presented in Table 5.
4. Discussion
CTEPH is a rare condition, but it can lead to right heart failure, multiorgan disfunction, and death if left untreated. The BPA therapy that has been used relatively recently in the treatment of patients with CTEPH [21,22] has been upgraded in the recent guidelines for CTEPH treatment, which currently recommend BPA as a part of a multimodal approach for patients who have inoperable lesions or have residual PH after PEA and distal obstructions amenable to BPA (class of recommendation IB). This procedure may also be applied to those patients who are operable but have a high proportion of distal disease, where a PEA procedure may generate a high risk for them. BPA can also be considered in some symptomatic patients with CTEPD without PH [8].
The differences observed in patients with CTEPH treated with BPA may vary significantly across populations due to genetic, environmental, and healthcare access factors. For example, in Japan, a notably higher proportion of female patients (75%) undergoing BPA has been reported, in contrast to the more balanced gender distributions observed in other regions [23]. Similarly, a registry study covering countries such as Russia, Kazakhstan, Turkey, Lebanon, and Saudi Arabia found that most patients presented with advanced disease at baseline, indicating potential delays in diagnosis and differences in disease progression compared to European cohorts [24]. These findings underscore the influence of regional variations, including genetic predispositions, environmental factors, and healthcare practices, on patient demographics and treatment outcomes. Consequently, while our study provides valuable insights into a European cohort, caution is warranted when extrapolating these findings to non-European populations, as local factors may substantially impact disease presentation and management outcomes.
A recent study that analyzed the preoperative computed tomography pulmonary angiography of patients who underwent PEA for CTEPH identified sex-specific differences in surgical cases [25]. Men had more vessels involved than women (mean 20.3 vs. 17.1, p = 0.004) and had fewer disease-free pulmonary segments (mean 4.9, SD 4.3 vs. 7.6, SD 5.5, p = 0.001). In addition, men had a greater number of webs, eccentric thickening, and occlusions. The distribution of lesion type did not significantly differ between the sexes at the main or lobar level, but men had significantly more lesions in the segmental vasculature while women had a higher proportion of subsegmental lesions (p < 0.001) despite no significant differences in baseline hemodynamics [25]. Although it was described that after PEA, women benefit less from the reduction in PVR (437 Dynes∙s∙cm−5 vs. 324 Dynes∙s∙cm−5 in males, p < 0.01), the overall 10-year survival after surgical treatment was similar (73% in females vs. 84% in males, p = 0.08) [26]. In the multivariate analysis, the female sex remained as an independent factor affecting the need for targeted PH medical therapy after PEA (HR 2.03, 95%CI 1.03–3.98, p = 0.04), which suggests other mechanisms may be responsible for the worse response to surgical treatment in females with proximal disease [26]. In addition, another study analyzed gender-dependent differences in CTEPH patients treated with PEA from a European registry [16]. The study showed that women had better survival rates than men regardless of whether they underwent PEA or not. However, data on other treatment modalities for CTEPH are also limited.
There is a lack of data on whether sex affects BPA results. Therefore, we aimed to evaluate whether differences exist between men and women with CTEPH and to evaluate if the outcomes of treatment with BPA differ regarding sex/gender. Women are known to be more susceptible to PH than men and, therefore, usually among patients with CTEPH, but on the other hand, women also have better survival [16,27,28]. In the case of CTEPH, the latest publication based on the European registry indicated an equal ratio of affected women and men [16], similar to the US registry [29]. The registry developed by the International CTEPH Association reported a general dominance of women (52.4%); however, in Europe, they made up slightly less than half (48.7%) of the patients with CTEPH [23]. Similar trends are corroborated by data from BPA registries, which consistently highlight variations in gender representation across different cohorts. The highest proportion of female participants was observed in the Brazilian registry, where women accounted for 87% [30] of the cohort. Similarly, in the Japanese registry, women constituted 80% of the participants [19], demonstrating a significant female predominance in this population. In contrast, in European BPA registries, the proportion of women ranged from 49% to 61% [14,31,32] (Figure 5). These differences may reflect regional variations in disease prevalence or referral patterns.
