1. Introduction
Advancements in critical care have significantly improved survival rates among patients admitted to intensive care units (ICUs). However, many ICU survivors experience long-term impairments in physical, cognitive, and psychological functions, a condition widely recognized as post-intensive care syndrome (PICS) [
1,
2]. PICS negatively affects quality of life, independence in daily living, and return to societal roles, while increasing risks of rehospitalization and long-term care needs [
3,
4]. Consequently, the focus of critical care has shifted from short-term survival to long-term recovery and functional reintegration [
5].
One of the major contributors to PICS is hospital-associated disability (HAD), which refers to a decline in activities of daily living (ADL) that develops during hospitalization in previously independent individuals [
6]. HAD frequently results from immobility, enforced bed rest, and acute illness-related deconditioning. Elderly patients and those with preexisting chronic conditions are particularly vulnerable. In ICU settings, where the severity of illness is high and invasive procedures are standard, the risk of HAD is especially elevated [
7,
8].
Although some cases of HAD are transient, many patients do not fully recover their function after discharge. HAD is associated with greater dependency, fall risk, depression, and social isolation [
6,
9]. Early mobilization during the ICU stay has been shown to preserve physical function and reduce ICU-acquired weakness [
10]. However, the incidence of HAD remains high in real-world clinical settings, and large-scale studies investigating its impact on post-discharge functional status are limited.
In this context, we aimed to investigate the relationship between in-hospital ADL decline (i.e., HAD) and functional impairment at 3 months after discharge using data from a nationwide, multicenter, prospective cohort study (J-RELIFE) involving 22 ICUs across Japan. Specifically, we focused on changes in the Barthel Index (BI) from ICU admission to discharge and their association with physical, cognitive, and psychological outcomes. We hypothesized that patients who experienced HAD during ICU stay would exhibit significantly higher rates of impaired functional status at three months post-discharge, even if they appeared functionally recovered at the time of hospital discharge. Establishing HAD as a prognostic marker, rather than a transient event, may support the development of targeted rehabilitation and discharge planning strategies.
2. Materials and Methods
2.1. Study Design and Setting
This study was a multicenter, prospective, observational cohort study conducted within the J-RELIFE project (UMIN000036503). The study was designed to investigate PICS, including physical, cognitive, and psychological impairments, and their associated risk factors in critically ill patients discharged from ICUs. Twenty-two tertiary hospitals with established ICU rehabilitation protocols participated in the study between October 2021 and December 2023. The study protocol was approved by the institutional review boards of all participating facilities, with central approval obtained from the Fujita Health University Ethics Committee (Approval ID: HM21-077). Written informed consent was obtained from all patients or their legally authorized representatives.
2.2. Participants
Eligible participants were adult patients (aged 40 years or older) admitted to the ICU who received mechanical ventilation for 48 h or more and were deemed eligible for ICU rehabilitation according to standard institutional criteria. The J-RELIFE study was designed to evaluate the implementation of rehabilitation in ICU settings. Therefore, only patients who received at least one rehabilitation session during their ICU stay were enrolled prospectively. Patients who did not undergo rehabilitation were excluded from the study cohort, in accordance with the predefined inclusion criteria. Patients were excluded if they (1) had central nervous system disorders causing disability; (2) had difficulty communicating in Japanese or had psychiatric conditions interfering with rehabilitation; (3) were unable to walk even with assistive devices before admission; (4) were under end-of-life care or died during hospitalization; or (5) were placed under COVID-19 quarantine, declined participation, had motor impairments that precluded walking assessment, or did not receive any rehabilitation sessions during their ICU stay.
2.3. Rehabilitation and Data Collection
All participating ICUs followed a standardized mobilization protocol categorized into five activity levels: level 1 (passive movement and respiratory therapy), level 2 (active range of motion), level 3 (sitting at bedside), level 4 (standing), and level 5 (ambulation) [
11,
12]. Rehabilitation was initiated as early as clinically feasible and provided daily when possible. Trained physiotherapists documented the start date of rehabilitation and the average daily duration.
Data were prospectively collected from ICU admission to three months post-discharge, including baseline demographics, comorbidities, ICU treatments, rehabilitation parameters, and functional assessments. Post-discharge follow-up was conducted using mailed questionnaires, including the Kihon Checklist (KCL), a validated screening tool for physical, cognitive, and psychological function in older adults [
13].
