Exacerbation of Asthma Among Pediatric Patients Presenting to the Emergency Department
Abstract
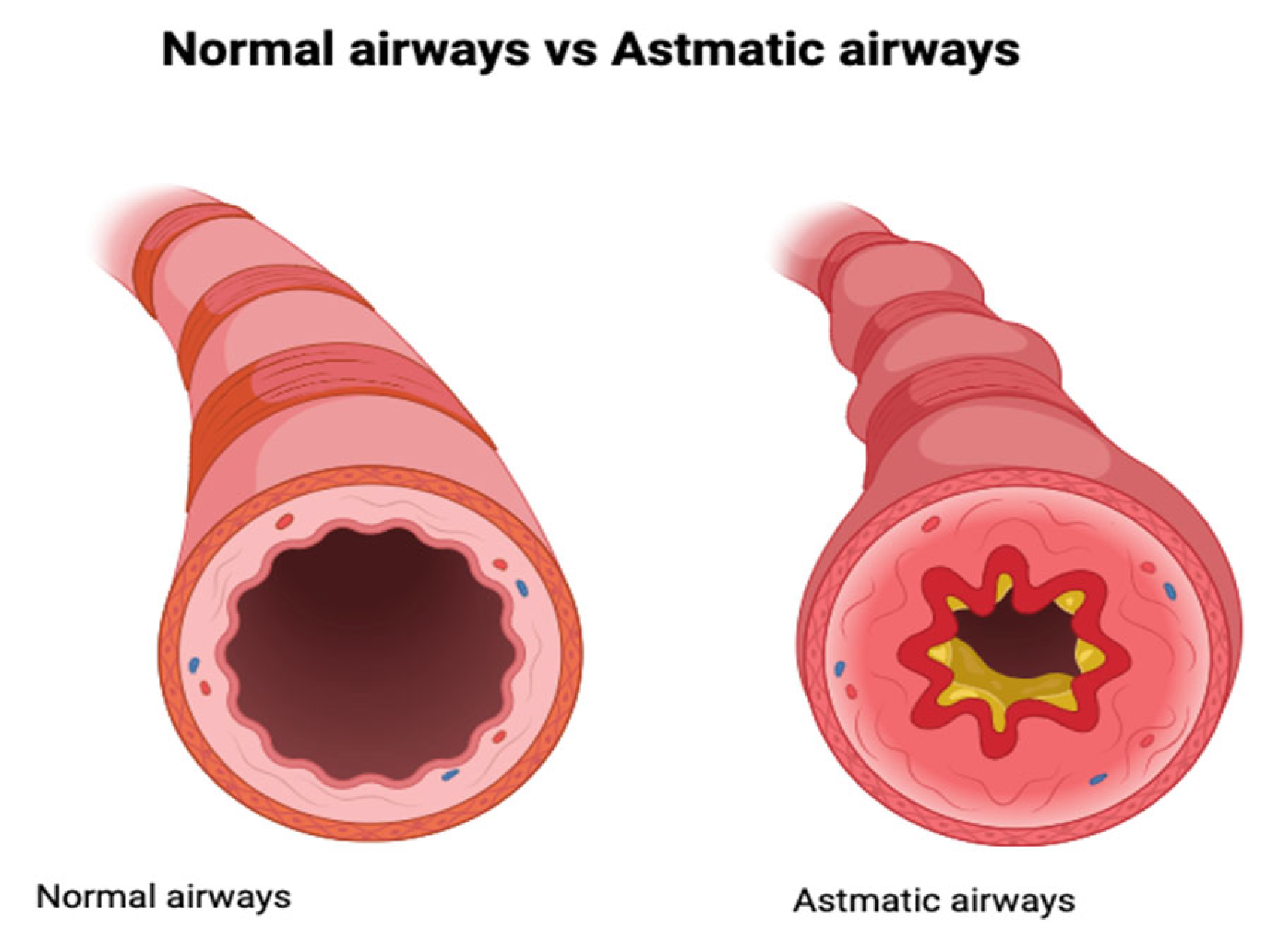
1. Introduction
2. Asthma in Children—Epidemiology and Risk Factors
2.1. Epidemiology of Asthma—Incidence, Prevalence and Comorbidities
2.2. Risk Factors for Asthma
3. Assessment of the Severity of Asthma Exacerbations in Children in the Emergency Department
3.1. The Pediatric Respiratory Assessment Measure (PRAM)
3.2. The Pediatric Asthma Severity Score (PASS) and the Modified Pulmonary Index Score (MPIS)
| Score | SpO2 Saturation Index | Accessory Muscle Use | I:E Ratio | Wheezing | Heart Rate | Respiratory Rate |
|---|---|---|---|---|---|---|
| 0 | ≥95% | none | 2:1 | none | <3 years: <120 ≥3 years: <100 | <6 years: ≤30 ≥6 years: ≤20 |
| 1 | 93–95% | mild | 1:1 | end-expiratory wheezes | <3 years: 120–140 ≥3 years: 100–120 | <6 years: 31–45 ≥6 years: 21–45 |
| 2 | 90–92% | moderate | 1:2 | inspiratory and expiratory wheezes, with good air entry | <3 years: 141–160 ≥3 years: 121–140 | <6 years: 46–60 ≥6 years: 36–50 |
| 3 | <90% | severe | 1:3 | inspiratory and expiratory wheezes, with reduced air entry | <3 years: >160 ≥3 years: >140 | <6 years: >60 ≥6 years: >50 |
3.3. The Asthma Clinical Score (ACS)
4. Treatment of Asthma Exacerbation in Children in the Emergency Department
4.1. Primary Medications Used in the Treatment of Asthma Exacerbations in the ED
4.1.1. Oxygen Therapy
4.1.2. Short-Acting Beta2-Mimetics (SABA)
4.1.3. Combination ICS/Formoterol in Children Aged 6–11 Years Old, Adolescents and Adults
4.1.4. Epinephrine in Children Aged 6–11 Years Old, Adolescents and Adults
4.1.5. Systemic Corticosteroids (SCS)
4.1.6. Inhaled Corticosteroids (ICS)
4.2. Other Therapeutic Options in Children Aged 6–11 Years Old, Adolescents and Adults
4.2.1. Ipratropium Bromide
4.2.2. Magnesium Sulfate
4.2.3. Helium Oxygen Therapy
4.2.4. Leukotriene Receptor Antagonists (LTRAs)
4.2.5. Non-Invasive Ventilation (NIV)
4.3. Not Recommended Medications in Children Aged 6–11 Years Old, Adolescents and Adults
| Under 5 Years of Age | Children Aged 6–11 Years | |
|---|---|---|
| Oxygen | Maintain ≥ 94% To avoid deterioration of blood oxygenation it could be combined with 2.5 mg SABA (or dissolved in 0.9% NaCl). | Maintain ≥ 94% |
| SABA (salbutamol) | Via pMDI 4 inhalations (100 µg per puff; in severe asthma 6 puffs); Via nebulizer the dose should be 2.5 mg; Further dosing is decided based on the patient’s clinical condition during observation [91]. | Via pMDI 4 to 10 inhalations (100 µg per puff) every 20 min for 1 h; After first hour it can be repeated every 3–4 h or 6–10 inhalations every 1–2 h; Via nebulizer the dose should be 2.5–5 mg for 30 min. It can be repeated up to (max) 4 doses per day [92]. |
| SCS | For methylprednisolone it is 1–2 mg/kg/day (max 20 mg/day for children < 2 years and 30 mg/day for children 2–5 years) for 3–5 days [52,93]; An alternative to methylprednisolone is dexamethasone in a single dose of 0.3–0.6 mg/kg (max. 12 mg) [54]. | For prednisolone it is 1–2 mg/kg up to a maximum of 40 mg/day for 3–5 days [94]. |
| Reliever Medication | Maintenance Treatment |
|---|---|
| Anti-inflammatory Reliever Medication |
|
| |
| Short-acting Bronchodilator Reliever Medication | Add-on maintenance medications |
|
|
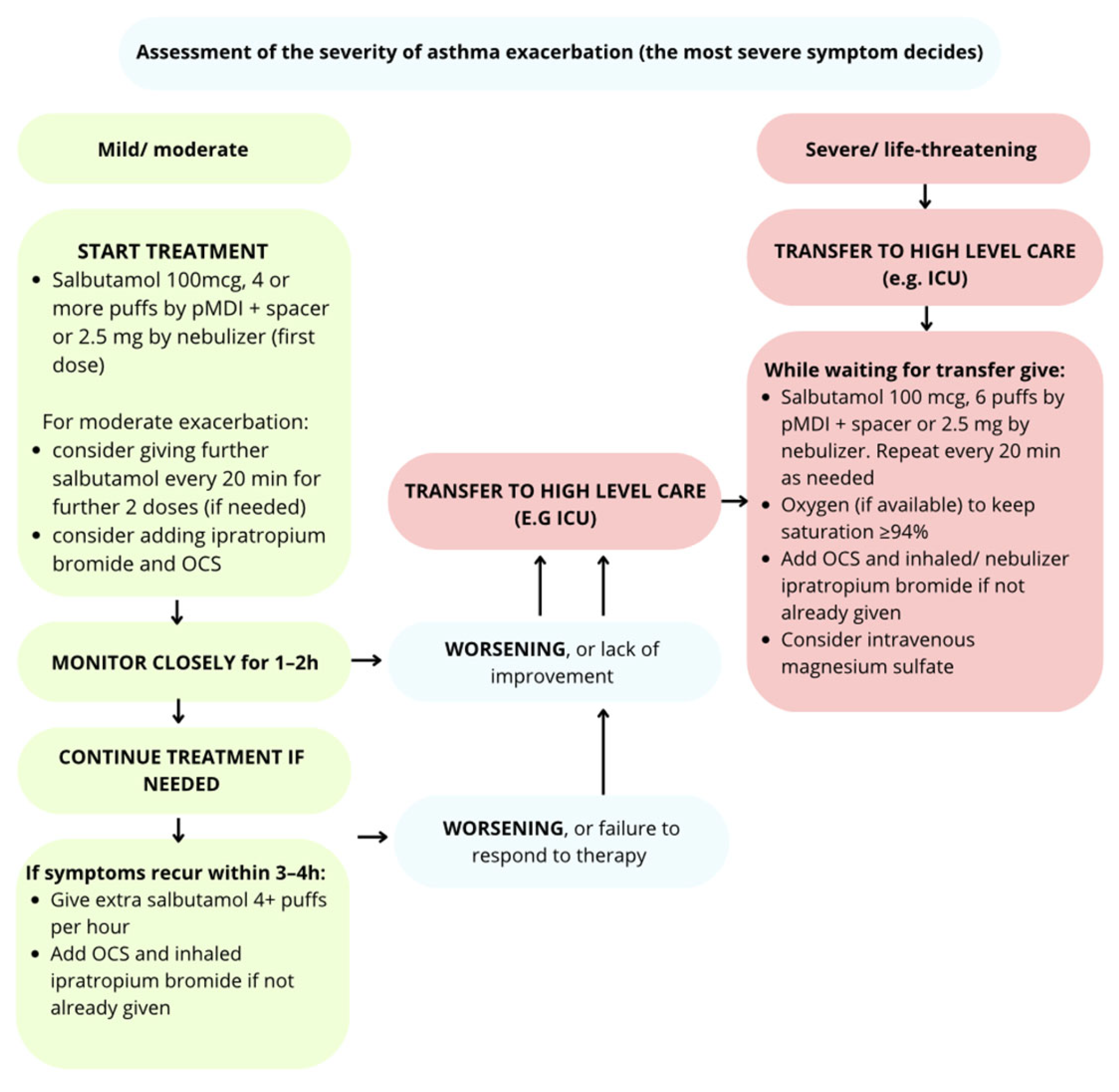
5. Treatment Algorithm for Asthma Exacerbations According to Severity
5.1. Management in Children Aged 6–11 Years, Adolescents and Adults
5.1.1. Mild/Moderate Exacerbation
5.1.2. Severe Exacerbation
5.1.3. Life-Threatening Exacerbation
5.2. Management Depending on the Severity of Exacerbation of Asthma in Children Under 5 Years of Age
5.2.1. Mild/Moderate Exacerbation
5.2.2. Severe Exacerbation
6. Criteria for Hospitalization and Discharge from the Emergency Department
7. The Role of Education, Prevention, and the Management Plan After Discharge from the Emergency Department
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ED | Emergency Department |
| ICU | Intensive Care Unit |
| PICU | Pediatric Intensive Care Unit |
| ICS | Inhaled Corticosteroids |
| GINA | Global Initiative for Asthma |
| RSV | Respiratory Syncytial Virus |
| PRAM | Paediatric Respiratory Assessment Measure |
| ASS | Asthma Severity Score |
| NAEPP | National Asthma Education and Prevention Program |
| SABA | Short- acting beta2-agonists |
| pMDI | Pressurised Metered Dose Inhaled |
| LABA | Long-acting beta2-agonists |
| OCS | Oral corticosteroids |
| SCS | Systemic corticosteroids |
| FEV1 | Forced Expiratory Volume in 1 s |
| LTRAs | Leukotriene receptor antagonists |
| NIV | Non- invasive ventilation |
| IMV | Invasive mechanical ventilation |
| Spo2 | Saturation of peripheal oxygen |
| NaCl | Sodium Chloride |
| PEFR | Peak Expiratory Flow Rate |
| CS | Corticosteroids |
| PEF | Peak Expiratory Flow |
| ER | Emergency Room |
| DALY | Disability-Adjusted Life-Years |