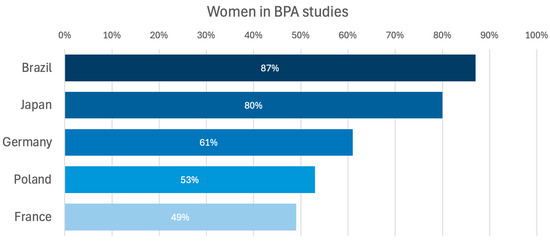
Figure 5.
Percentage of women represented in BPA studies [14,19,30,31,32]. BPA—balloon pulmonary angioplasty.
The European registry indicated that women had a lower prevalence of some CV risk factors than men, such as previous acute coronary syndrome, smoking, and COPD, but they more often suffered from obesity and had cancer or thyroid disease history [16]. The women and men included in our study did not differ in terms of comorbidities, except for the occurrence of COPD, which was much less frequently diagnosed in the women (26% vs. 0%). This disparity could introduce a bias in the assessment of dyspnea severity and other parameters both pre- and post-BPA. COPD is known to contribute to Group 3 PH, which may further complicate the interpretation of the observed differences between the sexes. While these findings highlight the complexity of pulmonary hemodynamics in patients with concomitant conditions, they also underscore the need for careful consideration of such confounding factors in future research.
As CTEPH is strongly associated with the occurrence of pulmonary embolism and an incomplete thrombus resolution [33], sex/gender differences related to coagulation should be also considered. Typical thrombogenic factors were not proven to increase in patients with CTEPH in contrast to plasma factor VIII [34,35]. This factor physiologically has higher values in women than in men [36], which may predispose women to developing CTEPH. In the Japanese BPA Registry, females represented 80% of the included patients, and previous episodes of acute pulmonary embolism and deep vein thrombosis were not frequent (15.3% and 43.7%, respectively) [19], as compared with reports from other Western countries (74.8% and 58.1%, respectively, in a European registry) [37].
Assessing the patients’ status at baseline, we found that the women tended to have worse hemodynamics values than the men, as indicated by higher sPAP and PVR and by lower SV. Creatinine levels were slightly higher in the men, but this probably resulted from the physiologically higher muscle mass in the men. In turn, clinical presentation in the women and men was quite similar—there were no significant differences between sex/gender in the results of the Dyspnea Borg Scale or in the distance walked during 6-MWT. Our population also did not differ in terms of WHO FC, and most patients were diagnosed with WHO FC class 3. This observation is consistent with the results described in other studies [23,28,38] and also with observations from the European CTEPH registry [16], indicating that women slightly more often have functional capacity class III/IV diagnosed than men. More severe courses of the disease in women than men with CTEPH were also reported by Wu et al. [39].
More severe baseline hemodynamic parameters in women compared with men together with similar clinical symptomatology suggest that at diagnosis, women are better adapted to the disease than men. On the other hand, it may be hypothesized that women will require more BPA sessions to achieve similar improvements in hemodynamics as men.
There are no clearly defined therapeutic targets for BPA treatment in patients with CTEPH. The guidelines suggest (i) achieving a good functional class (WHO-FC I–II), (ii) improving hemodynamic parameters, and (iii) enhancing quality of life [8]. However, some recent data from the ESC Working Group Statement defined a hemodynamic BPA treatment goal to achieve final mPAP < 30 mmHg [12]. Comparing the effects of BPA from two multicenter registries (Japanese and Polish) and from single-expert centers (German and French), it was demonstrated that only Japanese patients were able to reach the defined BPA treatment goal of mPAP below 30 mmHg [40]. This may be due to the intrinsic differences between European and Japanese patients, with European CTEPH patients having higher serum concentrations of C-reactive protein, fibrinogen, and myeloperoxidase and more red thrombus than Japanese CTEPH patients [41]. However, the high-volume representation of women in the Japanese registry may be also suggestive of gender-related outcomes in BPA treatment.
The control tests performed in the studied population after completion of BPA treatment revealed better hemodynamic status (especially regarding mSAP, sPAP, PCWP, and CI) in the women than in the men, although at baseline, the situation was the opposite. The detailed analysis demonstrated that after treatment, many parameters changed more in the women than in the men, with decreases in mPAP and PVR being the most pronounced. These were reflected particularly in the outcomes of the Dyspnea Borg Scale—compared with baseline at follow-up, in about 30% of the women, the ratings shifted towards points 0–2, and such an improvement was observed only in about 5% of the men. This difference did not achieve statistical significance in the results of the 6-MWT.
