Clinical Outcomes Associated with Oral Versus Intravenous Antibiotic Therapy in Emergency Department–Discharged Patients with Community-Acquired Pneumonia
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Patient Selection
2.3. Study Variables and Data Collection
2.4. Data Analysis
3. Results
3.1. Characteristics of Study Subjects
3.2. Main Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAP | Community-Acquired Pneumonia |
| ED | Emergency Department |
| IV | Intravenous |
| PO | oral administration |
| LOS | Length of Stay |
| CURB-65 | Confusion, Urea, Respiratory Rate, Blood Pressure, Age ≥65 |
| PSI | Pneumonia Severity Index |
| ICU | Intensive Care Unit |
| COPD | Chronic Obstructive Pulmonary Disease |
| BMI | Body Mass Index |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| HR | Heart Rate |
| RR | Respiratory Rate |
| BUN | Blood Urea Nitrogen |
| CrCl | Creatinine Clearance |
| eGFR | Estimated Glomerular Filtration Rate |
| WBC | White Blood Cells |
| HR | Hazard Ratio (in statistics section) |
| CV | Coefficient of Variation |
| OR | Odds Ratio |
| CI | Confidence Interval |
| RCTs | Randomized Controlled Trials |
| SPSS | Statistical Package for the Social Sciences |
| KAMC | King Abdulaziz Medical City |
| MNGHA | Ministry of National Guard Health Affairs |
| KAIMRC | King Abdullah International Medical Research Center |
References
- Regunath, H.; Oba, Y. Community-Acquired Pneumonia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430749/ (accessed on 19 July 2025).
- Gadsby, N.J.; Musher, D.M. The microbial etiology of community-acquired pneumonia in adults: From classical bacteriology to host transcriptional signatures. Clin. Microbiol. Rev. 2022, 35, e0001522. [Google Scholar] [CrossRef] [PubMed]
- Zaki, H.A.; Hamdi Alkahlout, B.; Shaban, E.; Mohamed, E.H.; Basharat, K.; Elsayed, W.A.E.; Azad, A. The Battle of the Pneumonia Predictors: A Comprehensive Meta-Analysis Comparing the Pneumonia Severity Index (PSI) and the CURB-65 Score in Predicting Mortality and the Need for ICU Support. Cureus 2023, 15, e42672. [Google Scholar] [CrossRef]