| BMI | Body mass index |
| NHIS | National Health Interview Survey |
| AAIRS | The Acute Asthma Intensity Research Score |
| PASS | Pediatric Asthma Severity Score |
| MPIS | Modified Pulmonary Index Score |
| ACS | Asthma Clinical Score |
| PFTs | Pulmonary Function Tests |
| AAP | Asthma Acting Plan |
| WAAPs | Written Asthma Action Plans |
| API | Asthma Predictive Index |
| mAPI | Modified Asthma Predictive Index |
| COPD | Chronic Obturatory Pulmonary Disease |
| SADCP | Safe Asthma Discharge Care Pathway |
References
- Akinbami, L.J.; Moorman, J.E.; Bailey, C.; Zahran, H.S.; King, M.; Johnson, C.A.; Liu, X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012, 94, 1–8. [Google Scholar]
- Licari, A.; Brambilla, I.; Marseglia, A.; De Filippo, M.; Paganelli, V.; Marseglia, G.L. Difficult vs. Severe Asthma: Definition and Limits of Asthma Control in the Pediatric Population. Front. Pediatr. 2018, 6, 170. [Google Scholar] [CrossRef]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention; Global Initiative for Asthma (GINA): Fontana, WI, USA, 2025. [Google Scholar]
- Pijnenburg, M.W.; Frey, U.; De Jongste, J.C.; Saglani, S. Childhood asthma: Pathogenesis and phenotypes. Eur. Respir. J. 2022, 59, 2100731. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Roth, M. Airway Wall Remodeling in Childhood Asthma-A Personalized Perspective from Cell Type-Specific Biology. J. Pers. Med. 2021, 11, 1229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cottini, M.; Lombardi, C.; Berti, A.; Comberiati, P. Small-airway dysfunction in paediatric asthma. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Dudek, J. Figure 1. Comparison of Normal Airways to Airways During Asthma Exacerbation [Figure]. Available online: https://app.biorender.com/illustrations/686b8ce8cde875f568399850BioRender.com (accessed on 10 July 2025).
- Zelicof Paul, A.; Rutherford, K.A.; Abuso, S.M. Emergency department management of pediatric acute asthma: An evidence-based review. Pediatr. Emerg. Med. Pract. 2023, 20, 1–28. [Google Scholar]
- von Mutius, E.; Smits, H.H. Primary prevention of asthma: From risk and protective factors to targeted strategies for prevention. Lancet 2020, 396, 854–866. [Google Scholar] [CrossRef] [PubMed]
- National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma; NIH Publication: Bethesda, MD, USA, 2007. [Google Scholar]
- Zheng, J.; Jin, Y.-J.; Wang, C.-H.; Feng, C.; Lai, X.-Y.; Hua, S.-Q.; Tai, J.-H. Global, regional, and national epidemiology of allergic diseases in children from 1990 to 2021: Findings from the Global Burden of Disease Study 2021. BMC Pulm. Med. 2025, 25, 54. [Google Scholar] [CrossRef]
- Zhou, W.; Tang, J. Prevalence and risk factors for childhood asthma: A systematic review and meta-analysis. BMC Pediatr. 2025, 25, 50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hurst, J.H.; Zhao, C.; Hostetler, H.P.; Ghiasi Gorveh, M.; Lang, J.E.; Goldstein, B.A. Environmental and clinical data utility in pediatric asthma exacerbation risk prediction models. BMC Med. Inf. Decis. Mak. 2022, 22, 108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mendy, A.; Mersha, T.B. Comorbidities in childhood-onset and adult-onset asthma. Ann. Allergy Asthma Immunol. 2022, 129, 327–334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sio, Y.Y.; Chew, F.T. Risk factors of asthma in the Asian population: A systematic review and meta-analysis. J. Physiol. Anthropol. 2021, 40, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caffrey Osvald, E.; Bower, H.; Lundholm, C.; Larsson, H.; Brew, B.K.; Almqvist, C. Asthma and all-cause mortality in children and young adults: A population-based study. Thorax 2020, 75, 1040–1046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frey, S.M.; Rowe, R.K.; Halterman, J.S. The prevalence of childhood asthma: Interpreting falling rates in the context of shifting measurement and the COVID-19 pandemic. Curr. Opin. Pulm. Med. 2023, 29, 197–201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ducharme, F.M.; Chalut, D.; Plotnick, L.; Savdie, C.; Kudirka, D.; Zhang, X.; Meng, L.; McGillivray, D. The Pediatric Respiratory Assessment Measure: A valid clinical score for assessing acute asthma severity from toddlers to teenagers. J. Pediatr. 2008, 152, 476–480.e1. [Google Scholar] [CrossRef] [PubMed]