Our results are even more interesting considering that there were no differences in the number of sessions, the number of treated vessels, or the required amount of contrast and radiation between the men and women. This may suggest that women respond better to CTEPH treatment with BPA than men.
The above observation seems to be in line with the previously reported better long-term survival in women compared with men. This phenomenon is suggested to be related to better right ventricular function in females than in males [42,43]. Unfortunately, echocardiographic data were unavailable in our study; hence, we were unable to assess right ventricular functions in our population. However, hemodynamic differences exist between different types of CTEPH with a worse condition in the central than the peripheral form of the disease [44]. In addition, in some studies, women tended to deteriorate more than males during follow-up [45].
Based on our results, we believe that the treatment strategy for CTEPH should be more tailored to specific patient characteristics, including sex. While the women in our cohort seem to exhibit more favorable outcomes with BPA, such as better hemodynamic responses, it is important to acknowledge that men may require a more individualized approach to achieve similar results. This could involve optimizing the BPA technique with more intensive and frequent treatment sessions to improve their outcomes. However, it is crucial to note that the current evidence is preliminary, and more data are needed to determine whether sex-specific treatment guidelines should be prioritized. Additionally, while some studies suggest better long-term survival in women, further investigation into survival time, symptom-free periods, and mortality is required to draw definitive conclusions on the need for earlier intervention for women. Consequently, clinical decision-making should be guided by a comprehensive assessment of each patient’s unique characteristics, and not solely on sex differences at this stage.
Strengths and Limitations of This Study
To our knowledge, this is the first very detailed study analyzing the impact of patients’ sex/gender on the results of BPA therapy in patients with CTEPH. As it was a retrospective (single center, small group) study, we were able to provide valuable data from real clinical practice. However, some assessments are missing, which is an inherent limitation to this type of research. The retrospective design of this study made it possible to rely exclusively on existing data, and the intervention was not blinded at any stage.
5. Conclusions
Although many studies demonstrated that BPA improves both the hemodynamic parameters and clinical status of patients with CTEPH [46,47], sex/gender-specific treatment results are unknown. Also, the current guidelines do not promote any sex/gender-specific approach, which implies that the diagnostic–therapeutic process is the same in women and men. Therefore, our data may be used to initiate further studies analyzing different scenarios of clinical management depending on sex/gender. We observed that at diagnosis, women had more severe hemodynamic parameters than men, which were accompanied by similar clinical symptomatology in both sexes. Despite this, women’s hemodynamic status was better after BPA therapy. The above outcomes may suggest that at diagnosis, women are better adapted to the disease than men, and women also respond better to BPA treatment. However, we are aware that these data are just preliminary, and research evidence from randomized controlled trials is needed to demonstrate whether the management of a patient with CTEPH should be sex-differentiated.
Author Contributions
Conceptualization, M.K., A.T. and S.D.; methodology, S.D.; validation, M.K. and M.F.; formal analysis, P.K. (Paweł Kurzyna); investigation, P.K. (Paweł Kurzyna) and A.W.; data curation, P.K. (Paweł Kurzyna); writing—original draft preparation, A.W. and P.K. (Paweł Kurzyna); writing—review and editing, M.K., A.T., S.D., P.K. (Piotr Kędzierski), P.S., M.P., M.B., A.G. and A.P.; visualization, P.K. (Paweł Kurzyna) and A.W.; supervision, M.K. and S.D.; project administration, P.K. (Paweł Kurzyna) and S.D.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by funds from the statutory activity of the Centre of Postgraduate Medical Education in Warsaw, Poland (grant number 501-1-054-25-25).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee (number: 79/PB-A/2015) issued by the Centre of Postgraduate Medical Education. Date obtained: 14/10/2015.