- Davis, D.; Thadhani, J.; Choudhary, V.; Nausheem, R.; Vallejo-Zambrano, C.R.; Arifuddin, B.M.; Ali, M.; Carson, B.J.; Kanwal, F.; Nagarajan, L.; et al. Advancements in the management of severe community-acquired pneumonia: A comprehensive narrative review. Cureus 2023, 15, e46893. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.A. Even more illness caused by smoking than previously estimated. JAMA Intern. Med. 2014, 174, 1928–1929. [Google Scholar] [CrossRef] [PubMed]
- Houck, P.M.; Bratzler, D.W.; Nsa, W.; Ma, A.; Bartlett, J.G. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch. Intern. Med. 2004, 164, 637–644. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows, Version 28.0; IBM Corp: Armonk, NY, USA, 2021. [Google Scholar]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- File, T.M.; Niederman, M.S. Antimicrobial therapy of community-acquired pneumonia. Infect. Dis. Clin. N. Am. 2005, 18, 993–1011. [Google Scholar] [CrossRef] [PubMed]
- Sartini, M.; Carbone, A.; Demartini, A.; Giribone, L.; Oliva, M.; Spagnolo, A.M.; Cremonesi, P.; Canale, F.; Cristina, M.L. Overcrowding in emergency department: Causes, consequences, and solutions—A narrative review. Healthcare 2022, 10, 1625. [Google Scholar] [CrossRef] [PubMed]
- Altyar, A.; Bakhsh, H.T.; Mohammed, A.; Skrepnek, G.; Patanwala, A.E. Administration of first dose antibiotic in the ED in patients with minor skin and soft tissue infections. Am. J. Emerg. Med. 2015, 33, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Alrashed, M.A.; Perona, S.J.; Borgstrom, M.C.; Ramirez-Moreno, E. Association between antibiotic administration before discharge and emergency department length of stay for urinary tract infection: A retrospective analysis. J. Am. Pharm. Assoc. 2024, 64, e55–e61. [Google Scholar] [CrossRef] [PubMed]
- Talan, D.A.; Takhar, S.S.; Krishnadasan, A.; Mower, W.R.; Pallin, D.J.; Garg, M.; Femling, J.; Rothman, R.E.; Moore, J.C.; Jones, A.E.; et al. Emergence of extended-spectrum β-lactamase urinary tract infections among hospitalized emergency department patients in the United States. Ann. Emerg. Med. 2021, 77, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Sulis, G.; Adam, P.; Nafade, V.; Gore, G.; Daniels, B.; Daftary, A.; Das, J.; Gandra, S.; Pai, M. Antibiotic prescription practices in primary care in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003139. [Google Scholar] [CrossRef] [PubMed]