- Al-Moamary, M.S.; Alhaider, S.A.; Allehebi, R.; Idrees, M.M.; Zeitouni, M.O.; Al Ghobain, M.O.; Alanazi, A.F.; Al-Harbi, A.S.; Yousef, A.A.; Alorainy, H.S.; et al. The Saudi initiative for asthma—2024 update: Guidelines for the diagnosis and management of asthma in adults and children. Ann. Thorac. Med. 2024, 19, 1–55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, M.D.; Nkoy, F.L.; Sheng, X.; Greene, T.; Stone, B.L.; Garvin, J. Direct concurrent comparison of multiple pediatric acute asthma scoring instruments. J. Asthma 2017, 54, 741–753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Douros, K.; Moriki, D.; Sardeli, O.; Boutopoulou, B.; Galani, A.; Papaevangelou, V.; Priftis, K.N. Assessment and management of asthma exacerbations in an emergency department unit. Allergol. Immunopathol. 2023, 51, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.H.; Saville, B.R.; Wang, W.; Hartert, T.V. Performance of the Acute Asthma Intensity Research Score (AAIRS) for acute asthma research protocols. Ann. Allergy Asthma Immunol. 2012, 109, 78–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryan, K.S.; Son, S.; Roddy, M.; Siraj, S.; McKinley, S.D.; Nakagawa, T.A.; Sochet, A.A. Pediatric asthma severity scores distinguish suitable inpatient level of care for children admitted for status asthmaticus. J. Asthma 2021, 58, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.G.; Haynes, K.E.; Gates, R.M.; Zimmerman, K.O.; Bartlett, K.W.; McLean, H.S.; Rehder, K.J. Initial Modified Pulmonary Index Score Predicts Hospital Length of Stay for Asthma Subjects Admitted to the Pediatric Intensive Care Unit. Respir. Care 2020, 65, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Nakamura, T.; Maeda, M.; Ishikawa, R.; Kamiya, T.; Imai, T. Utility of therapeutic strategy based on the modified Pulmonary Index Score for childhood asthma exacerbation. Allergy Asthma Proc. 2019, 40, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Oba, M.S.; Katsunuma, T.; Ishiguro, A.; Ohya, Y.; Nakamura, H. Modified pulmonary index score was sufficiently reliable to assess the severity of acute asthma exacerbations in children. Allergol. Int. 2014, 63, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Ohya, Y.; Mikami, M.; Uematsu, S.; Ishiguro, A. Clinical Utility of the Modified Pulmonary Index Score as an Objective Assessment Tool for Acute Asthma Exacerbation in Children. JMA J. 2018, 1, 57–66. [Google Scholar] [CrossRef]
- Andoh, A.A.; Truelove, A.; Helwig, S.; Nash, M.C.; Ulrich, L.; Shell, R.; Leonard, J.C. Performance of the Asthma Clinical Score in the Evaluation of Acute Asthma in the Emergency Department. Pediatr. Pulmonol. 2025, 60, e71084. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leatherman, J. Mechanical ventilation for severe asthma. Chest 2015, 147, 1671–1680. [Google Scholar] [CrossRef]
- Pollack, C.V., Jr.; Pollack, E.S.; Baren, J.M.; Smith, S.R.; Woodruff, P.G.; Clark, S.; Camargo, C.A. A prospective multicenter study of patient factors associated with hospital admission from the emergency department among children with acute asthma. Arch. Pediatr. Adolesc. Med. 2002, 156, 934–940. [Google Scholar] [CrossRef]
- Trottier, E.D.; Chan, K.; Allain, D.; Chauvin-Kimoff, L. Managing an acute asthma exacerbation in children. Paediatr. Child Health 2021, 26, 438. [Google Scholar] [CrossRef]
- Chien, J.W.; Ciufo, R.; Novak, R.; Skowronski, M.; Nelson, J.; Coreno, A.; McFadden, E. Uncontrolled oxygen administration and respiratory failure in acute asthma. Chest 2000, 117, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Perrin, K.; Wijesinghe, M.; Healy, B.; Wadsworth, K.; Bowditch, R.; Bibby, S.; Baker, T.; Weatherall, M.; Beasley, R. Randomised controlled trial of high concentration versus titrated oxygen therapy in severe exacerbations of asthma. Thorax 2011, 66, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Crocker, M.E.; Hossen, S.; Goodman, D.; Simkovich, S.M.; Kirby, M.; Thompson, L.M.; Rosa, G.; Garg, S.S.; Thangavel, G.; McCollum, E.D.; et al. Effects of high altitude on respiratory rate and oxygen saturation reference values in healthy infants and children younger than 2 years in four countries: A cross-sectional study. Lancet Glob. Health 2020, 8, e362–e373. [Google Scholar] [CrossRef]
- Rodrigo, C.; Rodrigo, G. Salbutamol treatment of acute severe asthma in the ED: MDI versus hand-held nebulizer. Am. J. Emerg. Med. 1998, 16, 637–642. [Google Scholar] [CrossRef]
- Iramain, R.; Castro-Rodriguez, J.A.; Jara, A.; Cardozo, L.; Bogado, N.; Morinigo, R.; De Jesús, R. Salbutamol and ipratropium by inhaler is superior to nebulizer in children with severe acute asthma exacerbation: Randomized clinical trial. Pediatr. Pulmonol. 2019, 54, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Lyttle, M.D.; O’Sullivan, R.; Doull, I.; Hartshorn, S.; Morris, I.; Powell, C.V.E. Variation in treatment of acute childhood wheeze in emergency departments of the United Kingdom and Ireland: An international survey of clinician practice. Arch. Dis. Child. 2015, 100, 121–125. [Google Scholar] [CrossRef]
- Use of Metered Dose Inhalers, Spacers, and Nebulizers: Practice Essentials, Overview, Preparation. Available online: https://emedicine.medscape.com/article/1413366-overview?form=fpf (accessed on 28 September 2025).
- Lemaçon, C.; Lopes, A.A. Inhalers or nebulisation of salbutamol in childhood asthma exacerbations in emergency departments. Respir. Med. 2025, 243, 108152. [Google Scholar] [CrossRef] [PubMed]