2.4. Outcome Measures
The primary outcome of this study was functional status three months after hospital discharge, assessed using the KCL. A total KCL score ≥ 8 was defined as global functional decline. Additionally, the prevalence of impairment in the individual KCL domains—physical function, nutritional status, oral function, cognitive function, and depression—was assessed. These domains represent key components of long-term frailty and disability in ICU survivors. The age threshold of 40 years was selected because the primary outcome measure is the KCL, a validated screening tool developed for use in middle-aged and older adults in Japan [
13]. Prior studies have demonstrated its predictive value for frailty, disability, and long-term care needs in this population. Moreover, functional decline following critical illness is not limited to the elderly but can also occur in adults from early middle age, especially among those with comorbidities. Therefore, including patients aged 40 years and older was deemed appropriate to capture both early- and late-onset post-ICU impairments while ensuring the applicability of the KCL.
Secondary outcomes included physical and psychological function at hospital discharge. Functional ability was assessed using the BI, the Short Physical Performance Battery (SPPB) [
14], the Medical Research Council (MRC) score [
15], and ambulatory status (independent, modified independent, dependent, or non-ambulatory). Psychological function was measured using the Hospital Anxiety and Depression Scale (HADS) [
16] and the Impact of Event Scale-Revised (IES-R) [
17]. HAD was defined as a decrease of ≥5 points in the BI from the pre-hospital baseline (assessed at ICU admission via patient or proxy interview) to hospital discharge [
18,
19]. Additional variables included ICU and hospital length of stay, mechanical ventilation duration, time to initiation of rehabilitation, and time to achievement of key mobilization milestones (sitting, standing, walking).
2.5. Covariates
We included clinically relevant covariates previously associated with functional outcomes in critically ill patients. Demographic variables included age and sex. To account for baseline disease burden and acute illness severity, we used the Charlson Comorbidity Index and APACHE II score at the time of ICU admission. Pre-hospital frailty was assessed using the Clinical Frailty Scale [
20].
Two discharge-related variables were also included: (1) ADL dependency at discharge, defined as a BI score < 85 [
19], and (2) psychological symptoms at discharge, defined by the presence of depression and/or anxiety according to the HADS [
16]. These covariates represent biological, functional, and psychological domains that may affect post-discharge recovery.
2.6. Statistical Analysis
Analyses were performed on complete cases, including only patients with available 3-month follow-up Kihon Checklist (KCL) data. Missing data was not imputed. All statistical analyses were conducted using R software (version 4.0.5; R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics were used to summarize baseline characteristics. Continuous variables were reported as medians with interquartile ranges (IQRs) and compared using the Mann–Whitney U test. Categorical variables were presented as counts and percentages and compared using Fisher’s exact test. To identify independent predictors of functional decline at three months post-discharge, multivariable logistic regression analysis was performed. Covariates included age, sex, Charlson Comorbidity Index, APACHE II score, Clinical Frailty Scale, ADL dependency at discharge, and psychological symptoms at discharge. Adjusted odds ratios (AORs) with 95% confidence intervals (CIs) were reported. To examine associations with all-cause mortality three months after ICU discharge, we conducted a relative risk analysis using a generalized linear model with a log link and binomial distribution. The same covariates were included to estimate adjusted relative risks (RRs) and 95% confidence intervals.
