Acute Pulmonary Edema in COVID-19: Clinical Predictors, Long-Term Pulmonary Sequelae, and Mortality in a Romanian Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Data Collection
2.4. Follow-Up and Assessment of Pulmonary Sequelae
2.5. Study Outcomes
2.6. Statistical Analysis
2.7. Ethical Considerations
2.8. Data Availability
3. Results
3.1. Baseline Demographic and Clinical Characteristics
3.2. Laboratory and Imaging Findings
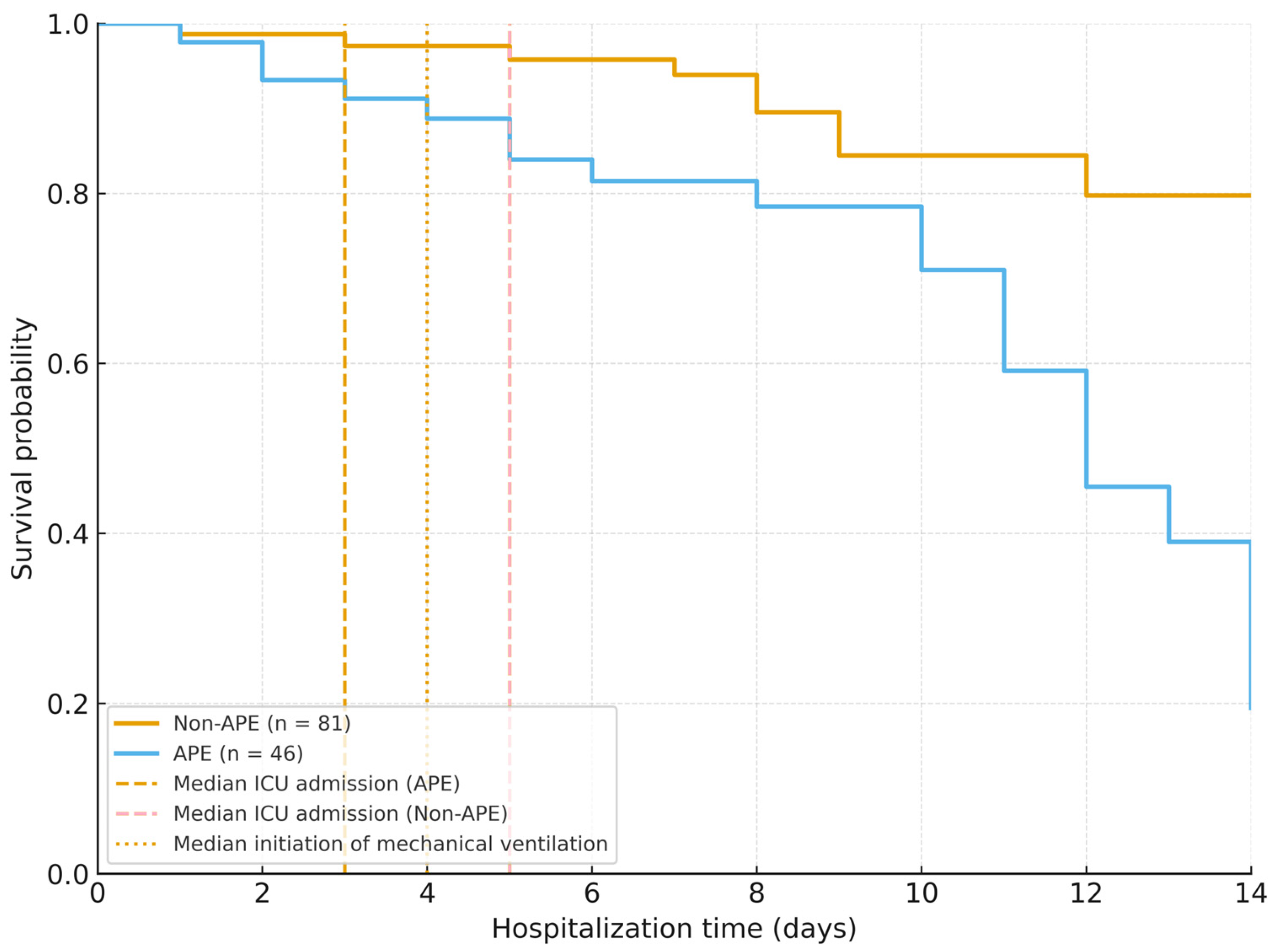
3.3. In-Hospital Outcomes and Mortality
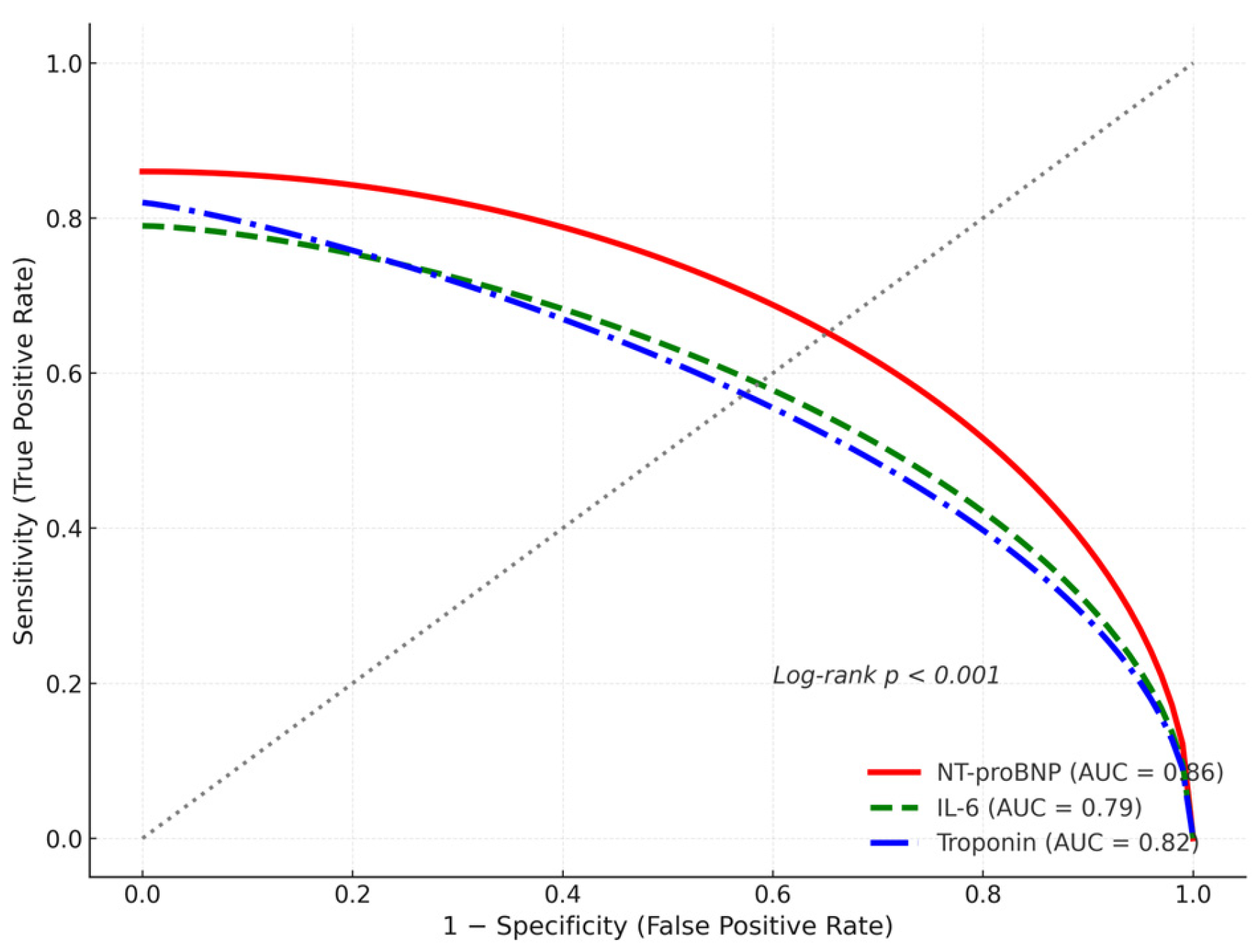
3.4. Predictors of Acute Pulmonary Edema and Mortality
3.5. Three-Month Follow-Up Outcomes
3.6. Summary of Key Findings
- APE occurred in 36.2% of hospitalized COVID-19 patients.