- Travers, A.; Jones, A.P.; Kelly, K.; Barker, S.J.; Camargo, C.A.; Rowe, B.H. Intravenous beta2-agonists for acute asthma in the emergency department. Cochrane Database Syst. Rev. 2001, 2012, CD002988. [Google Scholar] [CrossRef]
- Pollock, M.; Sinha, I.P.; Hartling, L.; Rowe, B.H.; Schreiber, S.; Fernandes, R.M. Inhaled short-acting bronchodilators for managing emergency childhood asthma: An overview of reviews. Allergy 2017, 72, 183–200. [Google Scholar] [CrossRef]
- Balanag, V.M.; Yunus, F.; Yang, P.C.; Jorup, C. Efficacy and safety of budesonide/formoterol compared with salbutamol in the treatment of acute asthma. Pulm. Pharmacol. Ther. 2006, 19, 139–147. [Google Scholar] [CrossRef]
- Jonkers, R.E.; Bantje, T.A.; Aalbers, R. Onset of relief of dyspnoea with budesonide/formoterol or salbutamol following methacholine-induced severe bronchoconstriction in adults with asthma: A double-blind, placebo-controlled study. Respir. Res. 2006, 7, 141. [Google Scholar] [CrossRef]
- Buhl, R. Budesonide/formoterol for the treatment of asthma. Expert. Opin. Pharmacother. 2003, 4, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Zetterström, O.; Buhl, R.; Mellem, H.; Perpiñá, M.; Hedman, J.; O’Neill, S.; Ekström, T. Improved asthma control with budesonide/formoterol in a single inhaler, compared with budesonide alone. Eur. Respir. J. 2001, 18, 262–268. [Google Scholar] [CrossRef]
- Bateman, E.D.; Bantje, T.A.; João Gomes, M.; Toumbis, M.G.; Huber, R.M.; Naya, I.; Eliraz, A. Combination therapy with single inhaler budesonide/formoterol compared with high dose of fluticasone propionate alone in patients with moderate persistent asthma. Am. J. Respir. Med. 2003, 2, 275–281. [Google Scholar] [CrossRef]
- Arun, J.J.; Lodha, R.; Kabra, S.K. Bronchodilatory effect of inhaled budesonide/formoterol and budesonide/salbutamol in acute asthma: A double-blind, randomized controlled trial. BMC Pediatr. 2012, 12, 21. [Google Scholar] [CrossRef]
- Rabe, K.F.; Atienza, T.; Magyar, P.; Larsson, P.; Jorup, C.; Lalloo, U.G. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: A randomised controlled, double-blind study. Lancet 2006, 368, 744–753. [Google Scholar] [CrossRef]
- Baggott, C.; Hardy, J.K.; Sparks, J.; Sabbagh, D.; Beasley, R.; Weatherall, M.; Fingleton, J. Epinephrine (adrenaline) compared to selective beta-2-agonist in adults or children with acute asthma: A systematic review and meta-analysis. Thorax 2022, 77, 563–572. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamaoki, J.; Nagase, H.; Yamaguchi, M.; Horiguchi, T.; Hozawa, S.; Ichinose, M.; Iwanaga, T.; Kondo, R.; Nagata, M.; et al. Japanese guidelines for adult asthma 2020. Allergol. Int. 2020, 69, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Manser, R.; Reid, D.; Abramson, M. Corticosteroids for acute severe asthma in hospitalised patients. Cochrane Database Syst. Rev. 2000, 2001, CD001740. [Google Scholar] [CrossRef]
- Rowe, B.H.; Spooner, C.; Ducharme, F.M.; Bretzlaff, J.A.; Bota, G.W. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst. Rev. 2001, 2001, CD002178. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.J.; McCoy, S.; Kennedy, U.; Fhailí, S.N.A.; Wakai, A.; Hayden, J.; Crispino, G.; Barrett, M.J.; Walsh, S.; O’Sullivan, R. A Randomized Trial of Single-Dose Oral Dexamethasone Versus Multidose Prednisolone for Acute Exacerbations of Asthma in Children Who Attend the Emergency Department. Ann. Emerg. Med. 2016, 67, 593–601.e3. [Google Scholar] [CrossRef] [PubMed]
- Dahan, E.; El Ghazal, N.; Nakanishi, H.; El Haddad, J.; Matar, R.H.; Tosovic, D.; Beran, A.; Than, C.A.; Stiasny, D. Dexamethasone versus prednisone/prednisolone in the management of pediatric patients with acute asthmatic exacerbations: A systematic review and meta-analysis. J. Asthma 2023, 60, 1481–1492. [Google Scholar] [CrossRef]
- Keeney, G.E.; Gray, M.P.; Morrison, A.K.; Levas, M.N.; Kessler, E.A.; Hill, G.D.; Gorelick, M.H.; Jackson, J.L. Dexamethasone for acute asthma exacerbations in children: A meta-analysis. Pediatrics 2014, 133, 493–499. [Google Scholar] [CrossRef]
- Paniagua, N.; Lopez, R.; Muñoz, N.; Tames, M.; Mojica, E.; Arana-Arri, E.; Mintegi, S.; Benito, J. Randomized Trial of Dexamethasone Versus Prednisone for Children with Acute Asthma Exacerbations. J Pediatr. 2017, 191, 190–196.e1. [Google Scholar] [CrossRef]
- Johnson, D.B.; Lopez, M.J.; Kelley, B. Dexamethasone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sonnenberg, L.K.; Sinclair, D. A Randomized Controlled Study Addressing Dexamethasone Tolerability in the Treatment of Acute Asthma in Children: Mary Poppins on Trial! J. Pharm. Pract. 2023, 36, 803–809. [Google Scholar] [CrossRef]
- Su, X.M.; Yu, N.; Kong, L.F.; Kang, J. Effectiveness of inhaled corticosteroids in the treatment of acute asthma in children in the emergency department: A meta-analysis. Ann. Med. 2014, 46, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M.L.; Milan, S.J.; Camargo, C.A., Jr.; Pollack, C.V.; Rowe, B.H. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database Syst. Rev. 2012, 2012, CD002308. [Google Scholar] [CrossRef] [PubMed]