3. Results
3.1. Baseline Characteristics
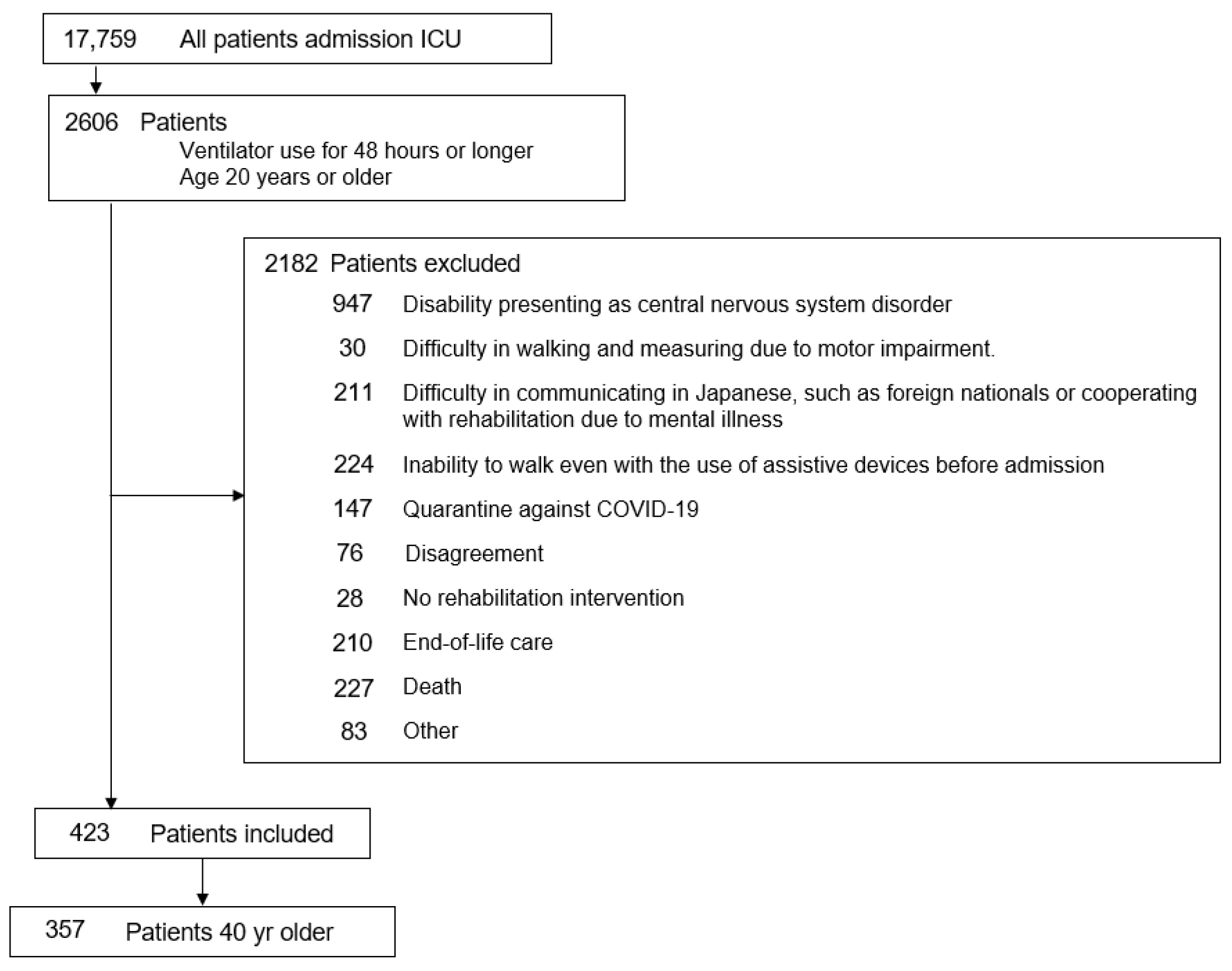
Of 17,759 ICU admissions, 2606 patients aged ≥ 20 years who received mechanical ventilation for ≥48 h were screened. After excluding 2182 patients due to clinical or functional limitations, 423 were eligible. Among them, 66 patients aged < 40 years were excluded, leaving 357 patients in the final analysis (
Figure 1).
Table 1 shows the baseline characteristics of 357 ICU survivors, stratified by the presence (n = 260) or absence (n = 97) of functional decline at 3 months post-discharge. Patients in the decline group were significantly older than those in the non-decline group (
p < 0.001) and had higher Charlson Comorbidity Index scores (
p < 0.001). The Clinical Frailty Scale was also higher in the decline group (
p = 0.014), despite comparable pre-admission BI scores.
Rehabilitation milestones were delayed in the decline group, as evidenced by significantly longer times to first sitting (
p = 0.008), standing (
p = 0.002), and marching (
p = 0.004) compared to the non-decline group. The rehabilitation time per session was also slightly shorter in the decline group (
p = 0.036). Time to initiate rehabilitation was modestly delayed in the decline group (
p = 0.045) (
Table 1).
3.2. Primary Outcomes
Table 2 presents the prevalence of impairments across six domains of the Kihon Checklist assessed at 3 months after hospital discharge. At this point, global functional decline—the primary outcome of the study —was defined as a total KCL score of 8 or greater and was observed in 72.8% of patients. The most frequent individual impairment was cognitive function decline (64.4%), followed by possible depression (48.2%) and physical function decline (47.3%). Declines in oral function and nutritional status were also noted in 28.3% and 15.7% of patients, respectively (
Table 2).
