Diagnostic Applications of Ultrasound Imaging in Dental Implantology: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
- Population (P): patients receiving dental implants;
- Intervention (I): diagnostic use of ultrasonography for the assessment of peri-implant tissues;
- Comparison (C): conventional imaging modalities such as radiography or CBCT;
- Outcome (O): diagnostic accuracy, clinical applicability, and usefulness of ultrasonography in preoperative planning and postoperative monitoring.
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Article Selection Process
2.4. Data Extraction and Quality Assessment
3. Results
3.1. Pre-Operative Diagnostic Applications
3.1.1. Soft Tissue Assessment and Phenotype Evaluation
3.1.2. Bone and Ridge Morphology Assessment
3.1.3. Vascular Perfusion and Soft Tissue Perfusion Mapping
3.1.4. Surgical and Prosthetic Planning
3.1.5. Summary
3.2. Post-Operative and Follow-Up Applications
3.2.1. Monitoring of Soft Tissue Healing and Thickness Changes
3.2.2. Assessment of Vascular Perfusion and Tissue Remodeling
3.2.3. Detection of Peri-Implant Complications
3.2.4. Long-Term Monitoring and Maintenance-Phase Evaluation
3.2.5. Summary
3.3. US Outcomes and Comparison with Conventional Diagnostic Methods
3.3.1. Comparison with CBCT and Radiography
3.3.2. Correlation with Intraoperative and Clinical Measurements
3.3.3. Doppler Ultrasonography for Perfusion and Inflammatory Assessment
3.4. Safety and Adverse Events
3.5. Limitations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| US | Ultrasonography/Ultrasound Imaging |
| RCT | Randomized Controlled Trial |
| CBCT | Cone-beam computed tomography |
| OPG | Orthopantomogram |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| Rob2 | Risk of Bias 2 |
| QUADAS-2 | Quality Assessment of Diagnostic Accuracy Studies—version 2 |
| JBI | Joanna Briggs Institute |
| HFUS | High-Frequency US |
| ICC | Intraclass Correlation Coefficients |
| CTG | Connective Tissue Graft |
| CAF | Coronally Advanced Flap |
| TUN | Tunnel Technique |
| STL | Standard Tessellation Language |
| CDV | Colour Doppler Velocity |
| PDI | Power Doppler Imaging |
| TKT | Thickness of keratinized tissue |
| CEJ | Cemento-enamel junction |
| MGJ | Mucogingival Junction |
| KM | Keratinized Mucosa width |
| MT | Mucosal Thickness |
| CBT | Crestal Bone Thickness |
| FBL | Facial Bone Level |
| BBD | Buccal bone dehiscence |
| STH | Supracrestal tissue height |
| STA | Soft tissue area |
| HSA | Hypoechoic supracrestal area |
| BRW | Bone ridge width |
| CBSQ | Crestal bone surface quality |
| GBR | Guided Bone Regeneration |
| FMT | Facial mucosal thickness |
References
- Moraschini, V.; Poubel, L.A.; Ferreira, V.F.; Barboza Edos, S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corbella, S.; Del Fabbro, M.; Taschieri, S.; De Siena, F.; Francetti, L. Clinical evaluation of an implant maintenance protocol for the prevention of peri-implant diseases in patients treated with immediately loaded full-arch rehabilitations. Int. J. Dent. Hyg. 2011, 9, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. S1), S304–S312. [Google Scholar] [CrossRef] [PubMed]