- Kearns, N.; Maijers, I.; Harper, J.; Beasley, R.; Weatherall, M. Inhaled Corticosteroids in Acute Asthma: A Systemic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2020, 8, 605–617.e6. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Pincheira, M.A.; Escobar-Serna, D.P.; Sossa-Briceño, M.P.; Rodriguez-Martinez, C.E. Adding nebulized corticosteroids to systemic corticosteroids for acute asthma in children: A systematic review with meta-analysis. Pediatr. Pulmonol. 2020, 55, 2508–2517. [Google Scholar] [CrossRef]
- Beckhaus, A.A.; Riutort, M.C.; Castro-Rodriguez, J.A. Inhaled versus systemic corticosteroids for acute asthma in children. A systematic review. Pediatr. Pulmonol. 2014, 49, 326–334. [Google Scholar] [CrossRef]
- Rowe, B.H.; Bota, G.W.; Fabris, L.; Therrien, S.A.; Milner, R.A.; Jacono, J. Inhaled budesonide in addition to oral corticosteroids to prevent asthma relapse following discharge from the emergency department: A randomized controlled trial. JAMA 1999, 281, 2119–2126. [Google Scholar] [CrossRef]
- Aaron, S.D. The use of ipratropium bromide for the management of acute asthma exacerbation in adults and children: A systematic review. J. Asthma 2001, 38, 521–530. [Google Scholar] [CrossRef]
- Rodrigo, G.J.; Castro-Rodriguez, J.A. Anticholinergics in the treatment of children and adults with acute asthma: A systematic review with meta-analysis. Thorax 2005, 60, 740–746. [Google Scholar] [CrossRef]
- Craig, S.S.; Dalziel, S.R.; Powell, C.V.; Graudins, A.; Babl, F.E.; Lunny, C. Interventions for escalation of therapy for acute exacerbations of asthma in children: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2020, 2020, CD012977. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Kumar, P.; Goyal, J.P.; Rajvanshi, N.; Prabhakaran, K.; Meena, J.; Gupta, A. Role of nebulised magnesium sulfate in treating acute asthma in children: A systematic review and meta-analysis. BMJ Paediatr. Open 2024, 8, e002638. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.; Dwan, K.; Milan, S.J.; Beasley, R.; Hughes, R.; A Knopp-Sihota, J.; Rowe, B.H. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst. Rev. 2012, 2012, CD003898. [Google Scholar] [CrossRef] [PubMed]
- Knightly, R.; Milan, S.J.; Hughes, R.; A Knopp-Sihota, J.; Rowe, B.H.; Normansell, R.; Powell, C. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst. Rev. 2017, 2017, CD003898. [Google Scholar] [CrossRef]
- Turker, S.; Dogru, M.; Yildiz, F.; Yilmaz, S.B. The effect of nebulised magnesium sulphate in the management of childhood moderate asthma exacerbations as adjuvant treatment. Allergol. Immunopathol. 2017, 45, 115–120. [Google Scholar] [CrossRef]
- Ambrożej, D.; Adamiec, A.; Forno, E.; Orzołek, I.; Feleszko, W.; Castro-Rodriguez, J.A. Intravenous magnesium sulfate for asthma exacerbations in children: Systematic review with meta-analysis. Paediatr. Respir. Rev. 2024, 52, 23–30. [Google Scholar] [CrossRef]
- Liu, X.; Yu, T.; Rower, J.E.; Campbell, S.C.; Sherwin, C.M.; Johnson, M.D. Optimizing the use of intravenous magnesium sulfate for acute asthma treatment in children. Pediatr. Pulmonol. 2016, 51, 1414–1421. [Google Scholar] [CrossRef]
- Rodrigo, G.J.; Castro-Rodriguez, J.A. Heliox-driven β2-agonists nebulization for children and adults with acute asthma: A systematic review with meta-analysis. Ann. Allergy Asthma Immunol. 2014, 112, 29–34. [Google Scholar] [CrossRef]
- Kim, I.K.; Phrampus, E.; Venkataraman, S.; Pitetti, R.; Saville, A.; Corcoran, T.; Gracely, E.; Funt, N.; Thompson, A. Helium/oxygen-driven albuterol nebulization in the treatment of children with moderate to severe asthma exacerbations: A randomized, controlled trial. Pediatrics 2005, 116, 1127–1133. [Google Scholar] [CrossRef]
- Rose, J.S.; Panacek, E.A.; Miller, P. Prospective randomized trial of heliox-driven continuous nebulizers in the treatment of asthma in the emergency department. J. Emerg. Med. 2002, 22, 133–137. [Google Scholar] [CrossRef]
- Watts, K.; Chavasse, R.J. Leukotriene receptor antagonists in addition to usual care for acute asthma in adults and children. Cochrane Database Syst. Rev. 2012, 2012, CD006100, Published 2012 May 16. [Google Scholar] [CrossRef] [PubMed]
- Todi, V.K.; Lodha, R.; Kabra, S.K. Effect of addition of single dose of oral montelukast to standard treatment in acute moderate to severe asthma in children between 5 and 15 years of age: A randomised, double-blind, placebo controlled trial. Arch. Dis. Child. 2010, 95, 540–543. [Google Scholar] [CrossRef]
- Nelson, K.A.; Smith, S.R.; Trinkaus, K.; Jaffe, D.M. Pilot study of oral montelukast added to standard therapy for acute asthma exacerbations in children aged 6 to 14 years. Pediatr. Emerg. Care 2008, 24, 21–27. [Google Scholar] [CrossRef]