- Pulia, M.; Redwood, R.; May, L. Antimicrobial stewardship in the emergency department. Emerg. Med. Clin. N. Am. 2018, 36, 853–872. [Google Scholar] [CrossRef] [PubMed]
- Sapozhnikov, J.; Albarillo, F.S.; Pulia, M.S. Optimizing antimicrobial stewardship in the emergency department. Emerg. Med. Clin. N. Am. 2024, 42, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Rae, N.; Singanayagam, A.; Chalmers, J.D. Oral versus intravenous clarithromycin for moderate severity community-acquired pneumonia: A randomized controlled trial. Clin. Microbiol. Infect. 2017, 23, 44–49. [Google Scholar] [PubMed]
- Teng, C.; Xu, C.; Liu, H.; Liu, Y.; Wang, X.; Zhang, X.; Zhao, L.; Li, M.; Chen, Y.; Huang, Z.; et al. Oral versus intravenous antibiotics for adults with community-acquired pneumonia: A systematic review and meta-analysis of randomized controlled trials. JAMA Netw. Open 2023, 6, e231264. [Google Scholar] [PubMed]
- Oosterheert, J.J.; Bonten, M.J.; Schneider, M.M.; Buskens, E.; Lammers, J.W.; Hustinx, W.M.; Kramer, M.H.; Prins, J.M.; Slee, P.H.; Kaasjager, K.; et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: Multicentre randomised trial. BMJ 2006, 333, 1193. [Google Scholar] [CrossRef] [PubMed]
- Kaal, M.E.; de Lange, D.W.; Bouman, C.S.; Linssen, C.F.; Koch, R.M.; Meesters, R.J.; van der Veen, A.; Vlaminckx, B.J.; van Vught, L.A.; Wessels, M.; et al. Reducing hospital length of stay by optimizing antimicrobial use in patients with community-acquired pneumonia: A multicenter prospective study. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 689–698. [Google Scholar] [PubMed]
- Tran-Le, Q.K.; Thai, T.T.; Tran-Ngoc, N.; Duong-Minh, N.; Nguyen-Ho, L.; Nguyen-Dang, K.; Nhat, P.T.H.; Pisani, L.; Vu-Hoai, N.; Le-Thuong, V. Lung ultrasound for the diagnosis and monitoring of pneumonia in a tuberculosis-endemic setting: A prospective study. BMJ Open 2025, 15, e094799. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]
- Froes, F.; Pereira, J.G.; Póvoa, P.; Torres, A.; Martin-Loeches, I.; Niederman, M.; Welte, T.; Woodhead, M.; Rello, J.; Chalmers, J.D.; et al. Outpatient management of community-acquired pneumonia. Curr. Opin. Pulm. Med. 2019, 25, 249–256. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Overall | Oral n = 162 (37.7) | Parenteral n = 268 (62.3) | p-Value |