- Chackartchi, T.; Romanos, G.E.; Sculean, A. Soft tissue-related complications and management around dental implants. Periodontol. 2000 2019, 81, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Clementini, M.; Rossetti, P.H.; Penarrocha, D.; Micarelli, C.; Bonachela, W.C.; Canullo, L. Systemic risk factors for peri-implant bone loss: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2014, 43, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, C.A. Diagnostic measures for monitoring and follow-up in periodontology and implant dentistry. Periodontol. 2000 2024, 95, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Gutmacher, Z.; Machtei, E.E.; Hirsh, I.; Zigdon-Giladi, H.; Horwitz, J. A comparative study on the use of digital panoramic and periapical radiographs to assess proximal bone height around dental implants. Quintessence Int. 2016, 47, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, I.N.R.; Vilela, N.; Naenni, N.; Jung, R.E.; Schwarz, F.; Romito, G.A.; Spin-Neto, R.; Pannuti, C.M. Methods for assessing peri-implant marginal bone levels on digital periapical radiographs: A meta-research. Dentomaxillofac. Radiol. 2025, 54, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.; Mendes, J.M.; Salazar, F.; Pacheco, J.J.; Rompante, P.; Câmara, M.I. Analysis of peri-implant bone defects by using cone beam computed tomography (CBCT): An integrative review. Oral Radiol. 2023, 39, 455–466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jacobs, R.; Vranckx, M.; Vanderstuyft, T.; Quirynen, M.; Salmon, B. CBCT vs other imaging modalities to assess peri-implant bone and diagnose complications: A systematic review. Eur. J. Oral Implantol. 2018, 11 (Suppl. S1), 77–92. [Google Scholar] [PubMed]
- Yeung, A.W.K.; AlHadidi, A.; Vyas, R.; Bornstein, M.M.; Watanabe, H.; Tanaka, R. Nonionizing diagnostic imaging modalities for visualizing health and pathology of periodontal and peri-implant tissues. Periodontol. 2000 2024, 95, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, V.; Chan, H.L.; MacEachern, M.; Kripfgans, O.D. Updates on ultrasound research in implant dentistry: A systematic review of potential clinical indications. Dentomaxillofac. Radiol. 2018, 47, 20180076. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, H.L.; Sinjab, K.; Chung, M.P.; Chiang, Y.C.; Wang, H.L.; Giannobile, W.V.; Kripfgans, O.D. Non-invasive evaluation of facial crestal bone with ultrasonography. PLoS ONE 2017, 12, e0171237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reda, R.; Zanza, A.; Cicconetti, A.; Bhandi, S.; Miccoli, G.; Gambarini, G.; Di Nardo, D. Ultrasound Imaging in Dentistry: A Literature Overview. J. Imaging 2021, 7, 238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evirgen, Ş.; Kamburoğlu, K. Review on the applications of ultrasonography in dentomaxillofacial region. World J. Radiol. 2016, 8, 50–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demirturk Kocasarac, H.; Angelopoulos, C. Ultrasound in Dentistry: Toward a Future of Radiation-Free Imaging. Dent. Clin. N. Am. 2018, 62, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Baum, G.; Greenwood, I.; Slawski, S.; Smirnow, R. Observation of internal structures of teeth by ultrasonography. Science 1963, 139, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Sáenz-Ravello, G.; Al-Nawas, B.; Schiegnitz, E.; Diaz, L.; Sagheb, K. The feasibility of ultrasonography for the measurement of periodontal and peri-implant phenotype: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2023, 25, 892–909. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.; Kripfgans, O.D. Ultrasonography for diagnosis of peri-implant diseases and conditions: A detailed scanning protocol and case demonstration. Dentomaxillofac. Radiol. 2020, 49, 20190445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bykhovsky, I.; Hildner, A.; Kripfgans, O.D.; Mengel, R. Sonography in the diagnosis of peri-implant bone defects: An in vitro study on native human mandibles. Clin. Oral Implant. Res. 2024, 35, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Eghbali, A.; De Bruyn, H.; Cosyn, J.; Kerckaert, I.; Van Hoof, T. Ultrasonic Assessment of Mucosal Thickness around Implants: Validity, Reproducibility, and Stability of Connective Tissue Grafts at the Buccal Aspect. Clin. Implant. Dent. Relat. Res. 2016, 18, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Sabri, H.; Nava, P.; Hazrati, P.; Alrmali, A.; Galindo-Fernandez, P.; Saleh, M.H.A.; Calatrava, J.; Barootchi, S.; Tavelli, L.; Wang, H.L. Comparison of Ultrasonography, CBCT, Transgingival Probing, Colour-Coded and Periodontal Probe Transparency With Histological Gingival Thickness: A Diagnostic Accuracy Study Revisiting Thick Versus Thin Gingiva. J. Clin. Periodontol. 2025, 52, 547–560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriguez Betancourt, A.; Kripfgans, O.D.; Meneghetti, P.C.; Mendonça, G.; Pereira, R.; Teixeira, W.; Zambrana, N.; Samal, A.; Chan, H.L. Intraoral ultrasonography image registration for evaluation of partial edentulous ridge: A methodology and validation study. J. Dent. 2024, 148, 105136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Donaldson, J.; Neves, V.C.M.; Scott, J. Are dental magnetic resonance imaging and ultrasonography techniques reliable alternatives for treatment planning dental implants? A systematic review and meta-analysis. Int. J. Implant. Dent. 2025, 11, 52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sönmez, G.; Kamburoğlu, K.; Gülşahı, A. Accuracy of high-resolution ultrasound (US) for gingival soft tissue thickness mesurement in edentulous patients prior to implant placement. Dentomaxillofac. Radiol. 2021, 50, 20200309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, S.; Mendiratta, A.K.; Shaikh, M.A.; Dora, H.; Shamsuddin, S.; Maqhbool, S.B. Comparison of gingival thickness by CBCT versus transgingival probing and estimation of cut-off values for gingival phenotype—A cross-sectional study in adults. Int. Orthod. 2024, 22, 100892. [Google Scholar] [CrossRef] [PubMed]
- Giovagnorio, F.; Drudi, F.M.; Valentini, C.; Paonessa, A. Ultrasonography in follow-up of soft tissue augmentation of the face with synthetic materials: A pilot study. Acta Radiol. 2004, 45, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Kripfgans, O.D.; Chan, H.L.; Vera Rodriguez, M.; Sabri, H.; Mancini, L.; Wang, H.L.; Giannobile, W.V.; Barootchi, S. Doppler ultrasonographic evaluation of tissue revascularization following connective tissue graft at implant sites. J. Clin. Periodontol. 