- Harmanci, K.; Bakirtas, A.; Turktas, I.; Degim, T. Oral montelukast treatment of preschool-aged children with acute asthma. Ann. Allergy Asthma Immunol. 2006, 96, 731–735. [Google Scholar] [CrossRef]
- Gupta, D.; Nath, A.; Agarwal, R.; Behera, D. A prospective randomized controlled trial on the efficacy of noninvasive ventilation in severe acute asthma. Respir. Care 2010, 55, 536–543. [Google Scholar]
- Pallin, M.; Naughton, M.T. Noninvasive ventilation in acute asthma. J. Crit. Care 2014, 29, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.J.; Mohammed Akram, R.; Carson, K.V.; Mysore, S.; A Labiszewski, N.; A Wedzicha, J.; Rowe, B.H.; Smith, B.J. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst. Rev. 2012, 2012, CD004360, Published 2012 Dec 12. [Google Scholar] [CrossRef]
- Jilani, T.N.; Preuss, C.V.; Sharma, S. Theophylline. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hendeles, L.; Weinberger, M. Avoidance of adverse effects during chronic therapy with theophylline. Eur. J. Respir. Dis. Suppl. 1980, 109, 103–119. [Google Scholar] [CrossRef]
- Normansell, R.; Sayer, B.; Waterson, S.; Dennett, E.J.; Del Forno, M.; Dunleavy, A. Antibiotics for exacerbations of asthma. Cochrane Database Syst. Rev. 2018, 2018, CD002741. [Google Scholar] [CrossRef] [PubMed]
- Graham, V.; Lasserson, T.; Rowe, B.H. Antibiotics for acute asthma. Cochrane Database Syst. Rev. 2001, 2001, CD002741. [Google Scholar] [CrossRef]
- Joseph, K.S.; Blais, L.; Ernst, P.; Suissa, S. Increased morbidity and mortality related to asthma among asthmatic patients who use major tranquillisers. BMJ 1996, 312, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Chalitsios, C.V.; Fogarty, A.W.; McKeever, T.M.; Shaw, D.E. Sedative medications: An avoidable cause of asthma and COPD exacerbations? Lancet Respir. Med. 2023, 11, e31–e32. [Google Scholar] [CrossRef]
- Joseph, K.S. Asthma mortality and antipsychotic or sedative use. What is the link? Drug Saf. 1997, 16, 351–354. [Google Scholar] [CrossRef]
- Cazzola, M.; Page, C.P.; Calzetta, L.; Matera, M.G. Pharmacology and therapeutics of bronchodilators. Pharmacol. Rev. 2012, 64, 450–504. [Google Scholar] [CrossRef]
- Kruszewski, J.; Chazan, R.; Kuziemski, K.; Rogala, B.; Szczeklik, W.; Dąbrowski, A.; Twardowska, M.; Kościelna, M.; Kryj-Radziszewska, E.; Oleszczyk, M.; et al. Management of asthma exacerbation in adults—Guidelines for primary care doctors. Pol. Arch. Intern. Medicine 2019, 129, 842–849. [Google Scholar] [CrossRef]
- Castro-Rodriguez, J.A.; Beckhaus, A.A.; Forno, E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: Systematic review with meta-analysis. Pediatr. Pulmonol. 2016, 51, 868–876. [Google Scholar] [CrossRef]
- Kayani, S.; Shannon, D.C. Adverse behavioral effects of treatment for acute exacerbation of asthma in children: A comparison of two doses of oral steroids. Chest 2002, 122, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamrani, A.; Al-Harbi, A.S.; Bagais, K.; Alenazi, A.; Alqwaiee, M. Management of asthma exacerbation in the emergency departments. Int. J. Pediatr. Adolesc. Med. 2019, 6, 61–67. [Google Scholar] [CrossRef]
- Gayen, S.; Dachert, S.; Lashari, B.H.; Gordon, M.; Desai, P.; Criner, G.J.; Cardet, J.C.; Shenoy, K. Critical Care Management of Severe Asthma Exacerbations. J. Clin. Med. 2024, 13, 859. [Google Scholar] [CrossRef] [PubMed]
- Newman, K.B.; Milne, S.; Hamilton, C.; Hall, K. A comparison of albuterol administered by metered-dose inhaler and spacer with albuterol by nebulizer in adults presenting to an urban emergency department with acute asthma. Chest 2002, 121, 1036–1041. [Google Scholar] [CrossRef]
- Dhuper, S.; Chandra, A.; Ahmed, A.; Bista, S.; Moghekar, A.; Verma, R.; Chong, C.; Shim, C.; Cohen, H.; Choksi, S. Efficacy and cost comparisons of bronchodilatator administration between metered dose inhalers with disposable spacers and nebulizers for acute asthma treatment. J. Emerg. Med. 2011, 40, 247–255. [Google Scholar] [CrossRef]
- Goldin, J.; Hashmi, M.F.; Cataletto, M.E. Asthma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Reddel, H.K.; Barnes, D.J. Pharmacological strategies for self-management of asthma exacerbations. Eur. Respir. J. 2006, 28, 182–199. [Google Scholar] [CrossRef]
- O’Driscoll, B.R.; Howard, L.S.; Earis, J.; Mak, V.; British Thoracic Society Emergency Oxygen Guideline Group; BTS Emergency Oxygen Guideline Development Group. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax 2017, 72 (Suppl. S1), ii1–ii90. [Google Scholar] [CrossRef] [PubMed]
- Cates, C.J.; Welsh, E.J.; Rowe, B.H. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst. Rev. 2013, 2013, CD000052. [Google Scholar] [CrossRef] [PubMed]
- Asthma Clinical Practice Guideline: Emergency Department Management. Available online: https://www.choa.org/-/media/Files/Childrens/medical-professionals/clinical-practice-guidelines/asthma-ed.pdf (accessed on 6 July 2025).