Informed Consent Statement
Patient consent was waived due to the retrospective character of this study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Proper Medical Writing Sp. z o. o. for their support in the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BPA | balloon pulmonary angioplasty |
| CTEPH | chronic thrombo-embolic pulmonary hypertension |
| CVD | cardiovascular diseases |
| RHC | right heart catherization |
| PEA | pulmonary endarterectomy |
| 6-MWT | 6 min walking test |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| M | male |
| F | female |
| BMI | body mass index |
| DOAC | direct oral anticoagulants |
| PH | pulmonary hypertension |
| SD | standard deviation |
| VKA | vitamin K antagonists |
| LMWH | low-molecular-weight heparin |
| WHO FC | World Health Organization functional class |
| mRAP | mean right atrial pressure |
| sPAP | systolic pulmonary arterial pressure |
| dPAP | diastolic pulmonary arterial pressure |
| mPAP | mean pulmonary artery pressure |
| PCWP | pulmonary capillary wedge pressure |
| CI | cardiac index |
| SV | stroke volume |
| SVI | stroke volume index |
| PVR | pulmonary vascular resistance |
| IQR | interquartile range |
| COPD | chronic obstructive pulmonary disease |
References
- Mensah, G.A.; Fuster, V. Sex and Gender Differences in Cardiovascular Health. J. Am. Coll. Cardiol. 2022, 79, 1385–1387. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.-A.; Galiè, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z.-C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.S.; Blair, C.; Oudiz, R.J.; Dufton, C.; Olschewski, H.; Despain, D.; Gillies, H.; Kawut, S.M. Baseline and Follow-up 6-Min Walk Distance and Brain Natriuretic Peptide Predict 2-Year Mortality in Pulmonary Arterial Hypertension. Chest 2013, 143, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; D’Armini, A.M.; Grimminger, F.; Grünig, E.; Hoeper, M.M.; Jansa, P.; Mayer, E.; Neurohr, C.; Simonneau, G.; Torbicki, A.; et al. Haemodynamic Effects of Riociguat in Inoperable/Recurrent Chronic Thromboembolic Pulmonary Hypertension. Heart 2017, 103, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.D.; Behr, J.; Collard, H.R.; Cottin, V.; Hoeper, M.M.; Martinez, F.J.; Corte, T.J.; Keogh, A.M.; Leuchte, H.; Mogulkoc, N.; et al. Riociguat for Idiopathic Interstitial Pneumonia-Associated Pulmonary Hypertension (RISE-IIP): A Randomised, Placebo-Controlled Phase 2b Study. Lancet Respir. Med. 2019, 7, 780–790. [Google Scholar] [CrossRef]
- Pugh, M.E.; Hemnes, A.R. Pulmonary Hypertension in Women. Expert. Rev. Cardiovasc. Ther. 2010, 8, 1549–1558. [Google Scholar] [CrossRef]
- Mair, K.M.; Johansen, A.K.Z.; Wright, A.F.; Wallace, E.; Maclean, M.R. Pulmonary Arterial Hypertension: Basis of Sex Differences in Incidence and Treatment Response. Br. J. Pharmacol. 2014, 171, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Leber, L.; Beaudet, A.; Muller, A. Epidemiology of Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension: Identification of the Most Accurate Estimates from a Systematic Literature Review. Pulm. Circ. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Delcroix, M.; Torbicki, A.; Gopalan, D.; Sitbon, O.; Klok, F.A.; Lang, I.; Jenkins, D.; Kim, N.H.; Humbert, M.; Jais, X.; et al. ERS Statement on Chronic Thromboembolic Pulmonary Hypertension. Eur. Respir. J. 2021, 57, 2002828. [Google Scholar] [CrossRef] [PubMed]