- APE was associated with markedly higher in-hospital mortality (43.5%).
- Independent mortality predictors: APE, elevated NT-proBNP, troponin, and IL-6.
- Pulmonary fibrosis and restrictive dysfunction persisted in ~40% of APE survivors at 3 months.
- The combination of high NT-proBNP (>2000 pg/mL) and IL-6 (>50 pg/mL) identified patients at highest risk of death and residual lung damage.
4. Discussion
4.1. Pathophysiology of APE in COVID-19
4.2. Predictors of APE and Mortality
4.3. Pulmonary Sequelae at Three Months
4.4. Comparison with Other Cohorts and Regional Context
4.5. Clinical Implications
4.6. Limitations
4.7. Strengths
4.8. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APE | Acute Pulmonary Edema |
| ARDS | Acute Respiratory Distress Syndrome |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| CKD | Chronic Kidney Disease |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRP | C-Reactive Protein |
| CT | Computed Tomography |
| DLCO | Diffusing Capacity of the Lung for Carbon Monoxide |
| ECLIA | Electrochemiluminescent Immunoassay |
| FEV1 | Forced Expiratory Volume in One Second |
| FVC | Forced Vital Capacity |
| HRCT | High-Resolution Computed Tomography |
| ICU | Intensive Care Unit |
| IL-6 | Interleukin-6 |
| IQR | Interquartile Range |
| LDH | Lactate Dehydrogenase |
| NT-proBNP | N-Terminal pro-Brain Natriuretic Peptide |
| NIV | Non-Invasive Ventilation |
| OR | Odds Ratio |
| RT-PCR | Reverse Transcriptase Polymerase Chain Reaction |
| SD | Standard Deviation |
| SpO2 | Peripheral Oxygen Saturation |
| VIF | Variance Inflation Factor |
References
- Pipitone, G.; Camici, M.; Granata, G.; Sanfilippo, A.; Di Lorenzo, F.; Buscemi, C.; Ficalora, A.; Spicola, D.; Imburgia, C.; Alongi, I.; et al. Alveolar-Arterial Gradient Is an Early Marker to Predict Severe Pneumonia in COVID-19 Patients. Infect. Dis. Rep. 2022, 14, 450–462. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.; Ashland, M.D.; Vasti, E.C.; Lu, Y.; Chang, A.Y.; Wang, P.; Daniels, L.B.; de Lemos, J.A.; Morrow, D.A.; Rodriguez, F.; et al. N-Terminal Pro-B-Type Natriuretic Peptide as a Biomarker for the Severity and Outcomes With COVID-19 in a Nationwide Hospitalized Cohort. J. Am. Heart Assoc. 2021, 10, e022913. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Trilogy of ACE2: A peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef]
- Mangalmurti, N.; Hunter, C.A. Cytokine storms: Understanding COVID-19. Immunity 2020, 53, 19–25. [Google Scholar] [CrossRef]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and cardiovascular disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef]
- Mojón-Álvarez, D.; Giralt, T.; Carreras-Mora, J.; Calvo-Fernández, A.; Izquierdo, A.; Soler, C.; Cabero, P.; Pérez-Fernández, S.; Vaquerizo, B.; Barquet, N.R. Baseline NT-proBNP levels as a predictor of short- and long-term prognosis in COVID-19 patients: A prospective observational study. BMC Infect. Dis. 2024, 24, 58. [Google Scholar] [CrossRef]