2025, 52, 68–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puzio, M.; Błaszczyszyn, A.; Hadzik, J.; Dominiak, M. Ultrasound assessment of soft tissue augmentation around implants in the aesthetic zone using a connective tissue graft and xenogeneic collagen matrix—1-year randomised follow-up. Ann. Anat. 2018, 217, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Yu, N.; Mancini, L.; Barootchi, S. Keratinized mucosa width assessment at implant sites using high-frequency ultrasonography. J. Periodontol. 2023, 94, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.; Sinjab, K.; Pan, Y.C.; Soki, F.; Chan, H.L.; Kripfgans, O. Comprehensive peri-implant tissue evaluation with ultrasonography and cone-beam computed tomography: A pilot study. Clin. Oral Implant. Res. 2021, 32, 777–785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nava, P.; Sabri, H.; Calatrava, J.; Zimmer, J.; Chen, Z.; Li, J.; Wang, H.L. Ultrasonography-Guided Dental Implant Surgery: A Feasibility Study. Clin. Implant. Dent. Relat. Res. 2025, 27, e13401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tavelli, L.; Barootchi, S. Soft tissue elasticity at teeth and implant sites. A novel outcome measure of the soft tissue phenotype. J. Periodontal Res. 2024, 59, 1130–1142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galarraga-Vinueza, M.E.; Barootchi, S.; Mancini, L.; Sabri, H.; Schwarz, F.; Gallucci, G.O.; Tavelli, L. Echo-intensity characterization at implant sites and novel diagnostic ultrasonographic markers for peri-implantitis. J. Clin. Periodontol. 2024, 51, 1586–1597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sinjab, K.; Kripfgans, O.D.; Ou, A.; Chan, H.L. Ultrasonographic evaluation of edentulous crestal bone topography: A proof-of-principle retrospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 110–117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Izzetti, R.; Vitali, S.; Gabriele, M.; Caramella, D. Feasibility of a combination of intraoral UHFUS and CBCT in the study of peri-implantitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, e89–e94. [Google Scholar] [CrossRef] [PubMed]
- Sirinirund, B.; Wang, I.C.; Ramadan, G.; Kripfgans, O.D.; Chan, H.L. Ridge augmentation planning, wound healing evaluation, and peri-implant tissue phenotype assessment with ultrasonography: A case report. Clin. Adv. Periodontics 2024, 14, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Bertram, S.; Emshoff, R. Sonography of periimplant buccal bone defects in periodontitis patients: A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Couso-Queiruga, E.; Raabe, C.; Belser, U.C.; Buser, D.; Avila-Ortiz, G.; Rodrigues, D.M.; Chappuis, V. Non-invasive assessment of peri-implant mucosal thickness: A cross-sectional study. J. Periodontol. 2023, 94, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Barootchi, S.; Majzoub, J.; Chan, H.L.; Giannobile, W.V.; Wang, H.L.; Kripfgans, O.D. Ultrasonographic tissue perfusion analysis at implant and palatal donor sites following soft tissue augmentation: A clinical pilot study. J. Clin. Periodontol. 2021, 48, 602–614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salmon, B.; Le Denmat, D. Intraoral ultrasonography: Development of a specific high-frequency probe and clinical pilot study. Clin. Oral Investig. 