|---|---|---|---|---|
| Age, years | 66.0 (52.0–76.0) | 67.0 (54.0–77.0) | 65.0 (51.0–75.0) | 0.3587 |
| Gender, Female | 223 (51.9) | 77 (47.5) | 146 (54.5) | 0.1624 |
| Weight, kg | 77.0 (66.0–92.1) | 76.0 (65.0–91.0) | 78.0 (68.0–94.0) | 0.2890 |
| Height, cm | 160.0 (153.0–166.0) | 160.0 (153.0–168.0) | 160.0 (153.0–166.0) | 0.6676 |
| BMI, kg/m2 | 30.3 (25.9–36.0) | 30.0 (25.6–35.0) | 30.8 (26.0–36.1) | 0.2926 |
| Vital signs and laboratory values | ||||
| SBP | 130.0 (117.0–143.0) | 133.0 (120.0–146.0) | 128.5 (116.0–141.5) | 0.0341 |
| DBP | 67.0 (59.0–76.0) | 66.0 (58.0–76.0) | 67.0 (60.0–77.0) | 0.2686 |
| HR | 94.0 (81.0–106.0) | 87.0 (78.0–100.0) | 96.0 (85.0–109.5) | 0.0002 |
| RR | 21.0 (20.0–23.0) | 20.0 (20.0–22.0) | 21.0 (20.0–24.0) | 0.0010 |
| O2 Saturation, % | 96.0 (95.0–98.0) | 96.0 (95.0–98.0) | 96.0 (94.0–98.0) | 0.0655 |
| Temperature, °C | 37.1 (36.8–38.1) | 37.0 (36.7–37.4) | 37.6 (37.0–38.6) | <0.0001 |
| WBC | 8.2 (6.0–11.4) | 8.2 (6.3–10.7) | 8.2 (5.7–11.9) | 0.6319 |
| CrCl | 69.0 (49.6–96.0) | 66.9 (43.9–94.2) | 72.0 (51.5–96.5) | 0.0825 |
| eGFR | 91.0 (69.0–109.0) | 86.0 (60.0–105.0) | 93.5 (71.0–109.0) | 0.0604 |
| BUN | 4.7 (3.4–6.0) | 5.0 (3.6–6.6) | 4.5 (3.2–5.8) | 0.0074 |
| Presenting complaint | ||||
| Cough | 350 (81.4) | 119 (73.5) | 231 (86.2) | 0.0010 |
| Fever | 284 (66.0) | 83 (51.2) | 201 (75.0) | <0.0001 |
| Shortness of breath | 271 (63.0) | 94 (58.0) | 177 (66.0) | 0.0950 |
| Mucus production | 135 (31.4) | 48 (29.6) | 87 (32.5) | 0.5396 |
| Chest pain | 49 (11.4) | 20 (12.3) | 29 (10.8) | 0.6297 |
| Confusion | 6 (1.4) | 3 (1.9) | 3 (1.1) | 0.5304 |
| Other | 116 (27.0) | 39 (24.1) | 77 (28.7) | - |
| Risk factor for recurrence | ||||
| Age > or equal to 65 | 226 (52.6) | 89 (54.9) | 137 (51.1) | 0.4422 |
| Hypertension | 226 (52.6) | 94 (58.0) | 132 (49.3) | 0.0776 |
| Diabetes | 225 (52.3) | 89 (54.9) | 136 (50.7) | 0.3990 |
| Obesity | 206 (47.9) | 70 (43.2) | 136 (50.7) | 0.1295 |
| Dyslipidemia | 126 (29.3) | 51 (31.5) | 75 (28.0) | 0.4402 |
| CAD | 72 (16.7) | 31 (19.1) | 41 (15.3) | 0.3017 |
| Asthma | 55 (12.8) | 17 (10.5) | 38 (14.2) | 0.2675 |
| Renal diseases | 45 (10.5) | 23 (14.2) | 22 (8.2) | 0.0493 |
