Recent Advancements in the Clinical Pathway of Respiration-Synchronized Hypoglossal Nerve Stimulation Therapy for Obstructive Sleep Apnea
Abstract
1. Introduction
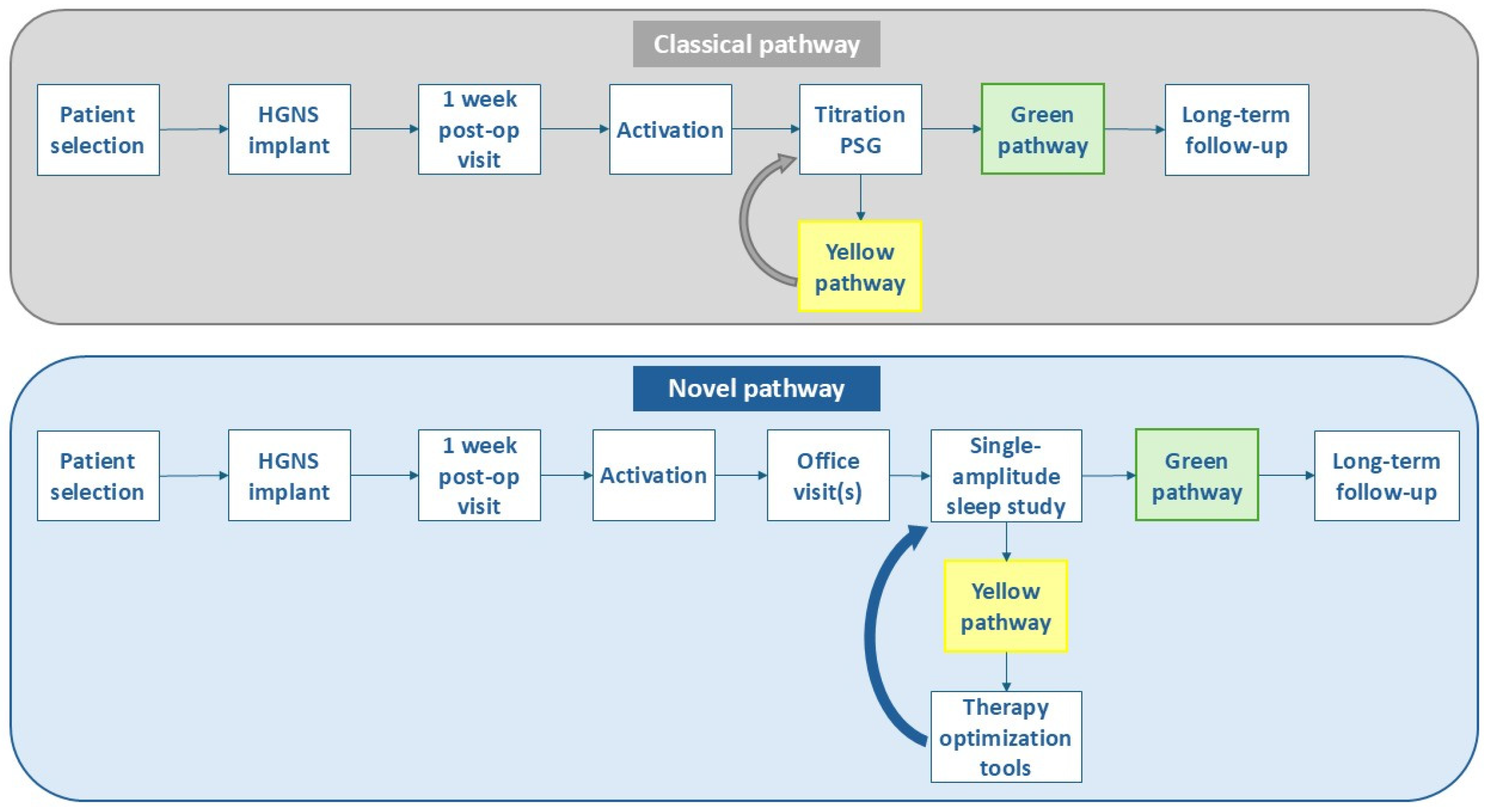
2. Methods
3. Patient Selection
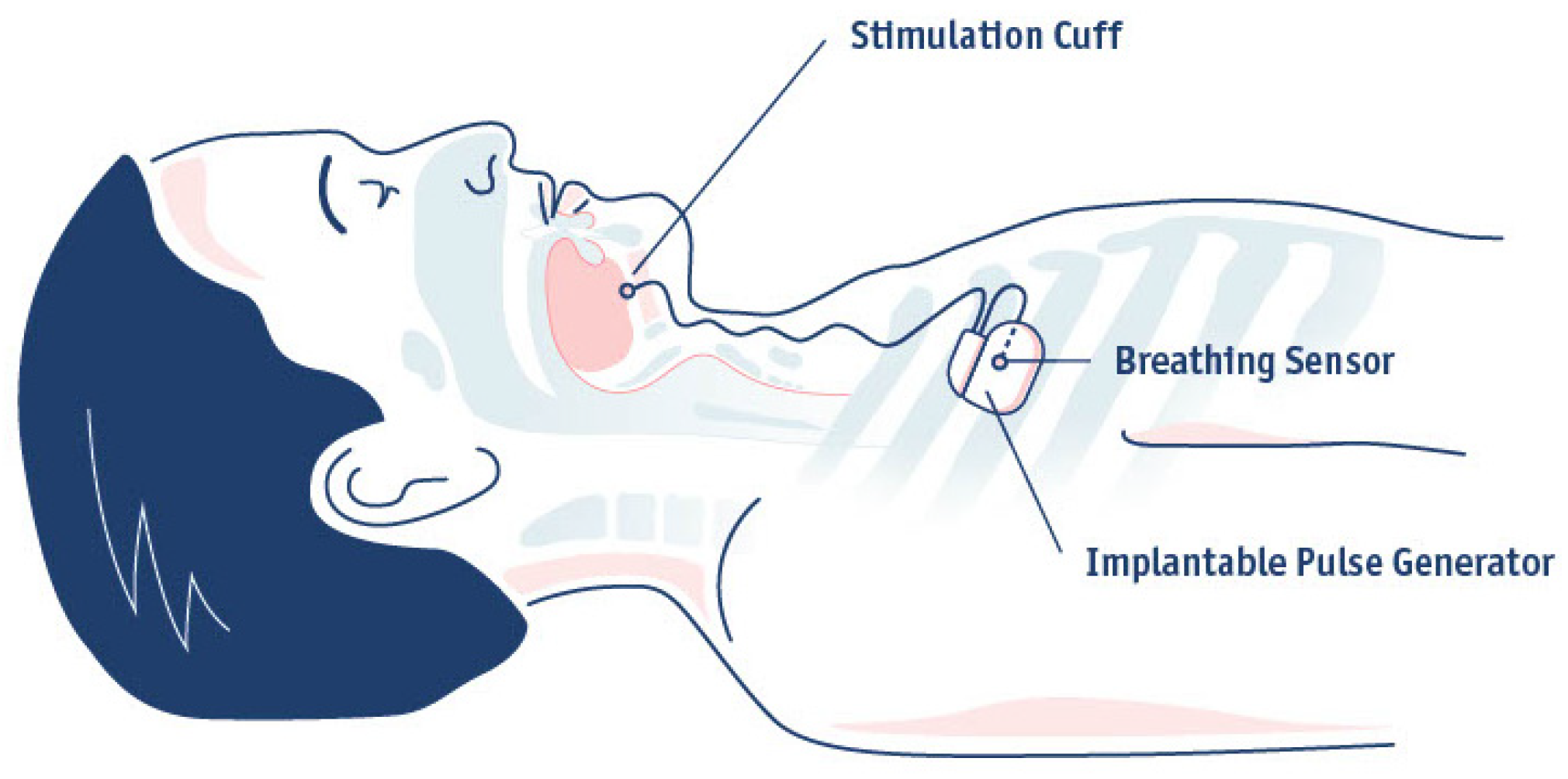
4. Implantation
5. Activation, Education and Acclimation
6. Defining HGNS Treatment Success
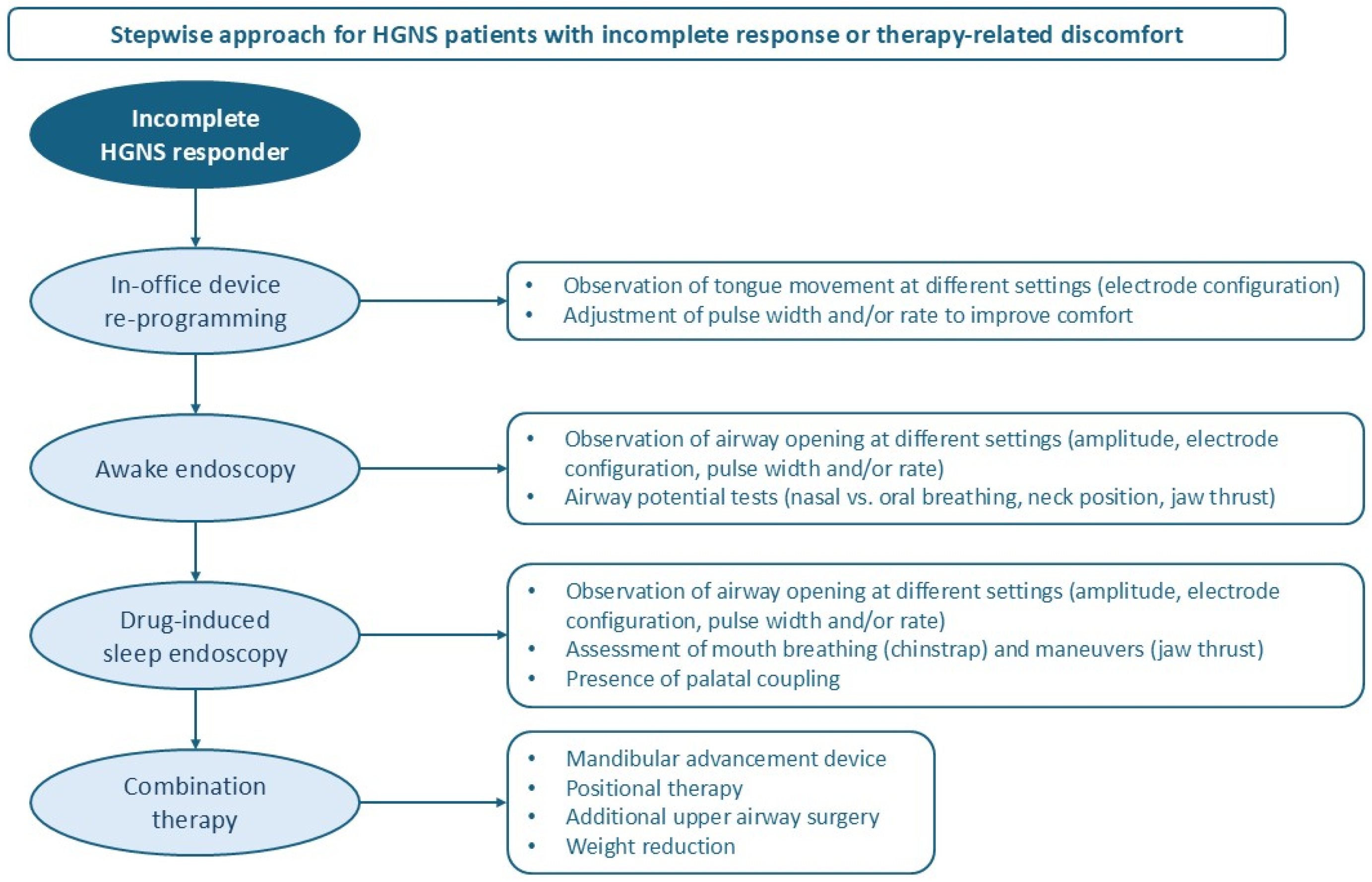
7. Optimization Tools for HGNS Patients in the Yellow Pathway
7.1. In-Office Device Reprogramming
7.2. Awake Endoscopy
7.3. Drug-Induced Sleep Endoscopy (DISE)
7.4. Combination Therapy
8. Long-Term Management
9. Limitations of Current Evidence
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHI | Apnea-hypopnea index |
| BMI | Body Mass Index |
| CCC | Complete concentric collapse |
| CPAP | Continuous Positive Airway Pressure |
| DISE | Drug-induced sleep endoscopy |
| FDA | Food and Drug Administration |
| HGNS | Hypoglossal nerve stimulation |
| HST | Home Sleep Test |
| IPG | Implantable Pulse Generator |
| MAD | Mandibular advancement device |
| MRI | Magnetic Resonance Imaging |
| OSA | Obstructive sleep apnea |
| PSG | Polysomnography |
References
- Strollo, P.J., Jr.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Cornelius, J.; Froymovich, O.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; Gillespie, M.B.; et al. Upper-airway stimulation for obstructive sleep apnea. N. Engl. J. Med. 2014, 370, 139–149. [Google Scholar] [CrossRef]
- Woodson, B.T.; Soose, R.J.; Gillespie, M.B.; Strohl, K.P.; Maurer, J.T.; de Vries, N.; Steward, D.L.; Baskin, J.Z.; Badr, M.S.; Lin, H.S.; et al. Three-Year Outcomes of Cranial Nerve Stimulation for Obstructive Sleep Apnea: The STAR Trial. Otolaryngol. Head Neck Surg. 2016, 154, 181–188. [Google Scholar] [CrossRef]
- Woodson, B.T.; Strohl, K.P.; Soose, R.J.; Gillespie, M.B.; Maurer, J.T.; De Vries, N.; Padhya, T.A.; Badr, M.S.; Lin, H.S.; Vanderveken, O.M.; et al. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngol. Head Neck Surg. 2018, 159, 194–202. [Google Scholar] [CrossRef]