- Kramm, T.; Wilkens, H.; Fuge, J.; Schäfers, H.J.; Guth, S.; Wiedenroth, C.B.; Weingard, B.; Huscher, D.; Pittrow, D.; Cebotari, S.; et al. Incidence and Characteristics of Chronic Thromboembolic Pulmonary Hypertension in Germany. Clin. Res. Cardiol. 2018, 107, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Andreassen, A.K.; Andersen, A.; Bouvaist, H.; Coghlan, G.; Escribano-Subias, P.; Jansa, P.; Kopec, G.; Kurzyna, M.; Matsubara, H.; et al. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: A Clinical Consensus Statement of the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. Eur. Heart J. 2023, 44, 2659–2671. [Google Scholar] [CrossRef] [PubMed]
- Araszkiewicz, A.; Darocha, S.; Pietrasik, A.; Pietura, R.; Jankiewicz, S.; Banaszkiewicz, M.; Sławek-Szmyt, S.; Biederman, A.; Mularek-Kubzdela, T.; Lesiak, M.; et al. Balloon Pulmonary Angioplasty for the Treatment of Residual or Recurrent Pulmonary Hypertension after Pulmonary Endarterectomy. Int. J. Cardiol. 2019, 278, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Darocha, S.; Pietura, R.; Pietrasik, A.; Norwa, J.; Dobosiewicz, A.; Piłka, M.; Florczyk, M.; Biederman, A.; Torbicki, A.; Kurzyna, M. Improvement in Quality of Life and Hemodynamics in Chronic Thromboembolic Pulmonary Hypertension Treated With Balloon Pulmonary Angioplasty. Circ. J. 2017, 81, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.; Wallenhorst, C.; Teal, S.; Cohen, A.T.; Peacock, A.J. Incidence and Risk Factors of Chronic Thromboembolic Pulmonary Hypertension Following Venous Thromboembolism, a Population-Based Cohort Study in England. Pulm. Circ. 2018, 8, 2045894018791358. [Google Scholar] [CrossRef]
- Barco, S.; Klok, F.A.; Konstantinides, S.V.; Dartevelle, P.; Fadel, E.; Jenkins, D.; Kim, N.H.; Madani, M.; Matsubara, H.; Mayer, E.; et al. Sex-Specific Differences in Chronic Thromboembolic Pulmonary Hypertension. Results from the European CTEPH Registry. J. Thromb. Haemost. 2020, 18, 151–161. [Google Scholar] [CrossRef]
- Kurzyna, M.; Araszkiewicz, A.; Błaszczak, P.; Grabka, M.; Hawranek, M.; Kopec, G.; Mroczek, E.; Zembala, M.; Torbicki, A.; Ochała, A. Summary of Recommendations for the Haemodynamic and Angiographic Assessment of the Pulmonary Circulation. Joint Statement of the Polish Cardiac Society’s Working Group on Pulmonary Circulation and Association of Cardiovascular Interventions. Pol. Heart J. (Kardiol. Pol.) 2015, 73, 63–68. [Google Scholar] [CrossRef]
- Siennicka, A.; Darocha, S.; Banaszkiewicz, M.; Kędzierski, P.; Dobosiewicz, A.; Błaszczak, P.; Peregud-Pogorzelska, M.; Kasprzak, J.D.; Tomaszewski, M.; Mroczek, E.; et al. Treatment of Chronic Thromboembolic Pulmonary Hypertension in a Multidisciplinary Team. Ther. Adv. Respir. Dis. 2019, 13, 1753466619891529. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Satoh, T.; Fukuda, T.; Sugimura, K.; Fukumoto, Y.; Emoto, N.; Yamada, N.; Yao, A.; Ando, M.; Ogino, H.; et al. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: Results of a Multicenter Registry. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e004029. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Ogawa, A.; Munemasa, M.; Mikouchi, H.; Ito, H.; Matsubara, H. Refined Balloon Pulmonary Angioplasty for Inoperable Patients with Chronic Thromboembolic Pulmonary Hypertension. Circ. Cardiovasc. Interv. 2012, 5, 748–755. [Google Scholar] [CrossRef]
- Medrek, S.; Safdar, Z. Epidemiology and Pathophysiology of Chronic Thromboembolic Pulmonary Hypertension: Risk Factors and Mechanisms. Methodist. Debakey Cardiovasc. J. 2016, 12, 195–198. [Google Scholar] [CrossRef]
