Atrial Cardiomyopathy: A “Distinct Clinical Entity” for a Deeper Understanding of Atrial Fibrillation and Cardioembolic Stroke
Abstract
1. Introduction
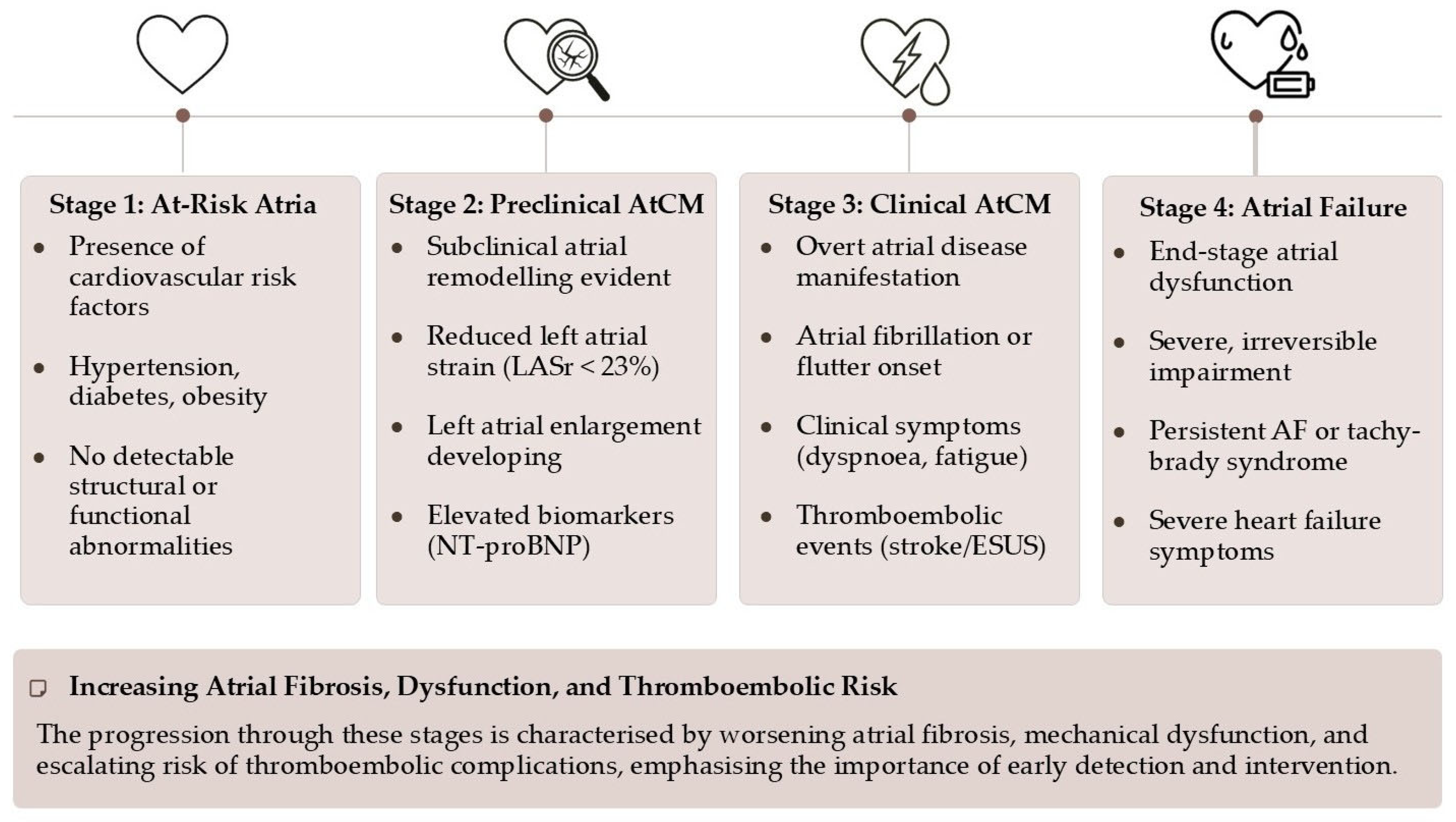
2. The Paradigm Shift: From Atrial Fibrillation to Atrial Cardiomyopathy
- Stage 1 (At-Risk Atria): Characterized by the presence of risk factors without any detectable structural, functional, or biomarker abnormalities. The atria are under stress, but remodeling has not yet begun.
- Stage 2 (Preclinical AtCM): This stage marks the onset of subclinical atrial remodeling. Structural and/or functional abnormalities are now detectable via imaging (e.g., atrial enlargement, reduced strain) or elevated biomarkers (e.g., NT-proBNP), but the patient remains asymptomatic and without a history of atrial arrhythmias or thromboembolism.
- Stage 3 (Clinical AtCM): The disease becomes clinically manifest. This stage is defined by the presence of symptoms (e.g., palpitations, dyspnea, fatigue attributable to atrial dysfunction), documented atrial arrhythmias (AF, atrial flutter), or an atrial-mediated thromboembolic event.