- Steffen, A.; Sommer, U.J.; Maurer, J.T.; Abrams, N.; Hofauer, B.; Heiser, C. Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath. 2020, 24, 979–984. [Google Scholar] [CrossRef]
- Suurna, M.V.; Steffen, A.; Boon, M.; Chio, E.; Copper, M.; Patil, R.D.; Green, K.; Hanson, R.; Heiser, C.; Huntley, C.; et al. Impact of Body Mass Index and Discomfort on Upper Airway Stimulation: ADHERE Registry 2020 Update. Laryngoscope 2021, 131, 2616–2624. [Google Scholar] [CrossRef] [PubMed]
- Vanderveken, O.M.; Beyers, J.; Op de Beeck, S.; Dieltjens, M.; Willemen, M.; Verbraecken, J.A.; De Backer, W.A.; Van de Heyning, P.H. Development of a Clinical Pathway and Technical Aspects of Upper Airway Stimulation Therapy for Obstructive Sleep Apnea. Front. Neurosci. 2017, 11, 523. [Google Scholar] [CrossRef] [PubMed]
- Soose, R.J.; Faber, K.; Greenberg, H.; Boon, M.; Woodson, T.; Strollo, P. Post-implant care pathway: Lessons learned and recommendations after 5 years of clinical implementation of hypoglossal nerve stimulation therapy. Sleep 2021, 44 (Suppl. S1), S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Thaler, E.; Schwab, R.; Maurer, J.; Soose, R.; Larsen, C.; Stevens, S.; Stevens, D.; Boon, M.; Huntley, C.; Doghramji, K.; et al. Results of the ADHERE upper airway stimulation registry and predictors of therapy efficacy. Laryngoscope 2020, 130, 1333–1338. [Google Scholar] [CrossRef]
- Tukanov, E.; Van Loo, D.; Dieltjens, M.; Verbraecken, J.; Vanderveken, O.M.; Op de Beeck, S. Baseline Characteristics Associated with Hypoglossal Nerve Stimulation Treatment Outcomes in Patients with Obstructive Sleep Apnea: A Systematic Review. Life 2024, 14, 1129. [Google Scholar] [CrossRef] [PubMed]
- Vanderveken, O.M.; Maurer, J.T.; Hohenhorst, W.; Hamans, E.; Lin, H.S.; Vroegop, A.V.; Anders, C.; de Vries, N.; Van de Heyning, P.H. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J. Clin. Sleep Med. 2013, 9, 433–438. [Google Scholar] [CrossRef]