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary post-mortem findings in COVID-19: A series of 38 cases. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I.; et al. Factors associated with hospitalization and critical illness among COVID-19 patients in New York City. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- Carfi, A.; Bernabei, R.; Landi, F. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 1-year pulmonary function and radiological outcomes in COVID-19 survivors. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef]
- Briciu, V.; Leucuta, D.-C.; Muntean, M.; Radulescu, A.; Cismaru, C.; Topan, A.; Herbel, L.; Horvat, M.; Calin, M.; Dobrota, R.; et al. Evolving clinical manifestations and outcomes in COVID-19 patients: A comparative analysis of SARS-CoV-2 variant waves in a Romanian hospital setting. Pathogens 2023, 12, 1453. [Google Scholar] [CrossRef]
- Santos, J.L.F.; Zanardi, P.; Alo, V.; Dos Santos, V.; Bovone, L.; Rodriguez, M.; Magdaleno, F.; De Langhe, V.; Villoldo, A.; Souvielle, R.M.; et al. Lung Injury in COVID-19 Has Pulmonary Edema as an Important Component and Treatment with Furosemide and Negative Fluid Balance (NEGBAL) Decreases Mortality. J. Clin. Med. 2023, 12, 1542. [Google Scholar] [CrossRef]
- Zhang, M.; Jiao, Z. Nonlinear Relationship Between Interleukin-6 and NT-proBNP at Admission in Hospitalized COVID-19 Patients. Infect. Drug Resist. 2023, 16, 6259–6267. [Google Scholar] [CrossRef]
- Zinellu, A.; Sotgia, S.; Carru, C.; Mangoni, A.A. B-Type Natriuretic Peptide Concentrations, COVID-19 Severity, and Mortality: A Systematic Review and Meta-Analysis With Meta-Regression. Front. Cardiovasc. Med. 2021, 8, 690790. [Google Scholar] [CrossRef]
- Yoo, J.; Grewal, P.; Hotelling, J.; Papamanoli, A.; Cao, K.; Dhaliwal, S.; Jacob, R.; Mojahedi, A.; Bloom, M.E.; Marcos, L.A.; et al. Admission NT-proBNP and outcomes in patients without history of heart failure hospitalized with COVID-19. ESC Heart Fail. 2021, 8, 4278–4287. [Google Scholar] [CrossRef]
- Jamoussi, A.; Messaoud, L.; Jarraya, F.; Rachdi, E.; Ben Mrad, N.; Yaalaoui, S.; Besbes, M.; Ayed, S.; Ben Khelil, J. Interleukin-6 Prediction of Mortality in Critically Ill COVID-19 Patients: A Prospective Observational Cohort Study. PLoS ONE 2023, 18, e0279935. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.; Pranata, R.; Akbar, M.R. Prognostic Performance of Troponin in COVID-19: A Diagnostic Meta-Analysis and Meta-Regression. IJC Heart Vasc. 2021, 32, 100731. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, Y.; Jing, F.; Feng, Y.; Ma, J.; Xue, P.; Dong, Z. Effects of acute-phase COVID-19-related indicators on pulmonary fibrosis and follow-up evaluation. Eur. J. Med. Res. 2024, 29, 585. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Jiang, W.; Yao, J.; Nicholson, C.J.; Li, R.H.; Sigurslid, H.H.; Wooster, L.; Rotter, J.I.; Guo, X.; Malhotra, R. Predictors of Mortality in Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. J. Med. Virol. 2020, 92, 1875–1883. [Google Scholar] [CrossRef]