- Stage 4 (Atrial Failure): This represents the end-stage of the disease, characterized by severe and largely irreversible structural and functional atrial derangement. It is defined as “the inability of the atria to guarantee an adequate cardiac output… either at rest or during exercise, in the presence of normal ventricular filling pressures”. This stage is associated with persistent arrhythmias, severe symptoms, and a markedly increased risk of mortality and stroke.
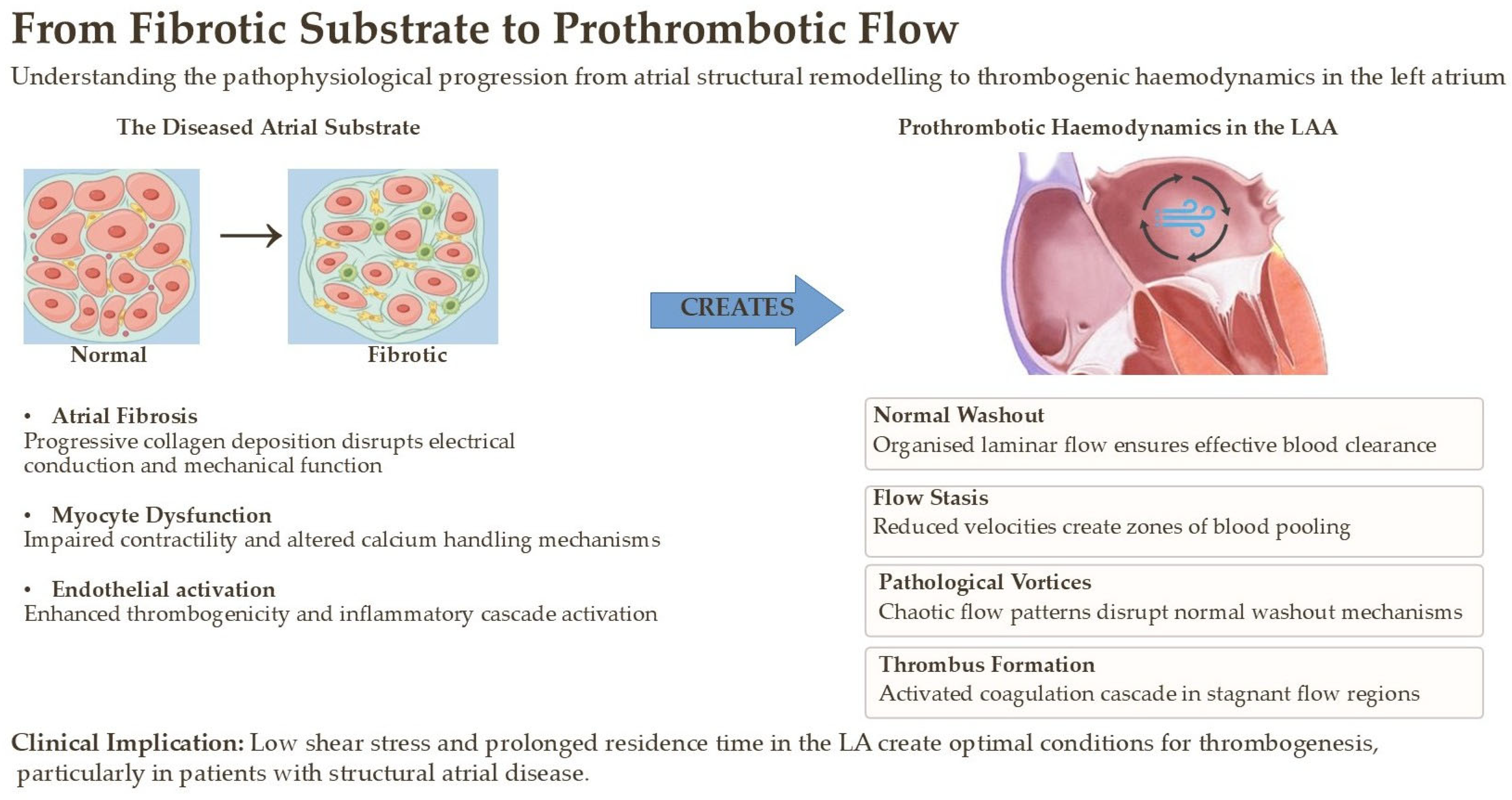
3. The Pathophysiological Substrate: Fibrosis as the Central Villain
- Blood Stasis (Abnormal Flow): Impaired atrial contractility and LAA dysfunction, direct consequences of fibrosis, lead to reduced blood flow velocity and prolonged residence time. This is particularly pronounced within the complex, trabeculated anatomy of the LAA, creating a sanctuary for thrombus formation.