3.3. Secondary Outcomes
Table 3 summarizes the physical and psychological status of patients at hospital discharge, stratified by functional outcome at 3 months. Patients in the functional decline group had significantly lower BI scores (median 95 vs. 100,
p < 0.001), lower MRC scores (58 vs. 60,
p = 0.015), and lower SPPB scores (10 vs. 11,
p = 0.004), particularly in the chair stand (2 vs. 4,
p = 0.001) and gait speed tests (4 vs. 4,
p = 0.037). Psychological assessments also revealed significantly higher HADS in the decline group, with 50.8% scoring ≥ 8 for depression and 40.0% for anxiety, compared to 29.9% and 24.7%, respectively, in the non-decline group (
p < 0.01). PTSD symptoms (IES-R ≥ 25) were more frequent in the decline group (21.9% vs. 11.3%,
p = 0.023). Additionally, functional decline during hospitalization (ΔBI ≥ 5) was more prevalent in the decline group (42.0% vs. 21.6%,
p < 0.001), and fewer were fully ambulatory at discharge (65.4% vs. 79.4%,
p = 0.014).
3.4. Multivariable Analysis
Table 4 shows the results of multivariable logistic regression analyses examining factors associated with functional decline at 3 months post-discharge. In Model 1, discharge ADL dependency (BI < 85) was significantly associated with functional decline (OR = 2.45, 95% CI: 1.24–4.82,
p = 0.009), as well as older age (
p = 0.004). In Model 2, HAD was a significant predictor (OR = 2.17, 95% CI: 1.23–3.83,
p = 0.007), and the Charlson Comorbidity Index also reached statistical significance (OR = 1.18, 95% CI: 1.01–1.38,
p = 0.042). In both models, depression or anxiety at discharge remained significantly associated with functional decline (ORs ~2.1,
p < 0.01). In Model 3, which included all variables, HAD (OR = 1.80, 95% CI: 1.00–3.24,
p = 0.049) and depression/anxiety (OR = 2.11, 95% CI: 1.27–3.49,
p = 0.004) remained independently associated with the outcome, whereas the effect of discharge ADL dependency was no longer statistically significant (OR = 1.88,
p = 0.077). Age was a consistent predictor across all models (
p < 0.05) (
Table 4).
Table 5 presents the results of the relative risk analysis for global functional decline at 3 months after hospital discharge. In the overall cohort (n = 357), hospital-acquired disability (HAD) was significantly associated with increased risk of decline (RR = 1.99, 95% CI: 1.71–2.31,
p < 0.001). Older age (≥65 years) and psychological symptoms at discharge (depression and/or anxiety) were also independent risk factors (RR = 1.13 and 1.35, respectively; both
p < 0.05). Patients with both HAD and psychological symptoms had a combined risk of 3.02 (95% CI: 2.49–3.67), while those with all three factors—HAD, older age, and psychological symptoms—had the highest observed risk (RR = 3.66, 95% CI: 2.95–4.55,
p < 0.001). Additionally, ADL dependency at discharge (Barthel Index < 85) was strongly associated with functional decline (RR = 2.92, 95% CI: 2.41–3.54,
p < 0.001). In the subgroup of patients who were functionally independent at discharge (BI ≥ 85, n = 268), similar associations were observed. HAD remained a robust predictor (RR = 3.98, 95% CI: 3.02–5.24,
p < 0.001), and the presence of all three risk factors conferred a markedly elevated risk of decline (RR = 10.17, 95% CI: 6.46–16.00,
p < 0.001) (
Table 5).
4. Discussion
In this multicenter prospective cohort study, we found that HAD, older age, and psychological distress at discharge were independently associated with global functional decline three months after discharge in ICU survivors. Notably, HAD alone was associated with a 4-fold increase in the odds of functional decline, and the cumulative presence of all three risk factors was associated with a 10-fold increase. These findings underscore the importance of monitoring and addressing physical and psychological vulnerabilities in critically ill patients not only during ICU stay, but also throughout the transition to post-acute care.