- Huyett, P.; Kent, D.T.; D’Agostino, M.A.; Green, K.K.; Soose, R.J.; Kaffenberger, T.M.; Woodson, B.T.; Huntley, C.; Boon, M.S.; Heiser, C.; et al. Drug-Induced Sleep Endoscopy and Hypoglossal Nerve Stimulation Outcomes: A Multicenter Cohort Study. Laryngoscope 2021, 131, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Nord, R.S.; Fitzpatrick, T.t.; Pingree, G.; Islam, A.; Chafin, A. Should lateral wall collapse be a contraindication for hypoglossal nerve stimulation? Am. J. Otolaryngol. 2024, 45, 104053. [Google Scholar] [CrossRef] [PubMed]
- Vena, D.; Op de Beeck, S.; Yang, H.; Sumner, J.; Mann, D.; Wang, T.Y.; Aishah, A.; Azarbarzin, A.; Messineo, L.; Calianese, N.; et al. Lateral wall collapse from sleep endoscopy and airflow shape predicts hypoglossal nerve stimulation efficacy in obstructive sleep apnea. Eur. Respir. J. 2025, 66, 2500236. [Google Scholar] [CrossRef]
- Op de Beeck, S.; Wellman, A.; Dieltjens, M.; Strohl, K.P.; Willemen, M.; Van de Heyning, P.H.; Verbraecken, J.A.; Vanderveken, O.M.; Sands, S.A.; Investigators, S.T. Endotypic Mechanisms of Successful Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2021, 203, 746–755. [Google Scholar] [CrossRef]
- Pordzik, J.; Petrowski, K.; Ludwig, K.; Seifen, C.; Matthias, C.; Gouveris, H. Difficulty Falling Asleep is Associated with Poorer Therapeutic Outcomes in Unilateral Hypoglossal Nerve Stimulation. Nat. Sci. Sleep 2024, 16, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Dhanda Patil, R.; Suurna, M.V.; Steffen, A.; Soose, R.; Coxe, J.; Chan, T.; Ishman, S.L. Relationship of Nocturnal Insomnia Symptoms and Outcomes After Hypoglossal Nerve Stimulation. OTO Open 2024, 8, e134. [Google Scholar] [CrossRef]
- Dhanda Patil, R.; Ishman, S.L.; Chang, J.L.; Thaler, E.; Suurna, M.V. Impact of Insomnia on Hypoglossal Nerve Stimulation Outcomes in the ADHERE Registry. Laryngoscope 2024, 134, 471–479. [Google Scholar] [CrossRef]
- Steffen, A.; Baptista, P.; Ebner, E.M.; Jeschke, S.; Konig, I.R.; Bruchhage, K.L. Insomnia affects patient-reported outcome in sleep apnea treated with hypoglossal nerve stimulation. Laryngoscope Investig. Otolaryngol. 2022, 7, 877–884. [Google Scholar] [CrossRef]