- Endothelial Dysfunction (Abnormal Vessel Wall): The chronic inflammation, oxidative stress, and mechanical stretch associated with AtCM activate the atrial endocardium. This activation leads to a prothrombotic state, characterized by the expression of adhesion molecules and the downregulation of anticoagulant factors like nitric oxide.
- Hypercoagulability (Abnormal Blood Constituents): The systemic inflammatory states that drive AtCM (e.g., diabetes, obesity) also contribute to a systemic hypercoagulable state by increasing levels of circulating pro-thrombotic factors like fibrinogen and plasminogen activator inhibitor-1 [3].
4. The Diagnostic Toolkit for Atrial Cardiomyopathy
4.1. Electrocardiographic Clues: Beyond AF Detection
- Advanced Interatrial Block (aIAB—Bayes’ Syndrome): Defined as a P-wave duration ≥ 120 ms combined with a biphasic (positive-negative) morphology in the inferior leads (II, III, aVF), aIAB signifies a conduction block in Bachmann’s bundle, forcing a slow, circuitous caudo-cranial activation of the left atrium. It is a potent marker of widespread atrial fibrosis and is strongly associated with incident AF, ischemic stroke, and cognitive decline. Its presence should alert the clinician to a high-risk atrial substrate.
- P-wave Terminal Force in Lead V1 (PTFV1): A classic marker of left atrial abnormality, an abnormally deep and wide terminal negative portion of the P-wave in V1 (≥−0.04 mm·s) reflects the delayed and aberrant depolarization of a diseased left atrium. It has been used as a key inclusion criterion for defining AtCM in major clinical trials investigating ESUS.
4.2. Echocardiographic Innovations: Left Atrial Strain
4.3. Advanced Imaging: Visualizing and Quantifying Fibrosis
5. From Anatomical Imaging to Functional Simulation: The Era of the Digital Twin in Atrial Cardiomyopathy
- Electrophysiological Modeling: By applying sophisticated mathematical models of cellular action potentials and electrical propagation (e.g., monodomain or bidomain models), it is possible to simulate the conduction of the electrical impulse across the atrial surface. These simulations can vividly demonstrate how regions of fibrosis slow down conduction velocity, create electrical heterogeneity, and establish the substrate for re-entrant circuits—the very mechanism of AF. This in silico approach allows researchers to test hypotheses about arrhythmogenesis and predict which fibrotic patterns are most likely to sustain AF [45].
- Biomechanical Modeling: The same patient-specific model can be used to simulate atrial mechanics and contractility. By assigning passive stiffness properties to fibrotic regions and active contractile properties to healthy myocardium, these models can predict regional wall motion, quantify myocardial stress and strain, and compute functional parameters like ejection fraction and reservoir strain. This provides a direct mechanistic link between the extent of fibrosis and the reduction in atrial function observed with echocardiography (LASr), moving from a mere correlation to a cause-and-effect simulation [46,47].
6. Clinical Manifestations of Atrial Cardiomyopathy
6.1. The Link to Embolic Stroke of Undetermined Source (ESUS)
6.2. Atrial Failure: The “Missing Diagnosis” in Heart Failure
7. The Hemodynamic Frontier: Flow Dynamics as a Lynchpin in Atrial Thrombogenesis
- Flow Stasis and Pathological Vorticity: In a healthy atrium, blood flow is organized into smooth, large-scale vortices that facilitate efficient “washout,” particularly within the LAA. In AtCM, non-contractile fibrotic regions and generalized hypokinesis disrupt these physiological flow patterns. This leads to zones of near-stasis, where blood velocity approaches zero, and the formation of abnormal, persistent, small-scale vortices. Within these regions, the residence time of blood elements, including activated platelets and coagulation factors, is dramatically prolonged, increasing the statistical probability of thrombus initiation and propagation. The complex, trabeculated morphology of the LAA makes it exceptionally vulnerable to these phenomena [59].
- Adverse Endocardial Shear Stress: Blood flowing across the endocardium exerts a frictional force known as wall shear stress (WSS). In healthy arteries and cardiac chambers, physiological, laminar WSS promotes endothelial quiescence through mechanotransduction pathways that upregulate antithrombotic factors like nitric oxide. The disturbed flow patterns in AtCM, however, generate pathological WSS profiles, characterized by either abnormally low WSS in regions of stasis or high oscillatory shear stress in areas of turbulent or recirculating flow. It is now well-established that these adverse WSS profiles are potent activators of endothelial pro-inflammatory and pro-thrombotic pathways, such as the NF-κB cascade, leading to a shift toward a thrombogenic endothelial phenotype [60].
8. Therapeutic Implications and Future Directions
- Upstream Therapies: The primary goal must be to prevent or reverse adverse atrial remodeling. This begins with aggressive, guideline-directed management of all underlying risk factors, including hypertension, diabetes, obesity, and sleep apnea. Beyond this, several drug classes have shown promise as “upstream” therapies due to their anti-fibrotic and anti-inflammatory properties. Renin–angiotensin–aldosterone system (RAAS) inhibitors have been shown to attenuate atrial fibrosis in experimental models [66]. More recently, SGLT2 inhibitors and GLP-1 receptor agonists have demonstrated remarkable cardiovascular benefits, part of which may be attributable to their favorable effects on atrial structure and function by reducing inflammation and oxidative stress [67,68].