- Guth, S.; D’armini, A.M.; Delcroix, M.; Nakayama, K.; Fadel, E.; Hoole, S.P.; Jenkins, D.P.; Kiely, D.G.; Kim, N.H.; Lang, I.M.; et al. Current Strategies for Managing Chronic Thromboembolic Pulmonary Hypertension: Results of the Worldwide Prospective CTEPH Registry. ERJ Open Res. 2021, 7, 00850-2020. [Google Scholar] [CrossRef] [PubMed]
- Öngen, H.G.; Akdeniz, B.; Düzenli, M.A.; Chernyavsky, A.; Dabar, G.; Idrees, M.; Khludeeva, E.; Kültürsay, H.; Lukianchikova, V.; Martynyuk, T.; et al. Diagnosis and Treatment Patterns of Chronic Thromboembolic Pulmonary Hypertension in Russia, Kazakhstan, Turkey, Lebanon, and Saudi Arabia: A Registry Study. Drugs Real World Outcomes 2024, 11, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Bambrick, M.; Grafham, G.; Lajkosz, K.; Donahoe, L.; de Perrot, M.; McInnis, M. Computed Tomography Identifies Sex-Specific Differences in Surgical Chronic Thromboembolic Pulmonary Hypertension. JHLT Open 2024, 6, 100130. [Google Scholar] [CrossRef]
- Chan, J.C.Y.; Man, H.S.J.; Asghar, U.M.; McRae, K.; Zhao, Y.; Donahoe, L.L.; Wu, L.; Granton, J.; de Perrot, M. Impact of Sex on Outcome after Pulmonary Endarterectomy for Chronic Thromboembolic Pulmonary Hypertension. J. Heart Lung Transplant. 2023, 42, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.N.; Pabelick, C.M. Sex Differences in the Pulmonary Circulation: Implications for Pulmonary Hypertension. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1253. [Google Scholar] [CrossRef] [PubMed]
- Bonderman, D.; Wilkens, H.; Wakounig, S.; Schäfers, H.J.; Jansa, P.; Lindner, J.; Simkova, I.; Martischnig, A.M.; Dudczak, J.; Sadushi, R.; et al. Risk Factors for Chronic Thromboembolic Pulmonary Hypertension. Eur. Respir. J. 2009, 33, 325–331. [Google Scholar] [CrossRef]
- Kerr, K.M.; Elliott, C.G.; Chin, K.; Benza, R.L.; Channick, R.N.; Davis, R.D.; He, F.; LaCroix, A.; Madani, M.M.; McLaughlin, V.V.; et al. Results From the United States Chronic Thromboembolic Pulmonary Hypertension Registry: Enrollment Characteristics and 1-Year Follow-Up. Chest 2021, 160, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.S.d.F.; Ferreira, M.G.; Melo, I.A.; de Sá, M.F.L.; Loureiro, C.M.C.; Abreu, R.; de Carvalho, P.H.A.; Viana, M.d.S.; Oliveira, V.; Ritt, L.E.F. Balloon Pulmonary Angioplasty in Patients with Chronic Thromboembolic Pulmonary Hypertension: Short- and Long-Term Results from a Cohort in Brazil. J. Bras. Pneumol. 2025, 50, e20240147. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.M.; Wiedenroth, C.B.; Kamp, J.C.; Breithecker, A.; Fuge, J.; Krombach, G.A.; Haas, M.; Hamm, C.; Kramm, T.; Guth, S.; et al. Balloon Pulmonary Angioplasty for Inoperable Patients with Chronic Thromboembolic Pulmonary Hypertension: The Initial German Experience. Eur. Respir. J. 2017, 49, 1602409. [Google Scholar] [CrossRef]
- Brenot, P.; Jaïs, X.; Taniguchi, Y.; Alonso, C.G.; Gerardin, B.; Mussot, S.; Mercier, O.; Fabre, D.; Parent, F.; Jevnikar, M.; et al. French Experience of Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1802095. [Google Scholar] [CrossRef]
- Lang, I.M.; Pesavento, R.; Bonderman, D.; Yuan, J.X.J. Risk Factors and Basic Mechanisms of Chronic Thromboembolic Pulmonary Hypertension: A Current Understanding. Eur. Respir. J. 2013, 41, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Boyer-Neumann, C.; Parent, F.; Eschwege, V.; Jaillet, H.; Meyer, D.; Simonneau, G. Thrombotic Risk Factors in Pulmonary Hypertension. Eur. Respir. J. 2000, 15, 395–399. [Google Scholar] [CrossRef]