- Rosenthal, M.E.; Lyons, M.M.; Schweller, J.; Yildiz, V.O.; Chio, E.G.; Khan, M.S. Association between anxiety, depression, and emotional distress and hypoglossal nerve stimulator adherence. Sleep Breath. 2022, 26, 141–147. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Premarket Approval (PMA)—Inspire Upper Airway Stimulation. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P130008 (accessed on 29 September 2025).
- U.S. Food and Drug Administration. Premarket Approval (PMA)—Inspire Upper Airway Stimulation. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P130008S039 (accessed on 29 September 2025).
- Bosschieter, P.F.N.; de Vries, N.; Mehra, R.; Manchanda, S.; Padhya, T.A.; Vanderveken, O.M.; Ravesloot, M.J.L.; Investigators, A.R. Similar effect of hypoglossal nerve stimulation for obstructive sleep apnea in 5 disease severity categories. J. Clin. Sleep Med. 2022, 18, 1657–1665. [Google Scholar] [CrossRef]
- Coca, K.K.; Heiser, C.; Huntley, C.; Boon, M.; de Vries, N.; Mamidala, M.; Gillespie, M.B. Hypoglossal Nerve Stimulation Usage by Therapy Nonresponders. Otolaryngol. Head Neck Surg. 2022, 166, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Wang, H.Z.; Jamro, E.L.; Lindburg, M.R.; Jackson, R.S.; Malhotra, R.K.; Lucey, B.P.; Landsness, E.C. Response to Hypoglossal Nerve Stimulation Changes With Body Mass Index and Supine Sleep. JAMA Otolaryngol. Head Neck Surg. 2024, 150, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Han, C.J.; Kaki, P.C.; Goldfarb, J.A.; Kaffenberger, T.M.; Molin, N.L.; Matzke, A.; Boon, M.S.; Huntley, C.T. Impact of Postoperative Weight Changes on Hypoglossal Nerve Stimulation Success for Obstructive Sleep Apnea. Otolaryngol. Head Neck Surg. 2025, 173, 761–768. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Inspire Upper Airway Stimulation—P130008/S089. Available online: https://www.fda.gov/medical-devices/recently-approved-devices/inspire-upper-airway-stimulation-p130008s089 (accessed on 21 January 2025).