2012, 16, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Barootchi, S.; Tavelli, L.; Majzoub, J.; Chan, H.L.; Wang, H.L.; Kripfgans, O.D. Ultrasonographic Tissue Perfusion in Peri-implant Health and Disease. J. Dent. Res. 2022, 101, 278–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thöne-Mühling, M.; Kripfgans, O.D.; Mengel, R. Ultrasonography for noninvasive and real-time evaluation of peri-implant soft and hard tissue: A case series. Int. J. Implant. Dent. 2021, 7, 95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tattan, M.; Sinjab, K.; Lee, E.; Arnett, M.; Oh, T.J.; Wang, H.L.; Chan, H.L.; Kripfgans, O.D. Ultrasonography for chairside evaluation of periodontal structures: A pilot study. J. Periodontol. 2020, 91, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]

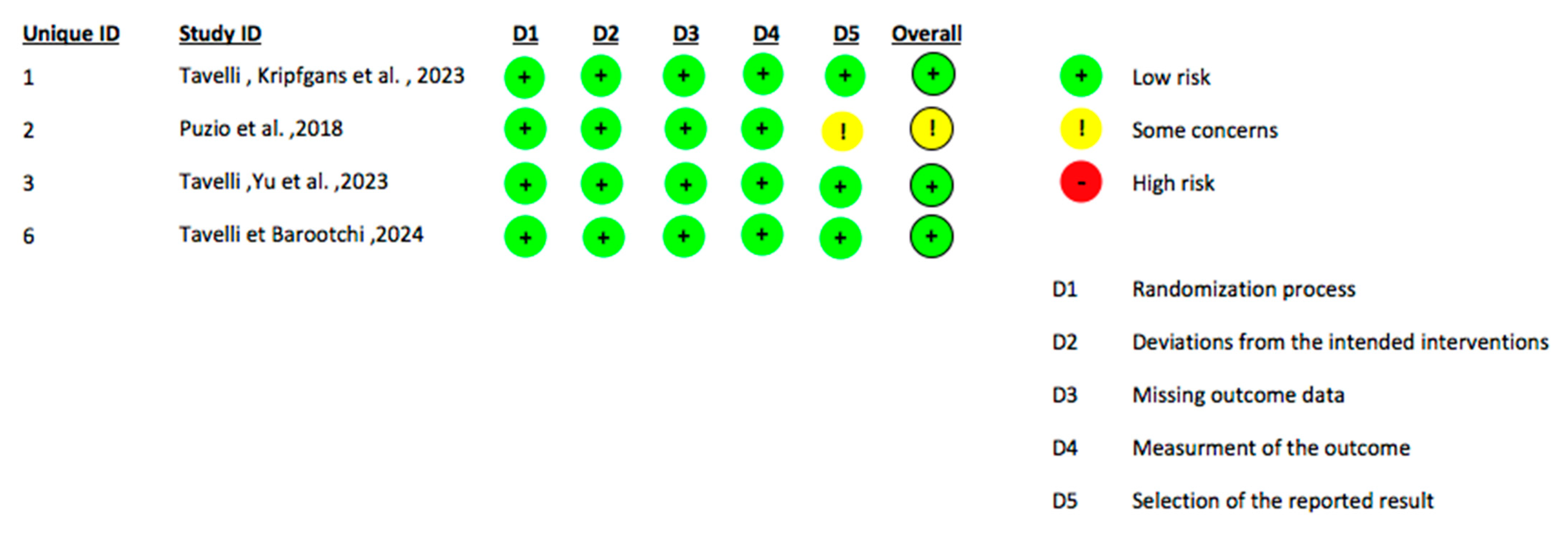
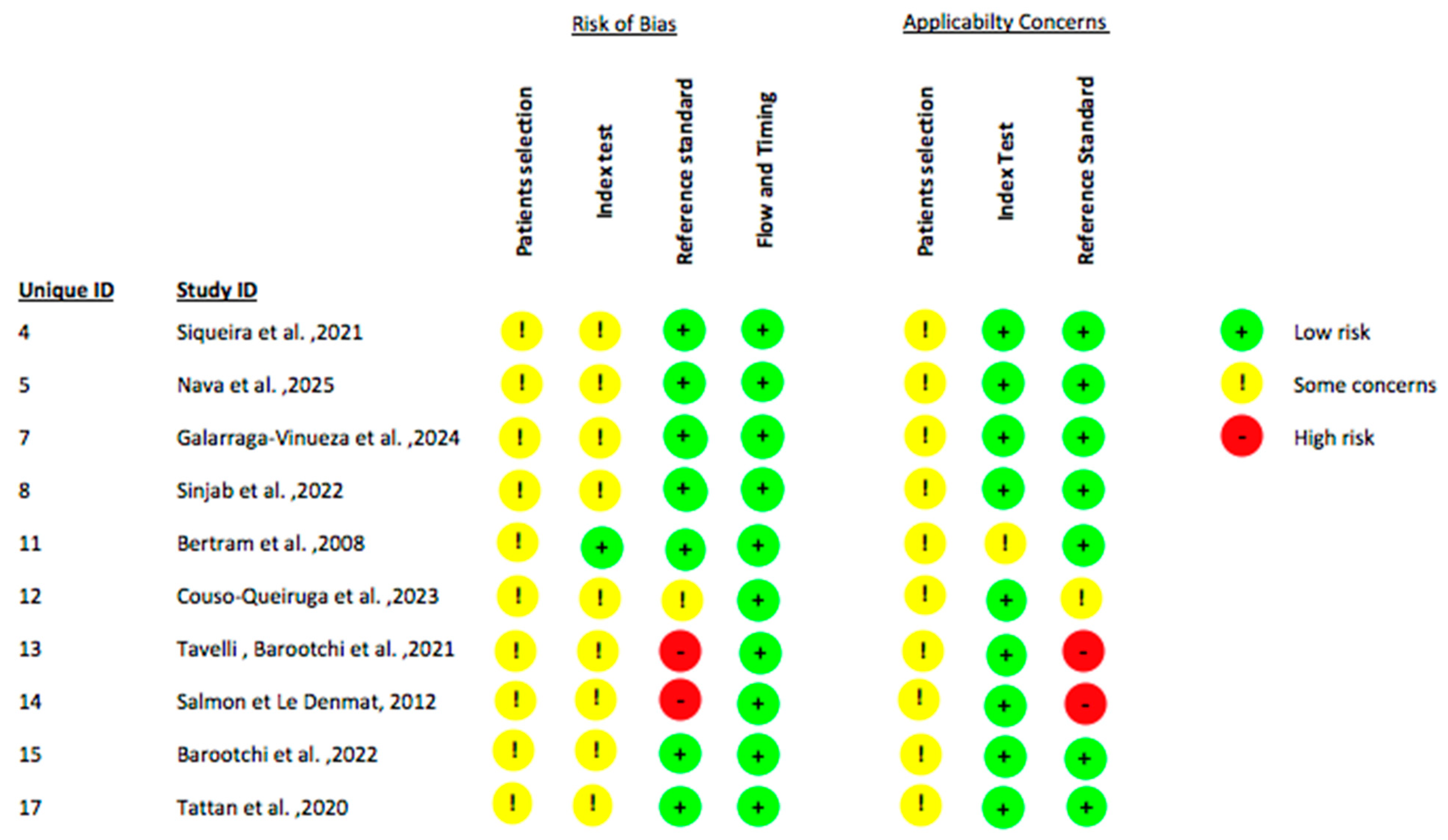
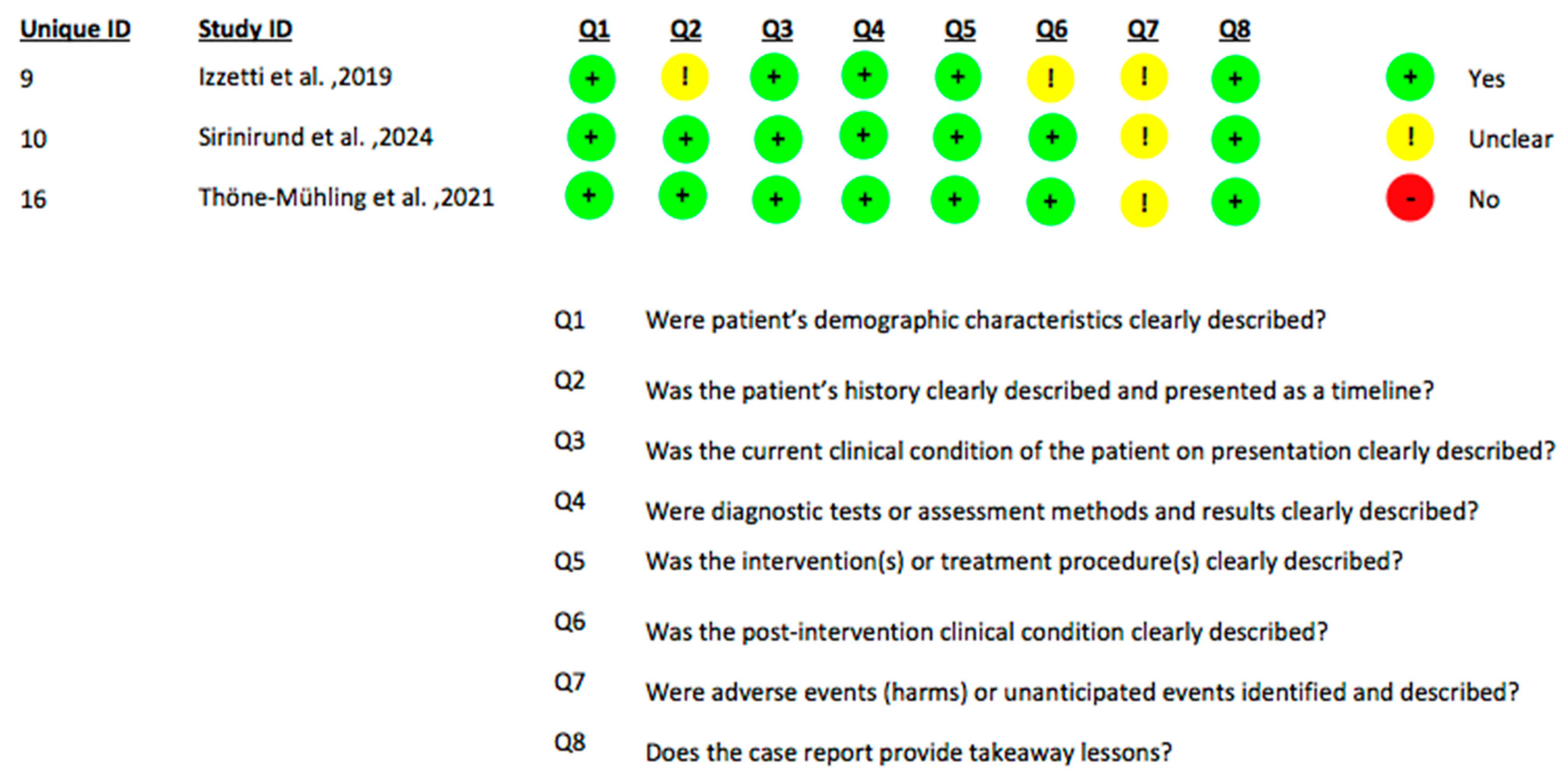
| Title | Authors | Study Design | Number of Patients | Ultrasound Type | Ultrasound Application | Surgical Procedure Evaluated with the Ultrasound | Outcomes Assessed | Follow Up | Comparison with Other Diagnostic Methods |
|---|---|---|---|---|---|---|---|---|---|
| Doppler ultrasonographic evaluation of tissue revascularization following connective tissue graft at implant sites | Tavelli, Kripfgans et al., 2025 [30] | RCT | 28 | High-frequency ultrasound (HFUS) system with Colour Doppler Velocity (CDV) and Power Doppler Imaging (PDI) | Both pre-operatively and post-operatively | CTG for peri-implant soft tissue augmentation using CAF or TUN | (1) Changes in the tissue perfusion (2) Early revascularization of the CTG (3) Direction of perfusion | Preoperative 1 week 1 month 6 months 12 months | No comparison |
| Ultrasound assessment of soft tissue augmentation around implants in the aesthetic zone using a connective tissue graft and xenogeneic collagen matrix—1-year randomised follow-up | Puzio et al., 2018 [31] | RCT | 57 | Pirop® ultrasonic device (Echoson, Puławy, Poland), A-scan probe, 20 MHz frequency, measurement range 0.25–6 mm, accuracy ±0.01 mm | Both pre-operatively and post-operatively | Soft tissue augmentation around implants in the aesthetic zone using either CTG or xenogeneic collagen matrix | (1) Thickness of keratinized tissue (TKT) at the cemento-enamel junction (CEJ) (2) Thickness of keratinized tissue (TKT) at the mucogingival junction (MGJ) | Preoperative 3 months 12 months | Direct periodontal probing with a manual probe |
| Keratinized mucosa width assessment at implant sites using high-frequency ultrasonography | Tavelli, Yu et al., 2023 [32] | RCT | 28 | Miniature HFUS transducer, 24 MHz, B-mode, high axial resolution (64 μm). | Both pre-operatively and post-operatively | CTG for peri-implant soft tissue augmentation using CAF or TUN | (1) Keratinized Mucosa width (KM) around dental implants (2) Mucosal Thickness (MT) around dental implants | Preoperative 12 months | Direct periodontal probing with a manual probe (PCP UNC 15) |
| Comprehensive peri-implant tissue evaluation with ultrasonography and cone-beam computed tomography: A pilot study | Siqueira et al., 2021 [33] | Retrospective pilot observational diagnostic accuracy study | 4 | High-resolution, 24 MHz, intraoral probe (ZS3, Mindray, Shenzhen, China) with B-mode and color-flow imaging | Pre-operatively | (1) Revision/implant removal surgery due to peri-implantitis (2) Second-stage uncovery surgery | (1) Crestal Bone Thickness (CBT) (2) Facial Bone Level (FBL) (3) Soft tissue characteristics (4) Blood flow within the soft tissue | No follow up. The evaluations were all performed at the time of the surgical procedure | (1) CBCT (2) Direct measurements (intraoperative photographs and a digital modeling) |