- Rethinking Anticoagulation: The central therapeutic dilemma is whether to anticoagulate patients with severe AtCM in the absence of documented AF. The neutral result of the ARCADIA trial highlights the need for better patient selection. Future trials are imperative and must employ more specific criteria to identify a population with a stroke risk high enough to warrant anticoagulation. This could involve combining biomarkers, for instance, requiring the presence of both severe mechanical dysfunction (e.g., LASr < 20%) and a significant structural substrate abnormality (e.g., severe LA enlargement or extensive fibrosis on LGE-CMR).
- Targeting Fibrosis Directly: As our understanding of the molecular pathways driving fibrosis deepens, novel anti-fibrotic therapies are on the horizon. The development of direct inhibitors of pathways like TGF-β, or agents such as pirfenidone and galectin-3 inhibitors, could one day offer the ability to halt or even reverse the progression of AtCM. In such a future, quantitative imaging with LGE-CMR would be essential not only for diagnosis but also for monitoring the efficacy of these targeted treatments. Future clinical trials of these agents must not only demonstrate efficacy in modifying the atrial substrate but also rigorously evaluate their long-term safety and potential off-target effects.
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial Fibrillation as an Independent Risk Factor for Stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, ehae176. [Google Scholar] [CrossRef]
- Watson, T.; Shantsila, E.; Lip, G.Y.H. Mechanisms of Thrombogenesis in Atrial Fibrillation: Virchow’s Triad Revisited. Lancet Lond. Engl. 2009, 373, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Diener, H.-C.; Coutts, S.B.; Easton, J.D.; Granger, C.B.; O’Donnell, M.J.; Sacco, R.L.; Connolly, S.J. Cryptogenic Stroke/ESUS International Working Group Embolic Strokes of Undetermined Source: The Case for a New Clinical Construct. Lancet Neurol. 2014, 13, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 333–340. [Google Scholar] [CrossRef]
- Sanna, T.; Diener, H.-C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; Rymer, M.M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. Cryptogenic Stroke and Underlying Atrial Fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef]
- Brambatti, M.; Connolly, S.J.; Gold, M.R.; Morillo, C.A.; Capucci, A.; Muto, C.; Lau, C.P.; Van Gelder, I.C.; Hohnloser, S.H.; Carlson, M.; et al. Temporal Relationship between Subclinical Atrial Fibrillation and Embolic Events. Circulation 2014, 129, 2094–2099. [Google Scholar] [CrossRef]
- Ning, Y.; Tse, G.; Luo, G.; Li, G. Atrial Cardiomyopathy: An Emerging Cause of the Embolic Stroke of Undetermined Source. Front. Cardiovasc. Med. 2021, 8, 674612. [Google Scholar] [CrossRef]
- Kamel, H.; Okin, P.M.; Elkind, M.S.V.; Iadecola, C. Atrial Fibrillation and Mechanisms of Stroke: Time for a New Model. Stroke 2016, 47, 895–900. [Google Scholar] [CrossRef]
- Weerts, J.; Țica, O.; Aranyo, J.; Basile, C.; Borizanova-Petkova, A.; Borovac, J.A.; Camilli, M.; Eichenlaub, M.; Fiori, E.; Van Loon, T.; et al. Atrial Cardiomyopathy: From Healthy Atria to Atrial Failure. A Clinical Consensus Statement of the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2025. [Google Scholar] [CrossRef]
- Papakonstantinou, P.E.; Rivera-Caravaca, J.M.; Chiarito, M.; Ehrlinder, H.; Iliakis, P.; Gąsecka, A.; Romiti, G.F.; Parker, W.A.E.; Lip, G.Y.H. Atrial Fibrillation versus Atrial Myopathy in Thrombogenesis: Two Sides of the Same Coin? Trends Cardiovasc. Med. 2025, 35, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE Expert Consensus on Atrial Cardiomyopathies: Definition, Characterization, and Clinical Implication. Ep Eur. 2016, 18, 1455–1490. [Google Scholar] [CrossRef] [PubMed]