Our findings are consistent with previous reports that have highlighted HAD as a key consequence of critical illness, especially in older adults and those undergoing prolonged immobilization [
6,
7]. HAD, characterized by a decline in ADL during hospitalization despite previously independent status, has been associated with increased mortality, readmission, and long-term care needs [
19]. While early mobilization during the ICU stay has been shown to mitigate the risk of HAD [
10,
11], our study suggests that even when patients regain basic ADL independence at discharge, a history of in-hospital functional decline may continue to influence post-discharge outcomes. This highlights the limitation of relying solely on discharge BI or SPPB scores when estimating long-term prognosis. These results aligned with previous reports highlighting the long-term impact of HAD in ICU survivors. For example, Schweickert et al. [
10] demonstrated that early mobilization during ICU stay improved short-term physical outcomes but did not fully prevent long-term functional limitations. Herridge et al. [
21] reported persistent physical disability lasting up to five years after ICU discharge, even in younger patients. These earlier studies underscored the prolonged recovery trajectory following critical illness but focused primarily on physical performance over time. Compared to those studies, our cohort was restricted to adults aged ≥ 40 years who were independent before ICU admission and received structured rehabilitation, allowing us to assess HAD as a discharge-time phenomenon more precisely. Additionally, while Schweickert et al. emphasized early interventions [
10] and Herridge et al. tracked long-term outcomes [
21], our study uniquely identifies discharge-time markers—such as HAD and psychological distress—that predict medium-term (3-month) global functional decline. Our use of the BI as a standardized ADL measure also allows for direct quantification of hospital-acquired changes, which some prior studies did not assess in detail.
Furthermore, the synergistic effect observed between HAD, older age, and depression/anxiety suggests a biopsychosocial interaction that shapes recovery trajectories after critical illness. The co-occurrence of HAD, psychological distress, and older age suggests a complex interplay between physiological vulnerability, emotional resilience, and aging-related decline. The presence of psychological symptoms at discharge may compound physical vulnerability, leading to disengagement from rehabilitation or reduced self-efficacy in resuming daily activities. Similarly, frail older adults may lack the physiological reserve to recover from acute functional losses [
20]. These findings align with the PICS framework, which describes persistent impairments across physical, cognitive, and mental domains after ICU discharge [
4,
22].
From a clinical standpoint, our findings support the need for more nuanced discharge planning that incorporates both objective physical performance measures and psychological status assessments. Patients with HAD, even if functionally independent at discharge, should be prioritized for early and structured post-discharge rehabilitation and follow-up. Integrating frailty screening and mood assessment into discharge protocols may improve the targeting of such interventions. Standard discharge checklists could be enhanced by incorporating screening for HAD and psychological symptoms. Moreover, patients with multiple risk factors may benefit from integrated care pathways with a long-term perspective, coordinated by interdisciplinary teams composed of physiotherapists, occupational therapists, mental health professionals, social workers, physicians, and nurses.
5. Limitations
Several limitations of our study warrant consideration. First, although the multicenter design enhances the external validity of our findings, differences in rehabilitation protocols across institutions may have introduced variability. While all participating centers adhered to standardized rehabilitation procedures, minor institutional variations likely remained and could have influenced patient outcomes. This potential confounding should be acknowledged when interpreting the results. Second, functional outcomes were assessed using self-reported questionnaires, which may be subject to response bias. These tools are also self-reported, raising concerns about reporting bias and potential under- or over-estimation of psychological distress. Similarly, pre-hospital ADL was assessed retrospectively via interview, which may be subject to recall bias, potentially limiting the accuracy of baseline functional status. Third, unmeasured factors such as nutritional status, social support, and psychosocial interventions may have influenced recovery. Future studies incorporating these aspects will be essential to provide a more comprehensive understanding of post-ICU recovery. As this was an observational study, our findings describe associations rather than causal relationships. Furthermore, although relative risk analysis was used to examine associations with functional decline, the outcome was assessed at a single time point (three months post-discharge), and the exact timing of the decrease was not recorded. As such, the estimated risk ratios should be interpreted as approximations of relative risk rather than actual time-to-event effects. While the Kihon Checklist is validated for middle-aged and older adults, excluding ICU survivors under 40 years of age may introduce selection bias and limit the generalizability of our findings to younger populations. Younger adults may have different patterns of recovery and risk factors for functional decline. Future studies should consider including validated outcome measures suitable for younger ICU survivors to assess post-ICU trajectories across age groups more comprehensively. Finally, our exclusion of patients with pre-existing central nervous system disorders, psychiatric illness, or communication difficulties, while necessary for accurate HAD classification, may have introduced selection bias. These populations are known to be at increased risk of post-ICU impairment, and their exclusion may lead to underestimation of the true prevalence and burden of functional decline after critical illness. Despite these limitations, this study adds necessary evidence to the growing body of literature on post-ICU outcomes. It supports conceptualizing HAD as a dynamic risk factor—not merely a transient in-hospital event—but as a long-term predictor of global functional decline. These insights may guide more effective transitional care models and rehabilitation strategies aimed at reducing disability and promoting recovery among ICU survivors.