- Stenerson, M.E.; Yu, P.K.; Kinane, T.B.; Skotko, B.G.; Hartnick, C.J. Long-term stability of hypoglossal nerve stimulation for the treatment of obstructive sleep apnea in children with Down syndrome. Int. J. Pediatr. Otorhinolaryngol. 2021, 149, 110868. [Google Scholar] [CrossRef]
- Kent, D.T.; Chio, E.G.; Weiner, J.S.; Heiser, C.; Suurna, M.V.; Weidenbecher, M. A Noninferiority Analysis of 3- vs 2-Incision Techniques for Hypoglossal Nerve Stimulator Implantation. Otolaryngol. Head Neck Surg. 2022, 167, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Heiser, C.; Thaler, E.; Boon, M.; Soose, R.J.; Woodson, B.T. Updates of operative techniques for upper airway stimulation. Laryngoscope 2016, 126 (Suppl. S7), S12–S16. [Google Scholar] [CrossRef]
- Inspire Medical Systems, Inc. Announces CE Mark Certification Under the European Union’s Medical Device Regulation for Inspire Therapy. Available online: https://investors.inspiresleep.com/news/news-details/2024/Inspire-Medical-Systems-Inc.-Announces-CE-Mark-Certification-under-the-European-Unions-Medical-Device-Regulation-for-Inspire-Therapy/default.aspx (accessed on 29 September 2025).
- U.S. Food and Drug Administration. Approval for Additional MRI Scanning Conditions Using 1.5T Full Body Coil Scanner for Model 3028 IPG, Model 4323, Model 4340, and Model 4063 Leads. 2022. Available online: https://device.report/m/a67aeb937e0aea03f67f6488745f6ed987dea5644dae6d3aed537e91ff24c9d4_pdf (accessed on 29 September 2025).
- Inspire Medical Systems, Inc. Announces FDA Approval of the Inspire V System. Available online: https://investors.inspiresleep.com/news/news-details/2024/Inspire-Medical-Systems-Inc.-Announces-FDA-Approval-of-the-Inspire-V-System/default.aspx#:~:text=MINNEAPOLIS%2C%20Aug.%2002%2C%202024%20%28GLOBE%20NEWSWIRE%29%20--%20Inspire,the%20associated%20Bluetooth%C2%AE%20patient%20remote%20and%20physician%20programmer (accessed on 25 July 2025).
- Chang, M.; Moore, V.; Eng, K.; Ryden, A.; Zeidler, M. Hypoglossal nerve stimulation over-titration. J. Clin. Sleep Med. 2024, 20, 999–1001. [Google Scholar] [CrossRef]
- Corr, A.; Sina, E.M.; Creighton, E.; Bautista, N.; Chandna, M.; Boon, M.; Huntley, C. Using Postactivation Adjustment as a Marker for Inferior Outcomes in Hypoglossal Nerve Stimulator Patients. Otolaryngol. Head Neck Surg. 2025, 172, 1063–1068. [Google Scholar] [CrossRef]
- Steffen, A.; Jeschke, S.; Soose, R.J.; Hasselbacher, K.; Konig, I.R. Impulse Configuration in Hypoglossal Nerve Stimulation in Obstructive Sleep Apnea: The Effect of Modifying Pulse Width and Frequency. Neuromodulation 2022, 25, 1312–1316. [Google Scholar] [CrossRef]
- Soose, R.J.; Araujo, M.; Faber, K.; Roy, A.; Lee, K.; Ni, Q.; Srivastava, J.; Strollo, P.J. Cluster analysis of upper airway stimulation adherence patterns and implications on clinical care. Sleep 2022, 45, zsac049. [Google Scholar] [CrossRef]
- Yu, P.K.; Huyett, P. Impact of the Connected Sleep Remote on Early Adherence to Hypoglossal Nerve Stimulation Therapy. Laryngoscope 2025, 135, 3944–3950. [Google Scholar] [CrossRef]
- Bosschieter, P.F.N.; Schoustra, E.; de Vries, N.; Steinbusch, M.J.L.; Kasius, K.M.; Ravesloot, M.J.L. Daytime polysomnography to perform titration for upper airway stimulation in patients with obstructive sleep apnea. Sleep Breath. 2022, 26, 707–715. [Google Scholar] [CrossRef]
- Dedhia, R.C.; Woodson, B.T. Standardized Reporting for Hypoglossal Nerve Stimulation Outcomes. J. Clin. Sleep Med. 2018, 14, 1835–1836. [Google Scholar] [CrossRef]
- Kaffenberger, T.M.; Sina, E.M.; Hambach, B.; Kaki, P.; Fuleihan, A.; Boon, M.; Huntley, C. How we measure hypoglossal nerve stimulator outcome matters: Titration vs single amplitude efficacy sleep studies. J. Clin. Sleep Med. 2025, 21, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kaffenberger, T.M.; Soose, R.J.; Strollo, P.J.; Vanderveken, O.M. Three-dimensional mean disease alleviation (3D-MDA): The next step in measuring sleep apnea treatment effectiveness. Sleep Med. 2025, 125, 27–30. [Google Scholar] [CrossRef]
- Steffen, A.; Konig, I.R.; Baptista, P.M.; Abrams, N.; Jeschke, S.; Hasselbacher, K. Home Sleep Testing to Direct Upper Airway Stimulation Therapy Optimization for Sleep Apnea. Laryngoscope 2021, 131, E1375–E1379. [Google Scholar] [CrossRef] [PubMed]
- Kent, D.; Huyett, P.; Yu, P.; Roy, A.; Mehra, R.; Rundo, J.V.; Stahl, S.; Manchanda, S. Comparison of clinical pathways for hypoglossal nerve stimulation management: In-laboratory titration polysomnography vs home-based efficacy sleep testing. J. Clin. Sleep Med. 2023, 19, 1905–1912. [Google Scholar] [CrossRef]
- Kaffenberger, T.M.; Soose, R.J.; Strollo, P.J.; Boon, M.; Huntley, C. Classifying the types of therapeutic sleep studies. J. Clin. Sleep Med. 2025, 21, 1143. [Google Scholar] [CrossRef] [PubMed]