- Jones, H.; Lawton, A.; Gupta, A. Asthma attacks in children—Challenges and opportunities. Indian J Pediatr. 2022, 89, 373–377. [Google Scholar] [CrossRef]
- Wade, T. Pediatric Office Emergencies: Asthma. Available online: https://www.tomwademd.net/linking-to-and-embedding-neurogal-mds-lithium-neurologist-reviews-evidence-and-top-brands/ (accessed on 7 November 2025).
- Jean, T.; Yang, S.-J.; Crawford, W.W.; Takahashi, S.H.; Sheikh, J. Development of a pediatric asthma predictive index for hospitalization. Ann. Allergy Asthma Immunol. 2019, 122, 283–288. [Google Scholar] [CrossRef]
- Chen, R.J.; McMahon, K.; Launico, M.V. Status Asthmaticus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 15 September 2025. [Google Scholar]
- Chalut, D.S.; Ducharme, F.M.; Davis, G.M. The Preschool Respiratory Assessment Measure (PRAM): A responsive index of acute asthma severity. J. Pediatr. 2000, 137, 762–768. [Google Scholar] [CrossRef]
- Thaweerujirot, C.; Daengsuwan, T. Comparison between pediatric respiratory assessment measure (PRAM) score and Wood’s asthma score to assess acute asthma exacerbation. Asian Pac. J. Allergy Immunol. 2018, 37, 123–129. Available online: https://www.apjai-journal.org/wp-content/uploads/2019/09/1.pdf (accessed on 20 July 2025).
- Smith, A.; França, U.L.; McManus, M.L. Trends in the use of noninvasive and invasive ventilation for severe asthma. Pediatrics 2020, 146, e20200534. [Google Scholar] [CrossRef]
- Smith, M.A.; Dinh, D.; Ly, N.P.; Ward, S.L.; McGarry, M.E.; Zinter, M.S. Changes in the use of invasive and noninvasive mechanical ventilation in pediatric asthma: 2009–2019. Ann. Am. Thorac. Soc. 2023, 20, 245–253. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Ebrahimi, H.K. Clinical guidelines on pediatric asthma exacerbation in emergency department, a narrative review. Eur. J. Transl. Myol. 2020, 30, 179–186. [Google Scholar] [CrossRef]
- Asthma Exacerbation in the Emergency Department or Urgent Care. Available online: https://www.childrensmercy.org/health-care-providers/evidence-based-practice/cpgs-cpms-and-eras-pathways/asthma-exacerbation-clinical-practice-guideline/asthma-reference-guide/12-asthma-exacerbations-in-the-emergency-department-or-urgent-care (accessed on 8 July 2025).
- Leung, J.S. Paediatrics: How to manage acute asthma exacerbations. Drugs Context 2021, 10, 2020-12. [Google Scholar] [CrossRef]
- Lee, M.O.; Sivasankar, S.; Pokrajac, N.; Smith, C.; Lumba-Brown, A. Emergency department treatment of asthma in children: A review. JACEP Open 2020, 1, 1552–1561. [Google Scholar] [CrossRef]
- Wohlleben, M.; Meleady, L.; Oei, K.; Seaton, C. 110 Improving Asthma Education in the Emergency Department: A Quality Improvement Initiative. Paediatr. Child Health 2020, 25 (Suppl. S2), e45–e46. [Google Scholar] [CrossRef]
- Wasilewski, Y.; Clark, N.M.; Evans, D.; Levison, M.J.; Levin, B.; Mellins, R.B. Factors associated with emergency department visits by children with asthma: Implications for health education. Am. J. Public Health 1996, 86, 1410–1415. [Google Scholar] [CrossRef]
- Deis, J.N.; Spiro, D.M.; Jenkins, C.A.; Buckles, T.L.; Arnold, D.H. Parental Knowledge and Use of Preventive Asthma Care Measures in Two Pediatric Emergency Departments. J. Asthma 2010, 47, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.; Arriaga, R.I.; Korman, R.; Zafar, F.; Stephens, C.; Kumari, P.; Jayaprakash, K.; Fitzpatrick, A.M.; Cooper, N.; Morris, C.R. Pediatric emergency department-based asthma education tools and parent/child asthma knowledge. Allergy Asthma Clin. Immunol. 2024, 20, 24. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, C.E.; Sossa, M.P.; Castro-Rodriguez, J.A. Factors associated to recurrent visits to the emergency department for asthma exacerbations in children: Implications for a health education programme. Allergol. Immunopathol. 2008, 36, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Al-Muhsen, S.; Horanieh, N.; Dulgom, S.; Al Aseri, Z.; Vazquez-Tello, A.; Halwani, R.; Al-Jahdali, H. Poor asthma education and medication compliance are associated with increased emergency department visits by asthmatic children. Ann. Thorac. Med. 2015, 10, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Dondi, A.; Calamelli, E.; Piccinno, V.; Ricci, G.; Corsini, I.; Biagi, C.; Lanari, M. Acute asthma in the pediatric emergency department: Infections are the main triggers of exacerbations. BioMed Res. Int. 2017, 2017, 9687061. [Google Scholar] [CrossRef]
- Martínez-Baz, I.; Navascués, A.; Casado, I.; Portillo, M.E.; Guevara, M.; Gómez-Ibáñez, C.; Burgui, C.; Ezpeleta, C.; Castilla, J. Effect of influenza vaccination in patients with asthma. CMAJ 2021, 193, E1120–E1128. [Google Scholar] [CrossRef] [PubMed]
- Bennett, N.M.; Pilishvili, T.; Whitney, C.G.; Moore, M.; Gierke, R.; Harris, A.M. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6–18 years with immunocompromising conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 2013, 62, 521–524. [Google Scholar]
- Asthma Predictive Index (API). Available online: https://www.mdcalc.com/calc/3381/asthma-predictive-index-api (accessed on 9 July 2025).