- Georgakopoulou, V.E.; Makrodimitri, S.; Gkoufa, A.; Apostolidi, E.; Provatas, S.; Papalexis, P.; Gamaletsou, M.N.; Lempesis, I.G.; Spandidos, D.A.; Sipsas, N.V. Lung function at three months after hospitalization due to COVID-19 pneumonia: Comparison of alpha, delta and omicron variant predominance periods. Exp. Ther. Med. 2024, 27, 83. [Google Scholar] [CrossRef]
- Tudoran, C.; Tudoran, M.; Lazureanu, V.E.; Marinescu, A.R.; Cut, T.G.; Oancea, C.; Pescariu, S.A.; Pop, G.N. Factors Influencing the Evolution of Pulmonary Hypertension in Previously Healthy Subjects Recovering from a SARS-CoV-2 Infection. J. Clin. Med. 2021, 10, 5272. [Google Scholar] [CrossRef]
- Suri, T.M.; Srivastava, G.; Kumar, S.; Surendranath, A.; Shaji, S.; Mittal, S.; Tiwari, P.; Hadda, V.; Madan, K.; Chauhan, A.; et al. Persistent pulmonary impairment after 2 years of COVID-19 infection: An observational study. Lung India 2024, 41, 405–410. [Google Scholar] [CrossRef]

| Variable | Total (n = 127) | APE (n = 46) | Non-APE (n = 81) | p-Value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 67.8 ± 10.9 | 71.2 ± 9.8 | 65.6 ± 10.7 | 0.002 |
| Male sex, n (%) BMI (kg/m2, mean ± SD) | 77 (60.6) 28.4 ± 4.6 | 30 (65.2) 28.8 ± 4.9 | 47 (58.0) 28.1 ± 4.4 | 0.41 0.48 |
| Current smokers, n (%) | 32 (25.2) | 14 (30.4) | 18 (22.2) | 0.32 |
| Fully vaccinated, n (%) | 74 (58.3) | 15 (32.6) | 59 (72.8) | <0.001 |
| Hypertension, n (%) | 78 (61.4) | 36 (78.3) | 43 (52.9) | 0.004 |
| Diabetes mellitus, n (%) | 33 (26.0) | 15 (32.6) | 18 (22.2) | 0.22 |
| Ischemic heart disease, n (%) | 21 (16.5) | 10 (21.7) | 11 (13.6) | 0.25 |
| Chronic heart failure, n (%) | 15 (11.8) | 12 (26.1) | 6 (7.4) | 0.006 |
| Chronic kidney disease, n (%) | 12 (9.4) | 8 (17.4) | 5 (6.1) | 0.048 |
| COPD or asthma, n (%) | 18 (14.2) | 8 (17.4) | 10 (12.3) | 0.44 |
| Obesity (BMI ≥ 30 kg/m2), n (%) | 49 (38.6) | 18 (39.1) | 31 (38.3) | 0.93 |
| Oxygen saturation on admission (% mean ± SD) | 88.0 ± 6.2 | 84.9 ± 6.7 | 90.1 ± 4.9 | <0.001 |
| Systolic BP (mm Hg, mean ± SD) | 136 ± 18 | 138 ± 17 | 135 ± 19 | 0.39 |
| Heart rate (bpm, mean ± SD) | 94 ± 16 | 96 ± 17 | 93 ± 15 | 0.41 |
| Respiratory rate (breaths/min, mean ± SD) | 24 ± 4 | 25 ± 4 | 23 ± 3 | 0.07 |
| Peripheral edema present, n (%) | 34 (26.8) | 20 (43.5) | 14 (17.3) | 0.002 |
| Jugular venous distention n (%) | 19 (15.0) | 12 (26.1) | 7 (8.6) | 0.014 |
| Length of hospital stay (days, mean ± SD) | 11.5 ± 5.4 | 14.7 ± 6.5 | 9.8 ± 4.2 | <0.001 |
| Parameter | APE (n = 46) | Non-APE (n = 81) | p-Value |
|---|---|---|---|
| Leukocytes (×109/L, mean ± SD) | 9.8 ± 3.1 | 8.7 ± 2.9 | 0.06 |
| Lymphocytes (×109/L, mean ± SD) | 1.05 ± 0.42 | 1.21 ± 0.39 | 0.08 |