- Vanderveken, O.M. The benefits of capturing patient-reported outcome measures for treatment of obstructive sleep apnea with hypoglossal nerve stimulation. J. Clin. Sleep Med. 2025, 21, 1147–1148. [Google Scholar] [CrossRef]
- Dedhia, R.C.; Shah, A.J.; Bliwise, D.L.; Quyyumi, A.A.; Strollo, P.J.; Li, Q.; Da Poian, G.; Clifford, G.D. Hypoglossal Nerve Stimulation and Heart Rate Variability: Analysis of STAR Trial Responders. Otolaryngol. Head Neck Surg. 2019, 160, 165–171. [Google Scholar] [CrossRef]
- Walia, H.K.; Thompson, N.R.; Strohl, K.P.; Faulx, M.D.; Waters, T.; Kominsky, A.; Foldvary-Schaefer, N.; Mehra, R. Upper Airway Stimulation vs Positive Airway Pressure Impact on BP and Sleepiness Symptoms in OSA. Chest 2020, 157, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Dedhia, R.C.; Bliwise, D.L.; Quyyumi, A.A.; Thaler, E.R.; Boon, M.S.; Huntley, C.T.; Seay, E.G.; Tangutur, A.; Strollo, P.J.; Gurel, N.; et al. Hypoglossal Nerve Stimulation and Cardiovascular Outcomes for Patients With Obstructive Sleep Apnea: A Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2024, 150, 39–48. [Google Scholar] [CrossRef]
- Heiser, C.; Maurer, J.T.; Steffen, A. Functional outcome of tongue motions with selective hypoglossal nerve stimulation in patients with obstructive sleep apnea. Sleep Breath. 2016, 20, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Steffen, A.; Kilic, A.; Konig, I.R.; Suurna, M.V.; Hofauer, B.; Heiser, C. Tongue motion variability with changes of upper airway stimulation electrode configuration and effects on treatment outcomes. Laryngoscope 2018, 128, 1970–1976. [Google Scholar] [CrossRef]
- Pawlak, D.; Bohorquez, D.; Konig, I.R.; Steffen, A.; Thaler, E.R. Effect of Electrode Configuration and Impulse Strength on Airway Patency in Neurostimulation for Obstructive Sleep Apnea. Laryngoscope 2021, 131, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Kent, D.T.; Budnick, H.A.; Green, K.K.; Huyett, P.; Schell, A.E.; Suurna, M.V. A Programming Algorithm for Optimizing Hypoglossal Nerve Stimulator Respiratory Entrainment. Laryngoscope 2025, 135, 1532–1536. [Google Scholar] [CrossRef]
- Wesson, T.; Saltagi, M.Z.; Manchanda, S.; Stahl, S.; Chernyak, Y.; Parker, N. A Protocolized Approach to Awake Endoscopy With Advanced Programming to Optimize Hypoglossal Nerve Stimulation. Otolaryngol. Head Neck Surg. 2024, 171, 927–929. [Google Scholar] [CrossRef]
- Meleca, J.B.; Kominsky, A.H. Reconfiguration of Upper Airway Stimulation Devices Utilizing Awake Endoscopy. Laryngoscope 2020, 130, 2494–2498. [Google Scholar] [CrossRef]
- Wesson, T.; Saltagi, M.Z.; Manchanda, S.; Stahl, S.; Chernyak, Y.; Parker, N. Treatment Outcomes in Awake Endoscopy with Advanced Programming in Hypoglossal Nerve Stimulation. Ann. Otol. Rhinol. Laryngol. 2025, 134, 110–116. [Google Scholar] [CrossRef]
- Kaffenberger, T.M.; Plawecki, A.; Kaki, P.; Boon, M.; Huntley, C. Troubleshooting Upper Airway Stimulation Therapy Using Drug-Induced Sleep Endoscopy. Otolaryngol. Head Neck Surg. 2024, 171, 588–595. [Google Scholar] [CrossRef]
- Lee, J.J.; Sahu, N.; Rogers, R.R.; Soose, R.J. Severe obstructive sleep apnea treated with combination hypoglossal nerve stimulation and oral appliance therapy. J. Dent. Sleep Med. 2015, 2, 185–186. [Google Scholar] [CrossRef]
- Lowery, M.M.; Rundo, J.V.; Walia, H.K.; Shah, V. Personalized multimodal management for severe obstructive sleep apnea in a patient intolerant of positive airway pressure with hypoglossal nerve stimulator and mandibular advancement device. J. Clin. Sleep Med. 2023, 19, 403–408. [Google Scholar] [CrossRef]
- Steffen, A.; Abrams, N.; Suurna, M.V.; Wollenberg, B.; Hasselbacher, K. Upper-Airway Stimulation Before, After, or Without Uvulopalatopharyngoplasty: A Two-Year Perspective. Laryngoscope 2019, 129, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Huyett, P.; Wellman, A.; Caruso, V.; Sumner, J.; Aishah, A.; Azarbarzin, A.; Sands, S.; Vena, D. Combination Tonsillectomy and Hypoglossal Nerve Stimulation for Sleep Apnea Patients With Oropharyngeal Lateral Wall Collapse. Otolaryngol. Head Neck Surg. 2024, 171, 1904–1910. [Google Scholar] [CrossRef]
- Heiser, C.; Steffen, A.; Boon, M.; Hofauer, B.; Doghramji, K.; Maurer, J.T.; Sommer, J.U.; Soose, R.; Strollo, P.J., Jr.; Schwab, R.; et al. Post-approval upper airway stimulation predictors of treatment effectiveness in the ADHERE registry. Eur. Respir. J. 2019, 53, 1801405. [Google Scholar] [CrossRef] [PubMed]
- Heiser, C.; Edenharter, G.; Bas, M.; Wirth, M.; Hofauer, B. Palatoglossus coupling in selective upper airway stimulation. Laryngoscope 2017, 127, E378–E383. [Google Scholar] [CrossRef] [PubMed]