- Corradi, D. Atrial Fibrillation from the Pathologist’s Perspective. Cardiovasc. Pathol. 2014, 23, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Coats, A.J.S.; Heymans, S.; Farmakis, D.; Anker, S.D.; Backs, J.; Bauersachs, J.; de Boer, R.A.; Čelutkienė, J.; Cleland, J.G.F.; Dobrev, D.; et al. Atrial Disease and Heart Failure: The Common Soil Hypothesis Proposed by the Heart Failure Association of the European Society of Cardiology. Eur. Heart J. 2021, 43, ehab834. [Google Scholar] [CrossRef]
- Savelieva, I.; Kakouros, N.; Kourliouros, A.; Camm, A.J. Upstream Therapies for Management of Atrial Fibrillation: Review of Clinical Evidence and Implications for European Society of Cardiology Guidelines. Part I: Primary Prevention. Europace 2011, 13, 308–328. [Google Scholar] [CrossRef]
- Pathak, R.K.; Middeldorp, M.E.; Lau, D.H.; Mehta, A.B.; Mahajan, R.; Twomey, D.; Alasady, M.; Hanley, L.; Antic, N.A.; McEvoy, R.D.; et al. Aggressive Risk Factor Reduction Study for Atrial Fibrillation and Implications for the Outcome of Ablation: The ARREST-AF Cohort Study. J. Am. Coll. Cardiol. 2014, 64, 2222–2231. [Google Scholar] [CrossRef]
- Spach, M.S.; Dolber, P.C. Relating Extracellular Potentials and Their Derivatives to Anisotropic Propagation at a Microscopic Level in Human Cardiac Muscle. Evidence for Electrical Uncoupling of Side-to-Side Fiber Connections with Increasing Age. Circ. Res. 1986, 58, 356–371. [Google Scholar] [CrossRef]
- Bisbal, F.; Baranchuk, A.; Braunwald, E.; Bayés de Luna, A.; Bayés-Genís, A. Atrial Failure as a Clinical Entity: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 222–232. [Google Scholar] [CrossRef]
- Chatterjee, S. Endothelial Mechanotransduction, Redox Signaling and the Regulation of Vascular Inflammatory Pathways. Front. Physiol. 2018, 9, 524. [Google Scholar] [CrossRef]
- Vogl, B.J.; Vitale, E.; Ahn, S.; Sularz, A.; Chavez Ponce, A.; Lo Russo, G.V.; Collins, J.; Bavo, A.M.; El Shaer, A.; Kramer, A.; et al. Flow Dynamic Factors Correlated with Device-Related Thrombosis After Left Atrial Appendage Occlusion. JACC Adv. 2024, 3, 101339. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. The Interactions between Inflammation and Coagulation. Br. J. Haematol. 2005, 131, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.I.; Mujtaba, M.; Floyd, J.S.; Chen, L.Y.; Soliman, E.Z. Electrocardiographic Markers of Atrial Cardiomyopathy and Risk of Heart Failure in the Multi-Ethnic Study of Atherosclerosis (MESA) Cohort. Front. Cardiovasc. Med. 2023, 10, 1143338. [Google Scholar] [CrossRef] [PubMed]
- Bayés de Luna, A.; Platonov, P.; Cosio, F.G.; Cygankiewicz, I.; Pastore, C.; Baranowski, R.; Bayés-Genis, A.; Guindo, J.; Viñolas, X.; Garcia-Niebla, J.; et al. Interatrial Blocks. A Separate Entity from Left Atrial Enlargement: A Consensus Report. J. Electrocardiol. 2012, 45, 445–451. [Google Scholar] [CrossRef]
- O’Neal, W.T.; Zhang, Z.-M.; Loehr, L.R.; Chen, L.Y.; Alonso, A.; Soliman, E.Z. Electrocardiographic Advanced Interatrial Block and Atrial Fibrillation Risk in the General Population. Am. J. Cardiol. 2016, 117, 1755–1759. [Google Scholar] [CrossRef]
- Martínez-Sellés, M.; Elosua, R.; Ibarrola, M.; de Andrés, M.; Díez-Villanueva, P.; Bayés-Genis, A.; Baranchuk, A.; Bayés-de-Luna, A. BAYES Registry Investigators Advanced Interatrial Block and P-Wave Duration Are Associated with Atrial Fibrillation and Stroke in Older Adults with Heart Disease: The BAYES Registry. EP Eur. 2020, 22, 1001–1008. [Google Scholar] [CrossRef]
- Tsang, T.S.M.; Barnes, M.E.; Gersh, B.J.; Bailey, K.R.; Seward, J.B. Left Atrial Volume as a Morphophysiologic Expression of Left Ventricular Diastolic Dysfunction and Relation to Cardiovascular Risk Burden. Am. J. Cardiol. 2002, 90, 1284–1289. [Google Scholar] [CrossRef]