| Ultrasonography-Guided Dental Implant Surgery: A Feasibility Study | Nava et al., 2025 [34] | Prospective diagnostic feasibility study | 9 | S3 Mindray ultrasound + L30-8 linear probe, 24 MHz, 32.1 μm resolution | Both pre-operatively and post-operatively | Fully guided implant placement using a tooth-supported surgical guide | (1) Soft tissue profile deviation (2) Implant planning | No follow-up. Evaluations were performed pre-operatively for planning and immediately post-operatively to verify implant position | (1) CBCT-based implant planning (2) Intraoperative verification (actual implant positions measured during surgery were compared with the US-based plan) |
| Soft tissue elasticity at teeth and implant sites. A novel outcome measure of the soft tissue phenotype | Tavelli & Barootchi 2024 [35] | RCT | 28 | Ultrasound device: ZS3 (Zonare/Mindray, Mahwah, NJ, USA) Transducer: L30-8, 24 MHz (Zonare/Mindray, Mahwah, NJ, USA) | Both pre-operatively and post-operatively | CTG for peri-implant soft tissue augmentation using CAF or TUN | (1) Mucosal thickness (2) Gingival thickness (3) Buccal bone dehiscence (BBD) (4) Tissue elasticity/strain ratios (SR1, SR2, SR3) | Preoperative 6 months 12 months | Direct periodontal probing with a manual probe |
| Echo-intensity characterization at implant sites and novel diagnostic ultrasonographic markers for peri-implantitis | Galarraga-Vinueza et al., 2024 [36] | Diagnostic accuracy study | 60 | High-frequency ultrasound (HFUS) (The authors did not provide details regarding the brand and model of the ultrasound device employed) | Post-operatively | Implant placement and loading | (1) Mucosal thickness (2) Keratinized tissue width (3) Mucosal recession (4) Supracrestal tissue height (STH) (5) Soft tissue area (STA) (6) Hypoechoic supracrestal area (HSA) (7) Proportion HSA/STA (8) Buccal bone dehiscence (9) Crown angle (10) Greyscale texture outcomes | The ultrasound was used once to image implants that had already been in function for at least 1 year | (1) Direct periodontal probing (2) Periapical radiographs |
| Ultrasonographic Evaluation of Edentulous Crestal Bone Topography: A Proof-of-Principle Retrospective Study | Sinjab et al., 2022 [37] | Retrospective diagnostic accuracy study. | 28 | Ultrasound device: ZS3 (Mindray of North America, Mountain View, CA, USA) Prototype probe (L25–8, 24-MHz transmit frequency, Mindray, Shenzhen, China) | Pre-operatively | Healed edentulous sites scheduled for implant placement | (1) Bone ridge width (BRW) (2) Crestal bone surface quality (CBSQ) | No follow up. The evaluations were all performed before the surgical procedure | CBCT |
| Feasibility of a combination of intraoral UHFUS and CBCT in the study of peri-implantitis | Izzetti et al., 2019 [38] | Case report | 1 | Vevo MD system (Fujifilm VisualSonics, Toronto, Canada) 70 MHz probe (ultra-high-frequency ultrasonography, UHFUS) | Post-operatively | Implant placement followed by a Guided Bone Regeneration (GBR)procedure (membrane + allogeneic bone graft) | (1) Width of the lesion (2) Degree of soft tissue alteration (3) Vascularity of the swollen mucosa (4) Identification of hypoechoic periosteal alterations and hyperechoic graft remnants | No follow up. The evaluations were all performed immediately after the onset of postoperative complications | CBCT |