- Bonderman, D.; Turecek, P.L.; Jakowitsch, J.; Weltermann, A.; Adlbrecht, C.; Schneider, B.; Kneussl, M.; Rubin, L.J.; Kyrle, P.A.; Klepetko, W.; et al. High Prevalence of Elevated Clotting Factor VIII in Chronic Thromboembolic Pulmonary Hypertension. Thromb. Haemost. 2003, 90, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Kain, K.; Carter, A.M.; Bamford, J.M.; Grant, P.J.; Catto, A.J. Gender Differences in Coagulation and Fibrinolysis in White Subjects with Acute Ischemic Stroke. J. Thromb. Haemost. 2003, 1, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Pepke-Zaba, J.; Delcroix, M.; Lang, I.; Mayer, E.; Jansa, P.; Ambroz, D.; Treacy, C.; D’Armini, A.M.; Morsolini, M.; Snijder, R.; et al. Chronic Thromboembolic Pulmonary Hypertension (CTEPH): Results from an International Prospective Registry. Circulation 2011, 124, 1973–1981. [Google Scholar] [CrossRef]
- Cruz-Utrilla, A.; Cristo-Ropero, M.J.; Calderón-Flores, M.; Velázquez, M.; López-Gude, M.J.; Ostolaza, Y.R.; Vela, J.L.P.; de la Cruz-Bertolo, J.; Bueno, H.; Ynsaurriaga, F.A.; et al. Sex Differences in Chronic Thromboembolic Pulmonary Hypertension. Treatment Options over Time in a National Referral Center. J. Clin. Med. 2021, 10, 4251. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, S.; Yan, X.X.; Peng, F.H.; Tan, J.S.; Guo, T.T.; Gao, X.; Hua, L. Chronic Thromboembolic Pulmonary Hypertension in Females: Clinical Features and Survival. J. Cardiovasc. Dev. Dis. 2022, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: Clinical Outcomes. Eur. Cardiol. Rev. 2023, 18, e11. [Google Scholar] [CrossRef] [PubMed]
- Chausheva, S.; Naito, A.; Ogawa, A.; Seidl, V.; Winter, M.P.; Sharma, S.; Sadushi-Kolici, R.; Campean, I.A.; Taghavi, S.; Moser, B.; et al. Chronic Thromboembolic Pulmonary Hypertension in Austria and Japan. J. Thorac. Cardiovasc. Surg. 2019, 158, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Keen, J.; Prisco, S.Z.; Prins, K.W. Sex Differences in Right Ventricular Dysfunction: Insights From the Bench to Bedside. Front. Physiol. 2021, 11, 623129. [Google Scholar] [CrossRef]
- Shigeta, A.; Tanabe, N.; Shimizu, H.; Hoshino, S.; Maruoka, M.; Sakao, S.; Tada, Y.; Kasahara, Y.; Takiguchi, Y.; Tatsumi, K.; et al. Gender Differences in Chronic Thromboembolic Pulmonary Hypertension in Japan. Circ. J. 2008, 72, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Kaldararova, M.; Simkova, I.; Bohacekova, M.; Reptova, A.; Hlavata, T.; Pacak, J.; Lindner, J.; Jansa, P. Central versus Peripheral CTEPH-Clinical and Hemodynamic Specifications. Medicina 2022, 58, 1538. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Yu, Y.Z.; Yuan, P.; Gong, S.G.; Wang, C.Y.; Li, Y.; Zhao, Q.H.; Jiang, R.; Wu, W.H.; He, J.; et al. Sex Differences of Hemodynamics during Acute Vasoreactivity Testing to Predict the Outcomes of Chronic Thromboembolic Pulmonary Hypertension. Clin. Respir. J. 2020, 14, 611–621. [Google Scholar] [CrossRef]
- Zoppellaro, G.; Badawy, M.R.; Squizzato, A.; Denas, G.; Tarantini, G.; Pengo, V. Balloon Pulmonary Angioplasty in Patients With Chronic Thromboembolic Pulmonary Hypertension—A Systematic Review and Meta-Analysis. Circ. J. 2019, 83, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.K.; Kennedy, S.A.; Tan, K.T.; de Perrot, M.; Bassett, P.; McInnis, M.C.; Thenganatt, J.; Donahoe, L.; Granton, J.; Mafeld, S. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: A Systematic Review and Meta-Analysis. Cardiovasc. Interv. Radiol. 2023, 46, 5–18. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).