- Singh, A.; Carvalho Singulane, C.; Miyoshi, T.; Prado, A.D.; Addetia, K.; Bellino, M.; Daimon, M.; Gutierrez Fajardo, P.; Kasliwal, R.R.; Kirkpatrick, J.N.; et al. Normal Values of Left Atrial Size and Function and the Impact of Age: Results of the World Alliance Societies of Echocardiography Study. J. Am. Soc. Echocardiogr. 2022, 35, 154–164.e3. [Google Scholar] [CrossRef]
- Pathan, F.; D’Elia, N.; Nolan, M.T.; Marwick, T.H.; Negishi, K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 59–70.e8. [Google Scholar] [CrossRef]
- Park, J.-H.; Hwang, I.-C.; Park, J.J.; Park, J.-B.; Cho, G.-Y. Left Atrial Strain to Predict Stroke in Patients with Acute Heart Failure and Sinus Rhythm. J. Am. Heart Assoc. 2021, 10, e020414. [Google Scholar] [CrossRef]
- Sugimoto, T.; Robinet, S.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Kacharava, G.; Athanassopoulos, G.D.; et al. Echocardiographic Reference Ranges for Normal Left Atrial Function Parameters: Results from the EACVI NORRE Study. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 630–638. [Google Scholar] [CrossRef]
- Fernandes, R.M.; Le Bihan, D.; Vilela, A.A.; Barretto, R.B.M.; Santos, E.S.; Assef, J.E.; Pedra, S.R.F.; Sousa, A.G.M.R.; Timerman, A. Association between Left Atrial Strain and Left Ventricular Diastolic Function in Patients with Acute Coronary Syndrome. J. Echocardiogr. 2019, 17, 138–146. [Google Scholar] [CrossRef]
- Zhang, M.J.; Ji, Y.; Wang, W.; Norby, F.L.; Parikh, R.; Eaton, A.A.; Inciardi, R.M.; Alonso, A.; Soliman, E.Z.; Mosley, T.H.; et al. Association of Atrial Fibrillation with Stroke and Dementia Accounting for Left Atrial Function and Size. JACC Adv. 2023, 2, 100408. [Google Scholar] [CrossRef]
- Maheshwari, A.; Norby, F.L.; Inciardi, R.M.; Wang, W.; Zhang, M.J.; Soliman, E.Z.; Alonso, A.; Johansen, M.C.; Gottesman, R.F.; Solomon, S.D.; et al. Left Atrial Mechanical Dysfunction and the Risk for Ischemic Stroke in People Without Prevalent Atrial Fibrillation or Stroke. Ann. Intern. Med. 2023, 176, 39–48. [Google Scholar] [CrossRef]
- Stassen, J.; van Wijngaarden, A.L.; Butcher, S.C.; Palmen, M.; Herbots, L.; Bax, J.J.; Delgado, V.; Ajmone Marsan, N. Prognostic Value of Left Atrial Reservoir Function in Patients with Severe Primary Mitral Regurgitation Undergoing Mitral Valve Repair. Eur. Heart J. Cardiovasc. Imaging 2022, 24, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M. Incremental Prognostic Role of Left Atrial Reservoir Strain in Asymptomatic Patients with Moderate Aortic Stenosis. Int. J. Cardiovasc. Imaging 2021, 37, 1913–1925. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; De Rosa, S.; Leo, I.; Strangio, A.; La Bella, S.; Sorrentino, S.; Mongiardo, A.; Spaccarotella, C.; Polimeni, A.; Indolfi, C. Early Reduction of Left Atrial Function Predicts Adverse Clinical Outcomes in Patients with Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement. Open Heart 2021, 8, e001685. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, N.; Ostrander, B.T.; Lu, D.-Y.; Ventoulis, I.; Haileselassie, B.; Goyal, S.; Greenland, G.V.; Vakrou, S.; Olgin, J.E.; Abraham, T.P.; et al. Low Left Atrial Strain Is Associated with Adverse Outcomes in Hypertrophic Cardiomyopathy Patients. J. Am. Soc. Echocardiogr. 2019, 32, 593–603.e1. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J.; Zhang, D.; Jia, F.; Lin, X.; Zhu, Y.; Zhuang, J.; Fang, L.; Chen, W. Prognostic Value of Left Atrial Mechanics in Cardiac Light-Chain Amyloidosis with Preserved Ejection Fraction: A Cohort Study. BMC Cardiovasc. Disord. 2022, 22, 175. [Google Scholar] [CrossRef]
- Daccarett, M.; Badger, T.J.; Akoum, N.; Burgon, N.S.; Mahnkopf, C.; Vergara, G.; Kholmovski, E.; McGann, C.J.; Parker, D.; Brachmann, J.; et al. Association of Left Atrial Fibrosis Detected by Delayed-Enhancement Magnetic Resonance Imaging and the Risk of Stroke in Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2011, 57, 831–838. [Google Scholar] [CrossRef]