| Ridge augmentation planning, wound healing evaluation, and peri-implant tissue phenotype assessment with ultrasonography: A case report | Sirinirund et al., 2024 [39] | Case report | 1 | Ultrasound device: (ZS3, Mindray of North America, Mountain View, CA, USA) L30-8 probe | Both pre-operatively and post-operatively | Tooth extraction: GBR with allograft + resorbable membrane. Implant placement with simultaneous GBR. Second-stage implant surgery with free gingival graft and crown delivery. | (1) Soft tissue thickness (2) Crestal bone width (3) Muscle attachment location (4) Soft-hard tissue interface morphology (5) Tissue perfusion (fractional color density with color/power Doppler) (6) Peri-implant tissue phenotype at 1 year after implant loading | Baseline 1 month 2.5 month 5 month 12 months | No comparison |
| Sonography of peri-implant buccal bone defects in periodontitis patients: A pilot study | Bertram et al., 2008 [40] | Diagnostic accuracy pilot study | 25 | Sonoace Pico (Sonoace GmbH, Marl, Germany) Linear B-scan 12.5 MHz small-part transducer | Pre-operatively | Periodontal flap surgery for patients with residual deep pockets (>6 mm) and peri-implant buccal bone loss. | Vertical distance between the upper implant thread and the most apical level of marginal buccal bone (linear bone loss measurement) | No follow up. Evaluations were all performed before and at the time of the surgical procedure | Direct periodontal probing with a manual probe |
| Non-invasive assessment of peri-implant mucosal thickness: A cross-sectional study | Couso-Queiruga et al., 2023 [41] | Cross-sectional diagnostic accuracy study | 50 | Non-ionizing ultrasound biometer (PIROP, Echo-Son, Pulawy, Poland) Probe frequency 20 MHz | Post-operatively | Implants already placed and restored before the study (diagnostic evaluation only) | Facial mucosal thickness (FMT) measured 3 mm apical to the mid-facial mucosal margin of each implant. | The ultrasound was used once to image implants that had already been in function for at least 1 year | (1) CBCT (2) CBC+STL |
| Ultrasonographic tissue perfusion analysis at implant and palatal donor sites following soft tissue augmentation: A clinical pilot study | Tavelli, Barootchi et al., 2021 [42] | Prospective clinical pilot diagnostic study | 5 | Power Doppler ultrasonography with a high-frequency linear transducer (20 MHz, 35-mm footprint) | Both pre-operatively and post-operatively | CAF combined with a subepithelial CTG for treatment of peri-implant soft tissue dehiscence defects at the implant site | Vascularization/perfusion indices at implant and palatal donor sites | Baseline 1 week 2 weeks 6 weeks | No comparison |
| Intraoral ultrasonography: development of a specific high-frequency probe and clinical pilot study | Salmon & Le Denmat 2012 [43] | Prospective clinical pilot feasibility study | 3 | Custom-made intraoral high-frequency B-mode ultrasound probe, 25 MHz linear transducer for periodontal, peri-implant and soft tissue imaging | Post-operatively | Implants already placed and restored before the study (diagnostic evaluation only) | Visualization of periodontal, peri-implant and soft tissue structures | The ultrasound was used once to image implants that had already been in function | No comparison |