- Lenze, N.R.; Alazawi, D.; Dailey, M.; Brown, C.; Hoff, P.T.; Goldstein, C.A. Evaluating changes in hypoglossal nerve stimulator use over time and long-term adherence. Sleep Breath. 2025, 29, 249. [Google Scholar] [CrossRef] [PubMed]
- Morse, E.; Suurna, M. Hypoglossal nerve stimulator patient usage: Patterns and trends over time. Laryngoscope Investig. Otolaryngol. 2022, 7, 1652–1658. [Google Scholar] [CrossRef]
- Chweya, C.M.; Alapati, R.; Bhargav, A.G.; Wright, R.; Nieves, A.B.; Holtkamp, K.; Rouse, D.; Larsen, C. Predictors of Hypoglossal Nerve Stimulator Usage: A Growth Curve Analysis Study. Otolaryngol. Head Neck Surg. 2025, 172, 2116–2123. [Google Scholar] [CrossRef]
- Zhu, Z.; Hofauer, B.; Wirth, M.; Heiser, C. Long-term changes of stimulation intensities in hypoglossal nerve stimulation. J. Clin. Sleep Med. 2020, 16, 1775–1780. [Google Scholar] [CrossRef]
| Optimization Tool for Yellow HGNS Patients | References | Study Design | Sample Size | Main Findings | Conclusion | Limitations |
|---|---|---|---|---|---|---|
| In-office device reprogramming | Heiser et al. [49] | Multicenter, retrospective study | 14 | An increased AHI reduction was found in patients with bilateral and right protrusion (Baseline: 29.6 ± 12.6/h; M06: 9.7 ± 12.6/h) compared to the mixed activation group (Baseline 49.6 ± 13.8/h; M06: 40.5 ± 4.1/h). | Postoperative tongue motions are associated with HGNS therapy outcome. |
|
| Steffen et al. [50] | Multicenter, prospective study | 35 | Changes in tongue motion patterns were frequently observed (58.8%) with different electrode configuration settings. Most of the patients alternated between right and bilateral protrusion (73.5%). | Alterations in electrode configuration modify tongue motion patterns and influence therapeutic outcomes of HGNS therapy. |
| |
| Pawlak et al. [51] | Single-center, prospective case series | 30 | The upper airway opening effects were visible in all HGNS patients during endoscopy, but the effect differed for the airway level and for the applied electrode configuration. | HGNS enlarges the upper airway at a variety of electrode configurations and voltages with similar opening effects in awake endoscopy and DISE in a large number of patients. |
| |
| Kent et al. [52] | Case report | 2 | Office-based adjustments to respiratory sensing parameters demonstrate improvements in patient tolerance in one case and HGNS efficacy in the other case. | Respiratory sensing changes in the office setting can impact patient comfort and HGNS response. |
| |
| Awake endoscopy (AE) with advanced programming | Wesson et al. [53] | Protocol/single-center case series | 5 | Of the first 5 consecutive patients that underwent AE with advanced HGNS programming, 2 patients (40%) showed an improvement in AHI, while 3 patients (60%) failed to show improvement. | A protocolized approach to AE with advanced HGNS programming can be integrated to optimize AHI reduction and/or patient comfort. |
|
| Meleca et al. [54] | Single-center, retrospective study | 60 | A total of 24 AEs were performed in 19 (32%) patients. The most common complaints and reasons for AE were perceived stimulus discomfort (42%), frequent awakenings (32%), and persistent fatigue or non-normalized AHI (21%). After first AE, there was a 0.87 (53%) and 0.93 (45%) V reduction in functional threshold (FT) and minimum therapeutic amplitude (MTA), respectively. | In-office AE with HGNS advanced programming serves as a useful tool to assess the pharyngeal airway and optimize the device settings. Reduction in the FT and MTA after AE may allow for improved device compliance by reducing discomfort and frequent awakenings. |
| |
| Wesson et al. [55] | Single-center, retrospective study | 17 | An improvement in AHI was noted in 65% of patients after performing AE with advanced HGNS programming, while 35% failed to improve. In one out of two patients with stimulation discomfort, an improvement in device usage could be achieved. | AE with advanced HGNS programming is a powerful tool to identify settings that could increase therapy efficacy and improve patient comfort. |
| |
| Drug-induced sleep endoscopy | Kaffenberger et al. [56] | Single-center, retrospective study | 34 | During DISE with HGNS, palatal coupling was observed in 7 patients (21%) with HGNS alone, 9 patients (26%) with jaw thrust alone, and 8 patients (24%) with both maneuvers combined. In 10 patients (29%), palatal coupling was absent with any maneuver. Based on DISE findings, 13 patients were recommended MAD therapy and 8 patients underwent further surgical interventions. | DISE may serve as a valuable tool to guide further treatment decisions in patients with suboptimal HGNS therapy efficacy as it identifies residual collapse. |
|