6. Conclusions
This multicenter prospective cohort study demonstrated that HAD, older age, and psychological distress at discharge are independent and cumulative predictors of global functional decline at three months after hospital discharge in ICU survivors. Notably, even patients who recovered their ADL independence by discharge remained at increased risk of post-discharge decline if they had experienced HAD during hospitalization. These findings emphasize the importance of not only early mobilization in the ICU but also comprehensive discharge assessment that includes physical, psychological, and frailty-related factors. Identifying high-risk patients at discharge may facilitate timely interventions and structured rehabilitation programs to mitigate long-term disability and enhance post-ICU recovery trajectories. Future research should focus on intervention strategies tailored to individuals with HAD and explore the effectiveness of multidisciplinary post-discharge care pathways.
Author Contributions
Conceptualization, Y.I.; methodology, Y.I. and S.W.; formal analysis, Y.I.; investigation, Y.Y., A.S., T.M., K.O. and the RELIFE Network; data curation, S.W. and Y.Y.; writing—original draft preparation, Y.I. and S.W.; writing—review and editing, R.K. and S.I.; visualization, S.W.; supervision, R.K. and S.I.; project administration, O.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Fujita Health University (protocol code HM21-077, initially approved on 23 June 2021 and revised on 13 February 2023). The revision updated the protocol by adding the names of newly participating institutions after the study had begun; there were no changes to the study methods. The ethics committees of all participating institutions also approved of the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study or their legal representatives.
Data Availability Statement
Data available on request due to restrictions (e.g., privacy, legal or ethical reasons). The data presented in this study are available upon request from the corresponding author due to ethical restrictions on patient confidentiality.
Acknowledgments
The authors would like to thank all collaborating ICUs and rehabilitation teams in the J-RELIFE network for their contributions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADL | Activities of Daily Living |
| ICU | Intensive Care Unit |
| PICS | Post-Intensive Care Syndrome |
| BI | Bathel Index |
| J-ReLIFE | Japanese Research on Rehabilitation and Risk Factors in Post-Intensive Care Patients |
| KCL | Kihon Checklist |
| HAD | Hospital-Acquired Disability |
| SPPB | Short Physical Performance Battery |
| MRC | Medical Research Council |
| HADS | Hospital Anxiety and Depression Scale |
| IES-R | Impact of Event Scale-Revised |
| IQR | Interquartile Range |
| APACHE II | Acute Physiology and Chronic Health Evaluation II |
| HR | Hazard Ratio |
| AOR | Adjusted Odds Ratio |
| CI | Confidence Interval |
References
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Christine, Z.; Anita, B.-D.; Berney, S.C.; Joseph, B.O.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef]
- Rawal, G.; Yadav, S.; Kumar, R. Post-intensive care syndrome: An overview. J. Transl. Int. Med. 2017, 5, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Pandharipande, P.P.; Girard, T.D.; Jackson, J.C.; Morandi, A.; Thompson, J.L.; Pun, B.T.; Brummel, N.E.; Hughes, C.G.; Vasilevskis, E.E.; Shintani, A.K.; et al. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef]
- Marra, A.; Pandharipande, P.P.; Girard, T.D.; Patel, M.B.; Hughes, C.G.; Jackson, J.C.; Thompson, J.L.; Chandrasekhar, R.; Ely, E.W.; Brummel, N.E. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit. Care Med. 2018, 46, 1393–1401. [Google Scholar] [CrossRef]
- Iwashyna, T.J. Survivorship will be the defining challenge of critical care in the 21st century. Ann. Intern. Med. 2010, 153, 204–205. [Google Scholar] [CrossRef] [PubMed]