- King, J.B.; Azadani, P.N.; Suksaranjit, P.; Bress, A.P.; Witt, D.M.; Han, F.T.; Chelu, M.G.; Silver, M.A.; Biskupiak, J.; Wilson, B.D.; et al. Left Atrial Fibrosis and Risk of Cerebrovascular and Cardiovascular Events in Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 70, 1311–1321. [Google Scholar] [CrossRef]
- Valinoti, M.; Fabbri, C.; Turco, D.; Mantovan, R.; Pasini, A.; Corsi, C. 3D Patient-Specific Models for Left Atrium Characterization to Support Ablation in Atrial Fibrillation Patients. Magn. Reson. Imaging 2018, 45, 51–57. [Google Scholar] [CrossRef]
- Paliwal, N.; Ali, R.L.; Salvador, M.; O’Hara, R.; Yu, R.; Daimee, U.A.; Akhtar, T.; Pandey, P.; Spragg, D.D.; Calkins, H.; et al. Presence of Left Atrial Fibrosis May Contribute to Aberrant Hemodynamics and Increased Risk of Stroke in Atrial Fibrillation Patients. Front. Physiol. 2021, 12, 657452. [Google Scholar] [CrossRef]
- Trayanova, N.A.; Lyon, A.; Shade, J.; Heijman, J. Computational Modeling of Cardiac Electrophysiology and Arrhythmogenesis: Toward Clinical Translation. Physiol. Rev. 2024, 104, 1265–1333. [Google Scholar] [CrossRef]
- Rodero, C.; Baptiste, T.M.G.; Barrows, R.K.; Lewalle, A.; Niederer, S.A.; Strocchi, M. Advancing Clinical Translation of Cardiac Biomechanics Models: A Comprehensive Review, Applications and Future Pathways. Front. Phys. 2023, 11, 1306210. [Google Scholar] [CrossRef]
- Telle, Å.; Bargellini, C.; Chahine, Y.; Del Álamo, J.C.; Akoum, N.; Boyle, P.M. Personalized Biomechanical Insights in Atrial Fibrillation: Opportunities & Challenges. Expert Rev. Cardiovasc. Ther. 2023, 21, 817–837. [Google Scholar] [CrossRef]
- Falanga, M.; Cortesi, C.; Chiaravalloti, A.; Monte, A.D.; Tomasi, C.; Corsi, C. A Digital Twin Approach for Stroke Risk Assessment in Atrial Fibrillation Patients. Heliyon 2024, 10, e39527. [Google Scholar] [CrossRef]
- Boyle, P.M.; Zghaib, T.; Zahid, S.; Ali, R.L.; Deng, D.; Franceschi, W.H.; Hakim, J.B.; Murphy, M.J.; Prakosa, A.; Zimmerman, S.L.; et al. Computationally Guided Personalized Targeted Ablation of Persistent Atrial Fibrillation. Nat. Biomed. Eng. 2019, 3, 870–879. [Google Scholar] [CrossRef]
- Aguado, A.M.; Olivares, A.L.; Yagüe, C.; Silva, E.; Nuñez-García, M.; Fernandez-Quilez, Á.; Mill, J.; Genua, I.; Arzamendi, D.; De Potter, T.; et al. In Silico Optimization of Left Atrial Appendage Occluder Implantation Using Interactive and Modeling Tools. Front. Physiol. 2019, 10, 237. [Google Scholar] [CrossRef]
- D’Alessandro, N.; Falanga, M.; Masci, A.; Severi, S.; Corsi, C. Preliminary Findings on Left Atrial Appendage Occlusion Simulations Applying Different Endocardial Devices. Front. Cardiovasc. Med. 2023, 10, 1067964. [Google Scholar] [CrossRef]
- Shen, M.J.; Arora, R.; Jalife, J. Atrial Myopathy. JACC Basic Transl. Sci. 2019, 4, 640–654. [Google Scholar] [CrossRef]
- Acampa, M.; Cartocci, A.; Domenichelli, C.; Tassi, R.; Guideri, F.; Lazzerini, P.E.; Martini, G. Markers of Atrial Cardiopathy in Severe Embolic Strokes of Undetermined Source. Front. Cardiovasc. Med. 2022, 9, 903778. [Google Scholar] [CrossRef]
- Kamel, H.; Longstreth, W.T.; Tirschwell, D.L.; Kronmal, R.A.; Broderick, J.P.; Palesch, Y.Y.; Meinzer, C.; Dillon, C.; Ewing, I.; Spilker, J.A.; et al. The AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke Randomized Trial: Rationale and Methods. Int. J. Stroke 2019, 14, 207–214. [Google Scholar] [CrossRef]
- Kamel, H.; Longstreth, W.T.; Tirschwell, D.L.; Kronmal, R.A.; Marshall, R.S.; Broderick, J.P.; Aragón García, R.; Plummer, P.; Sabagha, N.; Pauls, Q.; et al. Apixaban to Prevent Recurrence After Cryptogenic Stroke in Patients with Atrial Cardiopathy: The ARCADIA Randomized Clinical Trial. JAMA 2024, 331, 573–581. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Verbrugge, F.H.; Lin, G.; Borlaug, B.A. Atrial Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 76, 1051–1064. [Google Scholar] [CrossRef]