| Combination therapy | Lee et al. [57] | Case report | 1 | In a HGNS-treated patient with residual mild OSA and bothersome snoring, MAD therapy was initiated, resulting in a residual AHI of 2.1/h and resolution of snoring. | Combination of HGNS with MAD therapy may successfully treat severe OSA after incomplete response to monotherapy |
|
| Lowery et al. [58] | Case report | 1 | In a patient with residual REM supine OSA (AHI 17.8/h) with HGNS, the combination with MAD and positional therapy resulted in a residual AHI of 5.1/h. | Personalized combination therapy may help to optimize both AHI and subjective symptoms in patients with incomplete response to monotherapy. |
| |
| Patel et al. [24] | Single-center, retrospective study | 76 | In adjusted analyses, HGNS patients with BMI between 32 and 35 kg/m2 had 75% lower odds of responding to HGNS compared with those with a BMI ≤ 32 kg/m2 (odds ratio, 0.25; 95% CI, 0.07–0.94). Of 44 patients who slept in a supine position, 17 (39%) achieved a treatment response, with a clinically meaningful reduction in median (IQR) supine AHI from 46.3 (33.6–63.2) events/h pre-implantation to 21.8 (4.30–42.6) events/h post-implantation. | Higher BMI and supine sleeping position may decrease therapeutic response to HGNS. |
| |
| Steffen et al. [59] | Single-center, retrospective study | 25 in total (7 patients with soft palate surgery after HGNS) | Adjunctive uvulopalatopharyngoplasty with tonsillectomy was performed in HGNS non-responders with persistent soft palate obstruction during DISE, showing an AHI reduction from 49.4 to 13.3 events/hour at 2-year follow-up. | Adjunctive soft palate surgery can improve insufficient response in HGNS patients if the obstruction is identified at the level of the velum/oropharynx. |
| |
| Huyett et al. [60] | Single-center, case–control study | 19 | Linear regression demonstrated that adding tonsillectomy resulted in an additional 22.9% [7.5, 35.2] reduction in AHI [95% confidence interval, CI] (p = 0.006), and 8.6 [1.7,43.4] (p = 0.010) greater odds [95% CI] of a successful treatment response with HGNS. | Combining tonsillectomy with HGNS may represent a promising strategy to improve success rate in patients with oropharyngeal lateral wall collapse. |
| |
| Heiser et al. [61] | Multicenter, prospective observational study (ADHERE) | 227 | For each 1-unit increase in BMI, there was 9% reduced odds of treatment success at 1-year follow-up. | Reduced BMI is a predictor of HGNS treatment response. |
| |
| Suurna et al. [5] | Multicenter, prospective observational study (ADHERE) | 535 (BMI32 n = 438; BMI35 n = 97) | Surgical success was less likely in BMI35 versus BMI32 patients (59.8% vs. 72.2%, p = 0.02). AHI reduction in the BMI35 group was non-inferior to the BMI32 group. | Surgical response rate differs between BMI32 and BMI35 groups, while AHI and ESS reductions are similar between groups. |
| |
| Han et al. [25] | Retrospective study (institutional cohort and ADHERE) | 222 + 1949 patients from ADHERE | The patients who lost at least 2 BMI points had the greatest reduction in AHI (−24.77 ± 13.84, −22.58 ± 19.76 events/h) compared to patients who maintained stable BMI (−15.10 ± 20.33, −18.85 ± 18.11 events/h) or gained at least 2 + BMI points (−6.39 ± 22.52, −15.82 ± 19.63 events/h) following HGNS (p = 0.002 and 0.006, respectively). | Postoperative weight changes significantly correlate with HGNS response, showing the importance of adjunctive weight management after HGNS implantation. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Loo, D.; Tukanov, E.; Dieltjens, M.; Op de Beeck, S.; Van de Perck, E.; Verbraecken, J.; Vanderveken, O.M. Recent Advancements in the Clinical Pathway of Respiration-Synchronized Hypoglossal Nerve Stimulation Therapy for Obstructive Sleep Apnea. J. Clin. Med. 2025, 14, 8241. https://doi.org/10.3390/jcm14228241
Van Loo D, Tukanov E, Dieltjens M, Op de Beeck S, Van de Perck E, Verbraecken J, Vanderveken OM. Recent Advancements in the Clinical Pathway of Respiration-Synchronized Hypoglossal Nerve Stimulation Therapy for Obstructive Sleep Apnea. Journal of Clinical Medicine. 2025; 14(22):8241. https://doi.org/10.3390/jcm14228241
Chicago/Turabian StyleVan Loo, Dorine, Eldar Tukanov, Marijke Dieltjens, Sara Op de Beeck, Eli Van de Perck, Johan Verbraecken, and Olivier M. Vanderveken. 2025. "Recent Advancements in the Clinical Pathway of Respiration-Synchronized Hypoglossal Nerve Stimulation Therapy for Obstructive Sleep Apnea" Journal of Clinical Medicine 14, no. 22: 8241. https://doi.org/10.3390/jcm14228241
APA StyleVan Loo, D., Tukanov, E., Dieltjens, M., Op de Beeck, S., Van de Perck, E., Verbraecken, J., & Vanderveken, O. M. (2025). Recent Advancements in the Clinical Pathway of Respiration-Synchronized Hypoglossal Nerve Stimulation Therapy for Obstructive Sleep Apnea. Journal of Clinical Medicine, 14(22), 8241. https://doi.org/10.3390/jcm14228241