| Received paren. antibiotics within 90 days | 46 (10.7) | 23 (14.2) | 23 (8.6) | 0.0679 |
| Atrial fibrillation | 40 (9.3) | 16 (9.9) | 24 (9.0) | 0.7499 |
| COPD | 14 (3.3) | 4 (2.5) | 10 (3.7) | 0.4748 |
| Dementia | 9 (2.1) | 3 (1.9) | 6 (2.2) | 0.7859 |
| Stroke | 5 (1.2) | 1 (0.6) | 4 (1.5) | 0.4120 |
| GERD | 5 (1.2) | 1 (0.6) | 4 (1.5) | 0.4120 |
| Intervention | Overall | Oral n = 162 (37.7) | Parenteral n = 268 (62.3) |
|---|---|---|---|
| Emergency department resuscitation (NaCl 0.9%) | 198 (46.0) | 36 (22.2) | 162 (60.4) |
| Time to start fluid resuscitation, HH:MM | 12:54 ± 6:54 | 12:28 ± 6:58 | 13:00 ± 6:54 |
| Time to start parenteral antibiotic in ED, HH:MM | 13:39 ± 6:52 | NA | 13:39 ± 6:52 |
| Parenteral antibiotic administered in ED | |||
| Ceftriaxone | 102 (23.7) | NA | 102 (38.1) |
| Ceftriaxone + Azithromycin | 94 (21.9) | NA | 94 (35.1) |
| Moxifloxacin | 33 (7.7) | NA | 33 (12.3) |
| Azithromycin | 16 (3.7) | NA | 16 (6.0) |
| Piperacillin/tazobactam | 11 (2.6) | NA | 11 (4.1) |
| Piperacillin/tazobactam + Vancomycin | 3 (0.7) | NA | 3 (1.1) |
| Ceftriaxone + Clindamycin | 1 (0.2) | NA | 1 (0.4) |
| Ceftriaxone + Erythromycin | 1 (0.2) | NA | 1 (0.4) |
| Ceftriaxone + Moxifloxacin | 1 (0.2) | NA | 1 (0.4) |
| Moxifloxacin + Vancomycin | 1 (0.2) | NA | 1 (0.4) |
| Cefepime | 1 (0.2) | NA | 1 (0.4) |
| Ciprofloxacin | 1 (0.2) | NA | 1 (0.4) |
| Meropenem | 1 (0.2) | NA | 1 (0.4) |
| Ampicillin/sulbactam | 1 (0.2) | NA | 1 (0.4) |
| Prescribed oral antibiotics at discharge from ED | |||
| Moxifloxacin | 123 (28.6) | 53 (32.7) | 70 (26.1) |
| Cefuroxime + Azithromycin | 85 (19.8) | 24 (14.8) | 61 (22.8) |
| Amoxicillin/clavulanic acid + Azithromycin | 70 (16.3) | 12 (7.4) | 58 (21.6) |
| Amoxicillin/clavulanic acid | 62 (14.4) | 28 (17.3) | 34 (12.7) |
| Cefuroxime | 33 (7.7) | 18 (11.1) | 15 (5.6) |
| Azithromycin | 29 (6.7) | 17 (10.5) | 12 (4.5) |
| Amoxicillin/clavulanic acid + Doxycycline | 2 (0.5) | 0 (0.0) | 2 (0.7) |
| Amoxicillin/clavulanic acid + Moxifloxacin | 2 (0.5) | 1 (0.6) | 1 (0.4) |
| Azithromycin + Amoxicillin | 1 (0.2) | 0 (0.0) | 1 (0.4) |
| Azithromycin + Moxifloxacin | 3 (0.7) | 0 (0.0) | 3 (1.1) |
| Azithromycin + Clindamycin | 1 (0.2) | 0 (0.0) | 1 (0.4) |