- Melenovsky, V.; Hwang, S.-J.; Redfield, M.M.; Zakeri, R.; Lin, G.; Borlaug, B.A. Left Atrial Remodeling and Function in Advanced Heart Failure with Preserved or Reduced Ejection Fraction. Circ. Heart Fail. 2015, 8, 295–303. [Google Scholar] [CrossRef]
- Bosi, G.M.; Cook, A.; Rai, R.; Menezes, L.J.; Schievano, S.; Torii, R.; Burriesci, G. Computational Fluid Dynamic Analysis of the Left Atrial Appendage to Predict Thrombosis Risk. Front. Cardiovasc. Med. 2018, 5, 34. [Google Scholar] [CrossRef]
- Valvez, S.; Oliveira-Santos, M.; Piedade, A.P.; Gonçalves, L.; Amaro, A.M. Computational Flow Dynamic Analysis in Left Atrial Appendage Thrombus Formation Risk: A Review. Appl. Sci. 2023, 13, 8201. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Effects of Shear Stress on Endothelial Cells: Go with the Flow. Acta Physiol. 2017, 219, 382–408. [Google Scholar] [CrossRef]
- Zingaro, A.; Dede’, L.; Menghini, F.; Quarteroni, A. Hemodynamics of the Heart’s Left Atrium Based on a Variational Multiscale-LES Numerical Method. Eur. J. Mech. B Fluids 2021, 89, 380–400. [Google Scholar] [CrossRef]
- Corti, M.; Zingaro, A.; Dede’, L.; Quarteroni, A.M. Impact of Atrial Fibrillation on Left Atrium Haemodynamics: A Computational Fluid Dynamics Study. Comput. Biol. Med. 2022, 150, 106143. [Google Scholar] [CrossRef]
- Mazzi, V.; Gallo, D.; Calò, K.; Steinman, D.A.; Morbiducci, U. Linking Wall Shear Stress and Vorticity Topologies: Toward a Unified Theory of Cardiovascular Flow Disturbances. Phys. Fluids 2024, 36, 061905. [Google Scholar] [CrossRef]
- Govindarajan, V.; Rakesh, V.; Reifman, J.; Mitrophanov, A.Y. Computational Study of Thrombus Formation and Clotting Factor Effects under Venous Flow Conditions. Biophys. J. 2016, 110, 1869–1885. [Google Scholar] [CrossRef]
- Melidoro, P.; Sultan, A.R.A.; Qureshi, A.; Yacoub, M.H.; Elkhodary, K.L.; Lip, G.Y.H.; Montarello, N.; Lahoti, N.; Rajani, R.; Klis, M.; et al. Enhancing Stroke Risk Stratification in Atrial Fibrillation through Non-Newtonian Blood Modelling and Gaussian Process Emulation. J. Physiol. 2024. [Google Scholar] [CrossRef]
- Healey, J.S.; Baranchuk, A.; Crystal, E.; Morillo, C.A.; Garfinkle, M.; Yusuf, S.; Connolly, S.J. Prevention of Atrial Fibrillation with Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers: A Meta-Analysis. J. Am. Coll. Cardiol. 2005, 45, 1832–1839. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
| Modality | Key Parameters and Thresholds | Advantages | Limitations |
|---|---|---|---|
| ECG |
|
|
|
| Echocardiography |
|
|
|
| Cardiac MR (CMR) |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martignani, C.; Spadotto, A.; Carelli, M.; Massaro, G.; Bartoli, L.; Diemberger, I.; Biffi, M.; Corsi, C.; Zanuttigh, B. Atrial Cardiomyopathy: A “Distinct Clinical Entity” for a Deeper Understanding of Atrial Fibrillation and Cardioembolic Stroke. J. Clin. Med. 2025, 14, 8363. https://doi.org/10.3390/jcm14238363
Martignani C, Spadotto A, Carelli M, Massaro G, Bartoli L, Diemberger I, Biffi M, Corsi C, Zanuttigh B. Atrial Cardiomyopathy: A “Distinct Clinical Entity” for a Deeper Understanding of Atrial Fibrillation and Cardioembolic Stroke. Journal of Clinical Medicine. 2025; 14(23):8363. https://doi.org/10.3390/jcm14238363
Chicago/Turabian StyleMartignani, Cristian, Alberto Spadotto, Maria Carelli, Giulia Massaro, Lorenzo Bartoli, Igor Diemberger, Mauro Biffi, Cristiana Corsi, and Barbara Zanuttigh. 2025. "Atrial Cardiomyopathy: A “Distinct Clinical Entity” for a Deeper Understanding of Atrial Fibrillation and Cardioembolic Stroke" Journal of Clinical Medicine 14, no. 23: 8363. https://doi.org/10.3390/jcm14238363
APA StyleMartignani, C., Spadotto, A., Carelli, M., Massaro, G., Bartoli, L., Diemberger, I., Biffi, M., Corsi, C., & Zanuttigh, B. (2025). Atrial Cardiomyopathy: A “Distinct Clinical Entity” for a Deeper Understanding of Atrial Fibrillation and Cardioembolic Stroke. Journal of Clinical Medicine, 14(23), 8363. https://doi.org/10.3390/jcm14238363