| Ultrasonographic Tissue Perfusion in Peri-implant Health and Disease | Barootchi et al., 2022 [44] | Cross-sectional diagnostic case–control study | 21 | Color flow and power Doppler ultrasonography, high-frequency linear transducer, used to quantify peri-implant tissue perfusion (color velocity, color power) | Post-operatively | Implants already placed and restored before the study (diagnostic evaluation only) | Peri-implant tissue perfusion | The ultrasound was used once to image implants that had already been in function for at least 3 years | (1) Direct periodontal probing (2) Periapical radiographs |
| Ultrasonography for noninvasive and real- time evaluation of peri-implant soft and hard tissue: a case series | Thöne-Mühling et al., 2021 [45] | Case series | 3 | Mindray ZS3 system with L30-8 high-frequency intraoral probe (resolution ~60 µm), B-mode imaging; standoff pad used in some cases for buccal scans | Post-operatively | Implants already placed and restored before the study (diagnostic evaluation only) | (1) Crestal bone level (implant threads) (2) Detection of buccal bone loss (3) Soft tissue thickness at peri-implant sites (4) Visualization of implant–abutment connection + surrounding peri-implant tissues | The ultrasound was used once to image implants that had already been in function for at least 6 years | (1) Intraoral radiographs (2) Intraoperative surgical findings |
| Ultrasonography for chairside evaluation of periodontal structures: A pilot study | Tattan et al., 2020 [46] | Prospective diagnostic pilot study | 20 | Prototype 24 MHz intraoral B-mode US probe with gel standoff pad (axial res. 64 µm, lateral 192 µm) | Pre-operatively | Single-tooth implant placement with flap reflection | (1) Interdental papilla height (2) Mid-facial soft tissue height (3) Mucosal thickness (tooth and edentulous ridge) (4) Soft tissue height at ridge (5) Crestal bone level | No follow up. The evaluations were all performed before the surgical procedure | (1) Direct periodontal probing (2) CBCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barausse, C.; Tayeb, S.; Sansavini, M.; Pellegrino, G.; Felice, P. Diagnostic Applications of Ultrasound Imaging in Dental Implantology: A Systematic Review. J. Clin. Med. 2025, 14, 8239. https://doi.org/10.3390/jcm14228239
Barausse C, Tayeb S, Sansavini M, Pellegrino G, Felice P. Diagnostic Applications of Ultrasound Imaging in Dental Implantology: A Systematic Review. Journal of Clinical Medicine. 2025; 14(22):8239. https://doi.org/10.3390/jcm14228239
Chicago/Turabian StyleBarausse, Carlo, Subhi Tayeb, Martina Sansavini, Gerardo Pellegrino, and Pietro Felice. 2025. "Diagnostic Applications of Ultrasound Imaging in Dental Implantology: A Systematic Review" Journal of Clinical Medicine 14, no. 22: 8239. https://doi.org/10.3390/jcm14228239
APA StyleBarausse, C., Tayeb, S., Sansavini, M., Pellegrino, G., & Felice, P. (2025). Diagnostic Applications of Ultrasound Imaging in Dental Implantology: A Systematic Review. Journal of Clinical Medicine, 14(22), 8239. https://doi.org/10.3390/jcm14228239