| C-reactive protein (mg/L, median [IQR]) | 96.4 [64.7–138.5] | 84.5 [50.9–112.7] | 0.09 |
| D-dimer (ng/mL, median [IQR]) | 2280 [1340–4860] | 890 [530–1910] | 0.003 |
| IL-6 (pg/mL, median [IQR]) | 68.2 [38.1–110.4] | 34.7 [17.8–65.9] | 0.005 |
| Troponin I (ng/mL, median [IQR]) | 0.146 [0.07–0.31] | 0.031 [0.01–0.08] | <0.001 |
| NT-proBNP (pg/mL, median [IQR]) | 2890 [1340–5220] | 340 [110–890] | <0.001 |
| Serum creatinine (mg/dL, mean ± SD) | 1.31 ± 0.42 | 1.08 ± 0.36 | 0.012 |
| ALT (U/L, mean ± SD) | 38.7 ± 22.9 | 41.4 ± 19.3 | 0.47 |
| Albumin (g/dL, mean ± SD) | 3.22 ± 0.49 | 3.46 ± 0.41 | 0.018 |
| Lactate dehydrogenase (U/L, mean ± SD) | 391 ± 116 | 335 ± 104 | 0.022 |
| PaO2/FiO2 ratio (mean ± SD) | 242 ± 65 | 298 ± 58 | 0.001 |
| Bilateral alveolar infiltrates, n (%) | 42 (91.3) | 30 (37.0) | <0.001 |
| Pleural effusion, n (%) | 16 (34.7) | 7 (8.6) | 0.002 |
| Cardiomegaly on chest X-ray, n (%) | 19 (41.3) | 9 (11.1) | <0.001 |
| CT extent of lung involvement (% mean ± SD) | 49.2 ± 14.3 | 37.8 ± 12.6 | 0.004 |
| Ground-glass opacities, n (%) | 43 (93.5) | 72 (88.9) | 0.38 |
| Fibrotic or reticular changes at discharge, n (%) | 18 (39.1) | 14 (17.3) | 0.014 |
| Variable | Odds Ratio (OR) | 95% Confidence Interval (CI) | p-Value |
|---|---|---|---|
| Predictors of Acute Pulmonary Edema | |||
| Age (per year) | 1.06 | 1.02–1.10 | 0.004 |
| Hypertension | 2.94 | 1.24–6.97 | 0.014 |
| NT-proBNP (per pg/mL) | 1.0003 | 1.0001–1.0006 | 0.008 |
| IL-6 (per pg/mL) | 1.012 | 1.002–1.022 | 0.019 |
| Chronic heart failure | 1.84 | 0.87–3.92 | 0.11 |
| Chronic kidney disease | 1.63 | 0.74–3.59 | 0.21 |
| COPD or asthma | 1.25 | 0.59–2.62 | 0.55 |
| Model fit (Hosmer–Lemeshow) | χ2 = 5.27, p = 0.73 | — | — |
| Nagelkerke R2 = 0.41 | — | — | — |
| Predictors of In-Hospital Mortality | |||
| Presence of APE | 3.82 | 1.44–10.12 | 0.007 |
| NT-proBNP (per pg/mL) | 1.0004 | 1.0002–1.0008 | 0.003 |
| Troponin I (per ng/mL) | 1.09 | 1.03–1.17 | 0.005 |
| IL-6 > 50 pg/mL | 2.57 | 1.08–6.13 | 0.033 |
| Age (per year) | 1.04 | 1.00–1.08 | 0.06 |
| Hypertension | 1.62 | 0.78–3.39 | 0.19 |
| Chronic kidney disease | 1.91 | 0.88–4.13 | 0.10 |
| Model fit (Hosmer–Lemeshow) | χ2 = 6.11, p = 0.64 | — | — |
| Nagelkerke R2 = 0.47 | — | — | — |
| Characteristic | Total (n = 97) | APE (n = 26) | Non-APE (n = 71) | p-Value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 66.2 ± 10.5 | 68.4 ± 11.2 | 65.4 ± 10.1 | 0.21 |
| Male sex, n (%) | 57 (58.8) | 16 (61.5) | 41 (57.7) | 0.75 |
| BMI, kg/m2 (mean ± SD) | 29.8 ± 5.6 | 30.5 ± 6.1 | 29.5 ± 5.4 | 0.42 |
| Vaccinated, n (%) | 57 (58.8) | 14 (53.8) | 43 (60.6) | 0.57 |
| Current smoker, n (%) | 18 (18.6) | 5 (19.2) | 13 (18.3) | 0.92 |
| Hypertension, n (%) | 61 (62.9) | 17 (65.4) | 44 (62.0) | 0.77 |
| Ischemic heart disease, n (%) | 22 (22.7) | 7 (26.9) | 15 (21.1) | 0.57 |
| Heart failure, n (%) | 14 (14.4) | 5 (19.2) | 9 (12.7) | 0.43 |