- Covinsky, K.E.; Pierluissi, E.; Johnston, C.B. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA 2011, 306, 1782–1793. [Google Scholar] [CrossRef]
- Zisberg, A.; Shadmi, E.; Gur-Yaish, N.; Tonkikh, O.; Sinoff, G. Hospital-associated functional decline: The role of hospitalization processes beyond individual risk factors. J. Am. Geriatr. Soc. 2015, 63, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hermans, G.; Van Mechelen, H.; Clerckx, B.; Vanhullebusch, T.; Mesotten, D.; Wilmer, A.; Casaer, M.P.; Meersseman, P.; Debaveye, Y.; Van Cromphaut, S.; et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am. J. Respir. Crit. Care Med. 2014, 190, 410–420. [Google Scholar] [CrossRef]
- Fan, E.; Dowdy, D.W.; Colantuoni, E.; Mendez-Tellez, P.A.; Sevransky, J.E.; Shanholtz, C.; Himmelfarb, C.R.D.; Desai, S.V.; Ciesla, N.; Herridge, M.S.; et al. Physical complications in acute lung injury survivors: A two-year longitudinal prospective study. Crit. Care Med. 2014, 42, 849–859. [Google Scholar] [CrossRef]
- Schweickert, W.D.; Pohlman, M.C.; Pohlman, A.S.; Nigos, C.; Pawlik, A.J.; Esbrook, C.L.; Spears, L.; Miller, M.; Franczyk, M.; Deprizio, D.; et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet. 2009, 373, 1874–1882. [Google Scholar] [CrossRef]
- Morris, P.E.; Goad, A.; Thompson, C.; Taylor, K.; Harry, B.; Passmore, L.; Ross, A.; Anderson, L.; Baker, S.; Sanchez, M.; et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit. Care Med. 2008, 36, 2238–2243. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Liu, K.; Nakamura, K.; Kozu, R.; Horibe, T.; Ishii, K.; Yasumura, D.; Takahashi, Y.; Nanba, T.; Morita, Y.; et al. Association between early mobilization in the ICU and psychiatric symptoms after surviving a critical illness: A multi-center prospective cohort study. J. Clin. Med. 2022, 11, 2587. [Google Scholar] [CrossRef]
- Watanabe, D.; Yoshida, T.; Watanabe, Y.; Yamada, Y.; Miyachi, M.; Kimura, M. Validation of the Kihon Checklist and the frailty screening index for frailty defined by the phenotype model in older Japanese adults. BMC Geriatr. 2022, 22, 478. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Kleyweg, R.P.; van der Meché, F.G.; Schmitz, P.I. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain–Barré syndrome. Muscle Nerve. 1991, 14, 1103–1109. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.G.; Grant, D.M.; Read, J.P.; Clapp, J.D.; Coffey, S.F.; Miller, L.M.; Palyo, S.A. The Impact of Event Scale-Revised: Psychometric properties in a sample of motor vehicle accident survivors. J. Anxiety Disord. 2008, 22, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Morisawa, T.; Okamoto, H.; Matsumoto, N.; Saitoh, M.; Takahashi, T.; Fujiwara, T. Relationship Between Skeletal Muscle Quality and Hospital-Acquired Disability in Patients with Sepsis Admitted to the ICU: A Pilot Study. Crit. Care Explor. 2023, 5, e0835. [Google Scholar] [CrossRef] [PubMed]
- Church, S.; Rogers, E.; Rockwood, K.; Theou, O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020, 20, 393. [Google Scholar] [CrossRef]
- Katano, S.; Yano, T.; Ohori, K.; Kouzu, H.; Nagaoka, R.; Honma, S.; Shimomura, K.; Inoue, T.; Takamura, Y.; Ishigo, T.; et al. Barthel Index score predicts mortality in elderly heart failure—A goal of comprehensive cardiac rehabilitation. Circ. J. 2021, 86, 70–78. [Google Scholar] [CrossRef]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E.; et al. Functional disability 5 years after acute respiratory distress syndrome. N. Engl. J. Med. 2011, 364, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Hatakeyama, J.; Kondo, Y.; Hifumi, T.; Sakuramoto, H.; Kawasaki, T.; Taito, S.; Nakamura, K.; Unoki, T.; Kawai, Y.; et al. Post-intensive care syndrome: Its pathophysiology, prevention, and future directions. Acute Med. Surg. 2019, 6, 233–246. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).