- Modified Asthma Predictive Index (mAPI). Available online: https://www.mdcalc.com/calc/3382/modified-asthma-predictive-index-mapi (accessed on 9 July 2025).
- McGeachie, M.J. Childhood asthma is a risk factor for the development of chronic obstructive pulmonary disease. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 104–109. [Google Scholar] [CrossRef]
- Ducharme, F.; Zemek, R.; Chalut, D.; McGillivray, D.; Noya, F.; Resendes, S.; Khomenko, L.; Rouleau, R.; Zhang, X. Written action plan in pediatric emergency room improves asthma prescribing, adherence, and control. Am. J. Respir. Crit. Care Med. 2011, 183, 195–203. [Google Scholar] [CrossRef]
- Davis, J.; Fitzmaurice, L. Providing individualized written asthma action plans during the pediatric emergency department visit. J. Asthma 2020, 58, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Lakupoch, K.; Manuyakorn, W.; Preutthipan, A.; Kamalaporn, H. The effectiveness of newly developed written asthma action plan in improvement of asthma outcome in children. Asian Pac. J. Allergy Immunol. 2017, 36, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, F.; Masini, M.; Giovannini, M.; Barni, S.; Mori, F.; du Toit, G.; Bartha, I.; Lombardi, E. Asthma action plans: An international review focused on the pediatric population. Front. Pediatr. 2022, 10, 874935. [Google Scholar] [CrossRef]
- Hersey, S.; Retzke, J.; Allen, E.; Snyder, D.; Hardy, C.; Groner, J. A Primary Care-Based Quality Improvement Project to Reduce Asthma Emergency Department Visits. Pediatrics 2023, 152, e2023061355. [Google Scholar] [CrossRef]
- Kennedy, L.; Gallagher, G.; Maxwell, B.; Bartholme, B.; Fitzsimons, A.; Russell, C.; Mallon, O.; Hughes, J.; Beattie, S.; Vasi, V.; et al. Implementation of a Children’s Safe Asthma Discharge Care Pathway Reduces the Risk of Future Asthma Attacks in Children—A Retrospective Quality Improvement Report. Front. Pediatr. 2022, 10, 865476. [Google Scholar] [CrossRef]
- Asthma Management in Schools. Available online: https://www.aap.org/en/patient-care/school-health/management-of-chronic-conditions-in-schools/asthma-management-in-schools (accessed on 10 July 2025).

| Parameter | Exacerbation Severity | ||
|---|---|---|---|
| Mild/Moderate | Severe | Life-Threatening | |
| Body position | Sitting > lying | Sits hunched forwards | |
| Speech | Sentences | Words | |
| Level of consciousness | Not agitated | agitated | Drowsy, confused |
| Respiratory rate | Increased | >30/min | |
| Accessory muscle in use | No | Yes | Paradoxical breathing |
| Pulse rate | 100–120 bpm | >120 bpm | >120 bpm or bradycardia |
| SpO2 (on air) | 90–95% | <90% | |
| PEF, % predicted or best | >50% | ≤50% | Optionally |
| Symptoms | Exacerbation Severity | |
|---|---|---|
| Mild | Severe or Life Threatening | |
| Consciousness | - | Agitated, drowsy or confused |
| SaO2 | >94% | <92% |
| Speech | Sentences | Words/ Unable to speak or drink |
| Respiratory rate | ≤40/min | >40/min |
| Accessory muscle use | - | + |
| Pulse rate | <100/min | >180/min (0–3 years) >150/min (4–5 years) |
| Central cyanosis | - | + |
| Wheeze intensity | Variable | No wheezes |
| Score | Intercostal Retractions | Suprasternal Retractions | Wheezing | Vesicular Breath Sounds | SpO2 |
|---|---|---|---|---|---|
| 0 | none | none | none | present, normal | ≥95% |
| 1 | - | - | expiratory wheeze | absent in the basal regions | 92–94% |
| 2 | present | present | inspiratory and expiratory wheeze | generalized decreased breath sounds | <92% |
| 3 | audible without a stethoscope/silent chest with minimal air entry | minimal/absent breath sounds |
| Score | Respiratory Effort | Wheezing | Prolonged Expiration |
|---|---|---|---|
| 0 | absent/mild | absent/mild | normal/mildly prolonged |
| 1 | moderate | moderate | moderately prolonged |
| 2 | severe | loud wheezing or absent wheezing due to poor air exchange | significantly prolonged |
| Score | Tachypnea (According to Age) | Oxygen Requirement (to Maintain SpO2 ≥92%) | Presence of Wheezing | Vesicular Breath Sound | Types of Retractions: (Nasal Flaring, Supraclavicular, Suprasternal, Intercostal, Subcostal) |
|---|---|---|---|---|---|
| 0 | no | room air | none | normal | none |
| 1 | yes | ≤2 L/31% | end-expiratory or diffuse | moderate (diminished) | presence of one type of retraction |
| 2 | >2 L/31% and ≤4 L/50% | expiratory wheezes throughout the entire respiratory cycle | decreased | presence of two or more types of retractions | |
| 3 | >4 L/50% | inspiratory and expiratory wheezes | absent breath sounds (silent chest) | ||
| 4 | silent chest |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pełka, K.; Buzun, W.H.; Dudek, J.; Majcherczyk, K.; Klimek, O.; Chourasia, G.; Sokołowski, J.; Gogolewski, G. Exacerbation of Asthma Among Pediatric Patients Presenting to the Emergency Department. J. Clin. Med. 2025, 14, 8187. https://doi.org/10.3390/jcm14228187
Pełka K, Buzun WH, Dudek J, Majcherczyk K, Klimek O, Chourasia G, Sokołowski J, Gogolewski G. Exacerbation of Asthma Among Pediatric Patients Presenting to the Emergency Department. Journal of Clinical Medicine. 2025; 14(22):8187. https://doi.org/10.3390/jcm14228187
Chicago/Turabian StylePełka, Karolina, Wiktoria Hanna Buzun, Jakub Dudek, Krzysztof Majcherczyk, Oliwia Klimek, Goutam Chourasia, Janusz Sokołowski, and Grzegorz Gogolewski. 2025. "Exacerbation of Asthma Among Pediatric Patients Presenting to the Emergency Department" Journal of Clinical Medicine 14, no. 22: 8187. https://doi.org/10.3390/jcm14228187
APA StylePełka, K., Buzun, W. H., Dudek, J., Majcherczyk, K., Klimek, O., Chourasia, G., Sokołowski, J., & Gogolewski, G. (2025). Exacerbation of Asthma Among Pediatric Patients Presenting to the Emergency Department. Journal of Clinical Medicine, 14(22), 8187. https://doi.org/10.3390/jcm14228187