| Diabetes mellitus, n (%) | 28 (28.9) | 8 (30.8) | 20 (28.2) | 0.81 |
| COPD or asthma, n (%) | 12 (12.4) | 4 (15.4) | 8 (11.3) | 0.60 |
| Obesity (BMI ≥30), n (%) | 42 (43.3) | 12 (46.2) | 30 (42.3) | 0.74 |
| Chronic kidney disease, n (%) | 9 (9.3) | 3 (11.5) | 6 (8.5) | 0.66 |
| Chronic liver disease, n (%) | 5 (5.2) | 2 (7.7) | 3 (4.2) | 0.51 |
| History of thrombosis/embolism, n (%) | 7 (7.2) | 3 (11.5) | 4 (5.6) | 0.33 |
| Parameter | APE Survivors (n = 26) | Non-APE Survivors (n = 71) | p-Value |
|---|---|---|---|
| Persistent dyspnea, n (%) | 24 (92.3) | 16 (22.5) | <0.001 |
| Fatigue, n (%) | 21 (80.8) | 15 (21.1) | <0.001 |
| Cough, n (%) | 14 (53.8) | 11 (15.5) | 0.001 |
| FVC (% predicted, mean ± SD) | 77.9 ± 14.1 | 83.3 ± 13.2 | 0.07 |
| FEV1 (% predicted, mean ± SD) | 75.6 ± 15.3 | 80.4 ± 14.0 | 0.11 |
| DLCO (% predicted, mean ± SD) | 66.4 ± 15.9 | 74.3 ± 15.7 | 0.039 |
| Restrictive or mixed ventilatory defect, n (%) | 17 (65.4) | 8 (11.3) | <0.001 |
| Fibrotic or reticular CT changes, n (%) | 18 (69.2) | 14 (19.7) | <0.001 |
| Persistent NT-proBNP elevation (>125 pg/mL), n (%) | 11 (42.3) | 8 (11.3) | 0.002 |
| Persistent IL-6 elevation (>10 pg/mL), n (%) | 9 (34.6) | 10 (14.1) | 0.03 |
| Residual dyspnea correlated with NT-proBNP (r) | 0.42 | — | 0.002 |
| Residual dyspnea correlated with IL-6 (r) | 0.39 | — | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateescu, D.-M.; Gavrilescu, D.-M.; Marginean, A.; Cotet, I.-G.; Guse, E.-C.; Muresan, C.-O.; Toma, A.-O.; Iurciuc, S.; Ilie, A.-C.; Enache, A. Acute Pulmonary Edema in COVID-19: Clinical Predictors, Long-Term Pulmonary Sequelae, and Mortality in a Romanian Cohort Study. J. Clin. Med. 2025, 14, 8188. https://doi.org/10.3390/jcm14228188
Mateescu D-M, Gavrilescu D-M, Marginean A, Cotet I-G, Guse E-C, Muresan C-O, Toma A-O, Iurciuc S, Ilie A-C, Enache A. Acute Pulmonary Edema in COVID-19: Clinical Predictors, Long-Term Pulmonary Sequelae, and Mortality in a Romanian Cohort Study. Journal of Clinical Medicine. 2025; 14(22):8188. https://doi.org/10.3390/jcm14228188
Chicago/Turabian StyleMateescu, Diana-Maria, Dragos-Mihai Gavrilescu, Andrei Marginean, Ioana-Georgiana Cotet, Elena-Cristina Guse, Camelia-Oana Muresan, Ana-Olivia Toma, Stela Iurciuc, Adrian-Cosmin Ilie, and Alexandra Enache. 2025. "Acute Pulmonary Edema in COVID-19: Clinical Predictors, Long-Term Pulmonary Sequelae, and Mortality in a Romanian Cohort Study" Journal of Clinical Medicine 14, no. 22: 8188. https://doi.org/10.3390/jcm14228188
APA StyleMateescu, D.-M., Gavrilescu, D.-M., Marginean, A., Cotet, I.-G., Guse, E.-C., Muresan, C.-O., Toma, A.-O., Iurciuc, S., Ilie, A.-C., & Enache, A. (2025). Acute Pulmonary Edema in COVID-19: Clinical Predictors, Long-Term Pulmonary Sequelae, and Mortality in a Romanian Cohort Study. Journal of Clinical Medicine, 14(22), 8188. https://doi.org/10.3390/jcm14228188