| Cefuroxime + Doxycycline | 6 (1.4) | 2 (1.2) | 4 (1.5) |
| Cefuroxime + Clarithromycin | 2 (0.5) | 0 (0.0) | 2 (0.7) |
| Cefuroxime + Augmentin | 1 (0.2) | 0 (0.0) | 1 (0.4) |
| Moxifloxacin + Metronidazole | 2 (0.5) | 2 (1.2) | 0 (0.0) |
| Ciprofloxacin | 3 (0.7) | 1 (0.6) | 2 (0.7) |
| Doxycycline | 3 (0.7) | 3 (1.9) | 0 (0.0) |
| Amoxicillin | 1 (0.2) | 0 (0.0) | 1 (0.4) |
| Cefprozil | 1 (0.2) | 1 (0.6) | 0 (0.0) |
| Outcome | Overall | Oral n = 162 (37.7) | Parenteral n = 268 (62.3) | p-Value |
|---|---|---|---|---|
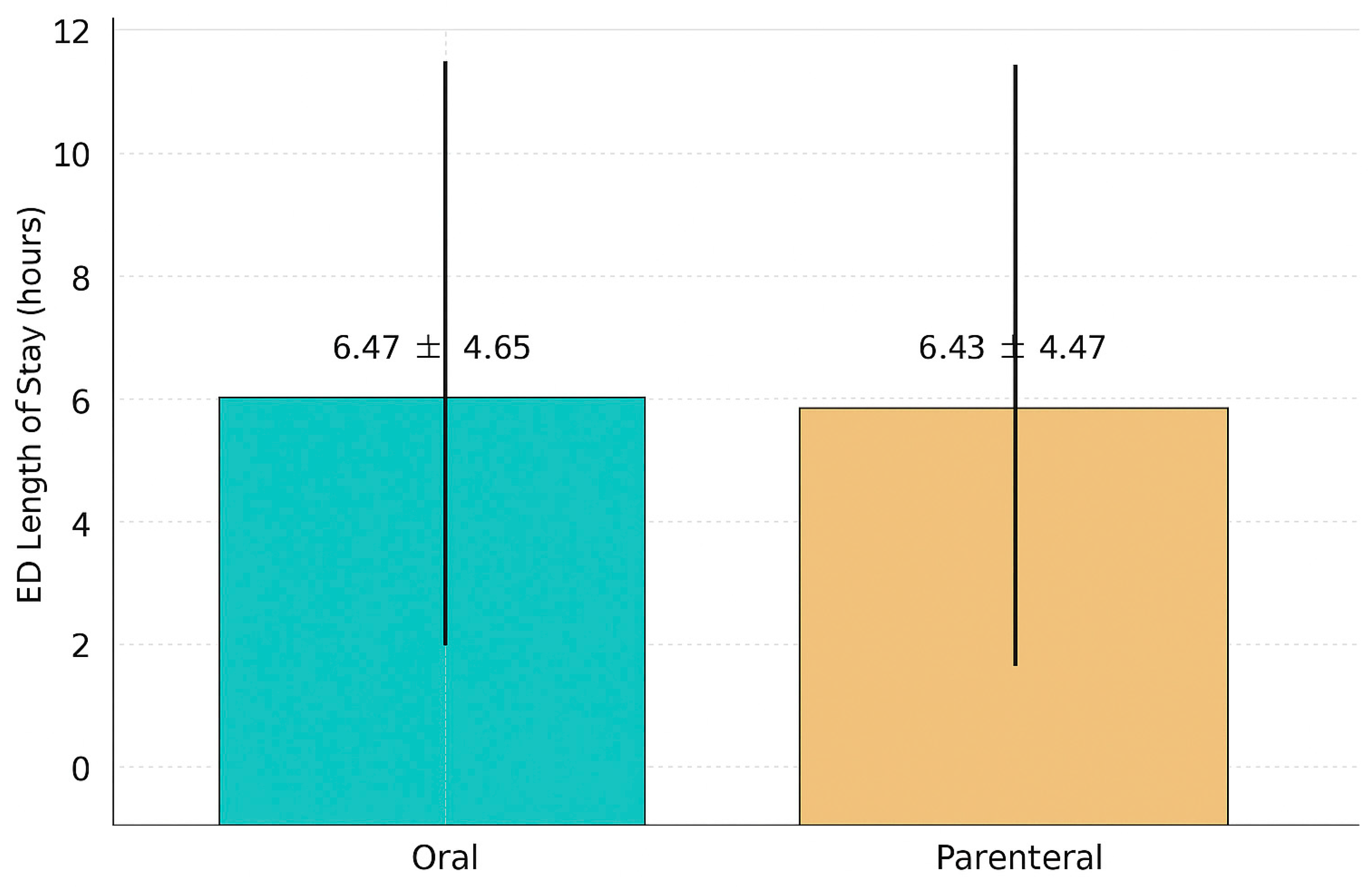
| Mean ED LOS, HH:MM | 6:28 ± 4:39 | 6:31 ± 4:56 | 6:26 ± 4:28 | 0.5559 |
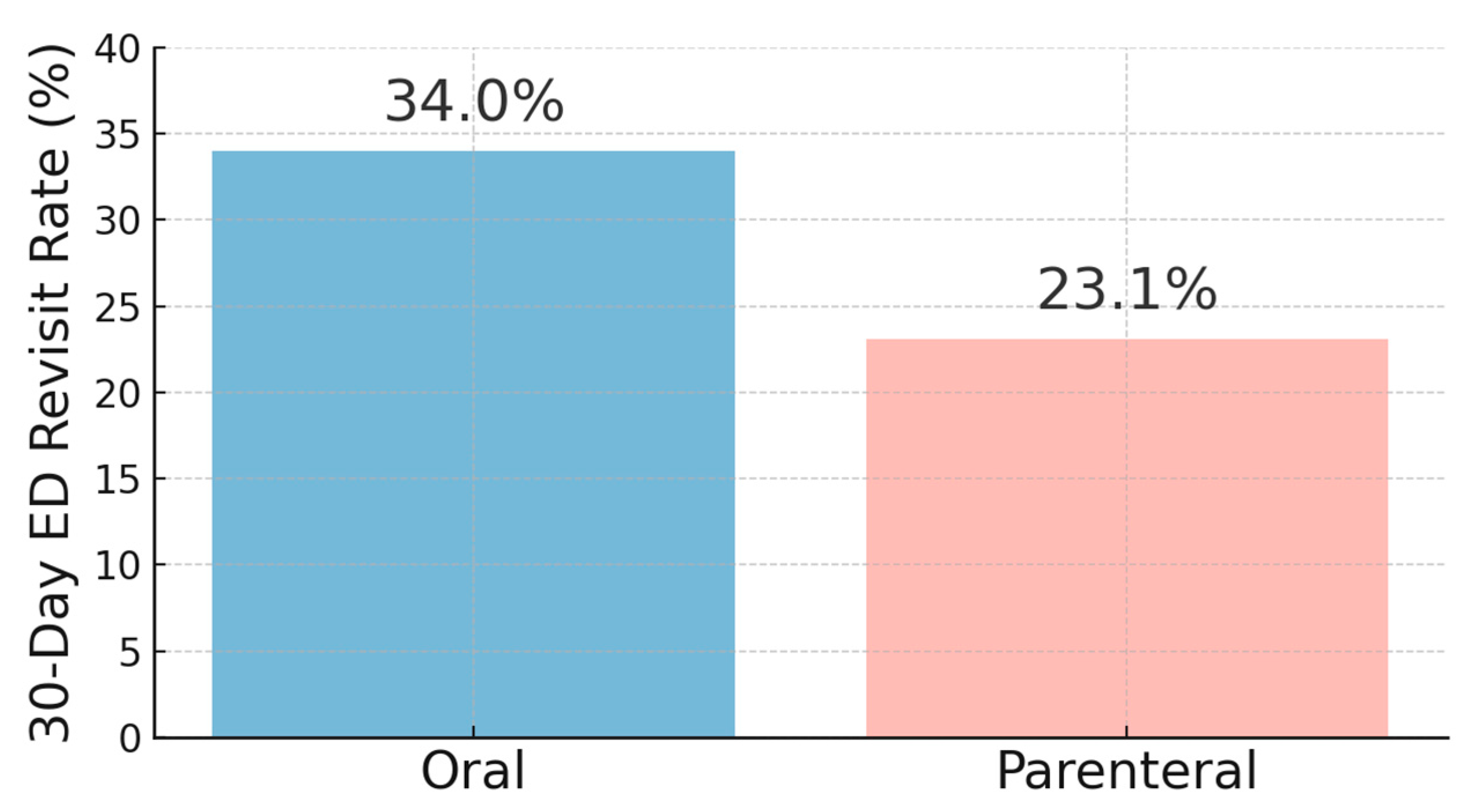
| 30-Day ED Revisit Rates | 117 (27.2) | 55 (34.0) | 62 (23.1) | 0.0146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrashed, M.; Alyousef, S.; Alamri, B.; Yousef, O.; AlJarallah, H.; Alshehri, A.; Almohammed, O.A.; Aljabri, A. Clinical Outcomes Associated with Oral Versus Intravenous Antibiotic Therapy in Emergency Department–Discharged Patients with Community-Acquired Pneumonia. J. Clin. Med. 2025, 14, 8167. https://doi.org/10.3390/jcm14228167
Alrashed M, Alyousef S, Alamri B, Yousef O, AlJarallah H, Alshehri A, Almohammed OA, Aljabri A. Clinical Outcomes Associated with Oral Versus Intravenous Antibiotic Therapy in Emergency Department–Discharged Patients with Community-Acquired Pneumonia. Journal of Clinical Medicine. 2025; 14(22):8167. https://doi.org/10.3390/jcm14228167
Chicago/Turabian StyleAlrashed, Mohammed, Saleh Alyousef, Bader Alamri, Omar Yousef, Hisham AlJarallah, Abdulmajeed Alshehri, Omar A. Almohammed, and Ahmed Aljabri. 2025. "Clinical Outcomes Associated with Oral Versus Intravenous Antibiotic Therapy in Emergency Department–Discharged Patients with Community-Acquired Pneumonia" Journal of Clinical Medicine 14, no. 22: 8167. https://doi.org/10.3390/jcm14228167
APA StyleAlrashed, M., Alyousef, S., Alamri, B., Yousef, O., AlJarallah, H., Alshehri, A., Almohammed, O. A., & Aljabri, A. (2025). Clinical Outcomes Associated with Oral Versus Intravenous Antibiotic Therapy in Emergency Department–Discharged Patients with Community-Acquired Pneumonia. Journal of Clinical Medicine, 14(22), 8167. https://doi.org/10.3390/jcm14228167






