Psoriatic Arthritis: From Diagnosis to Treatment
Abstract
1. Introduction
2. Clinical Case
3. Epidemiology
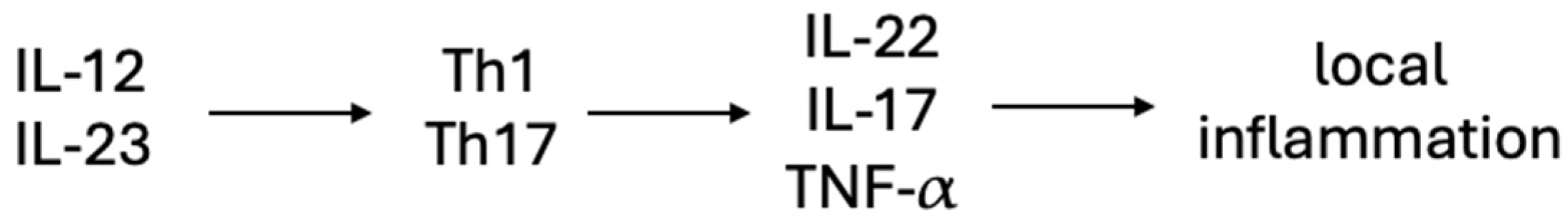
4. Etiology and Pathogenesis
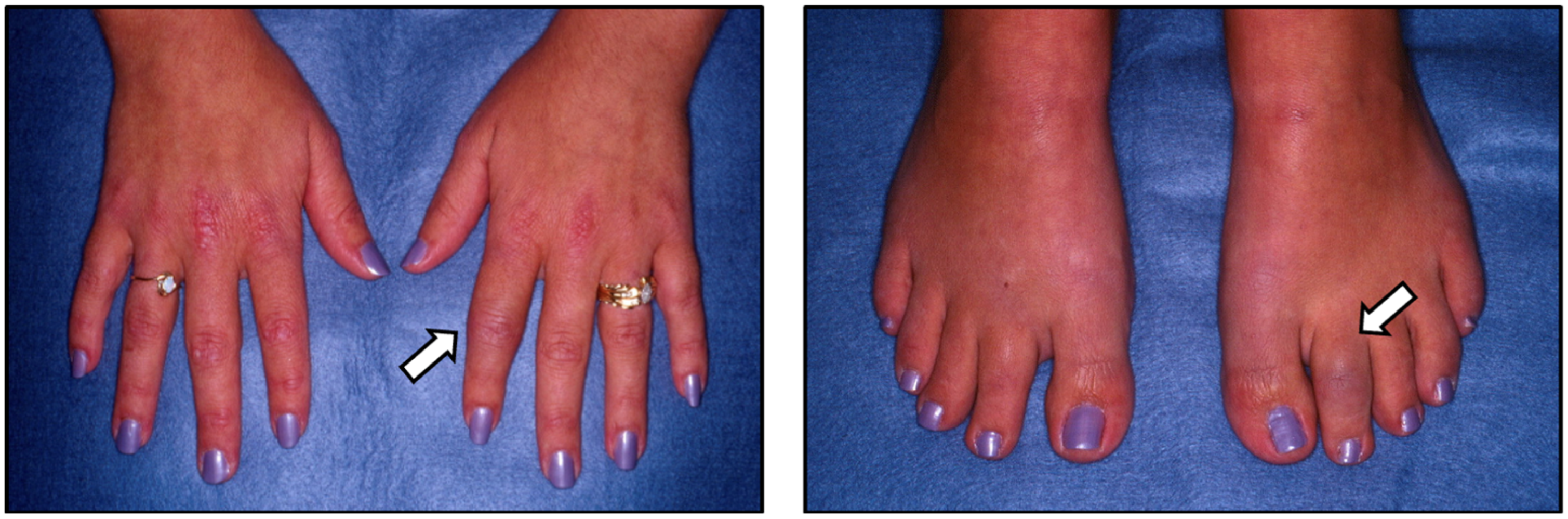
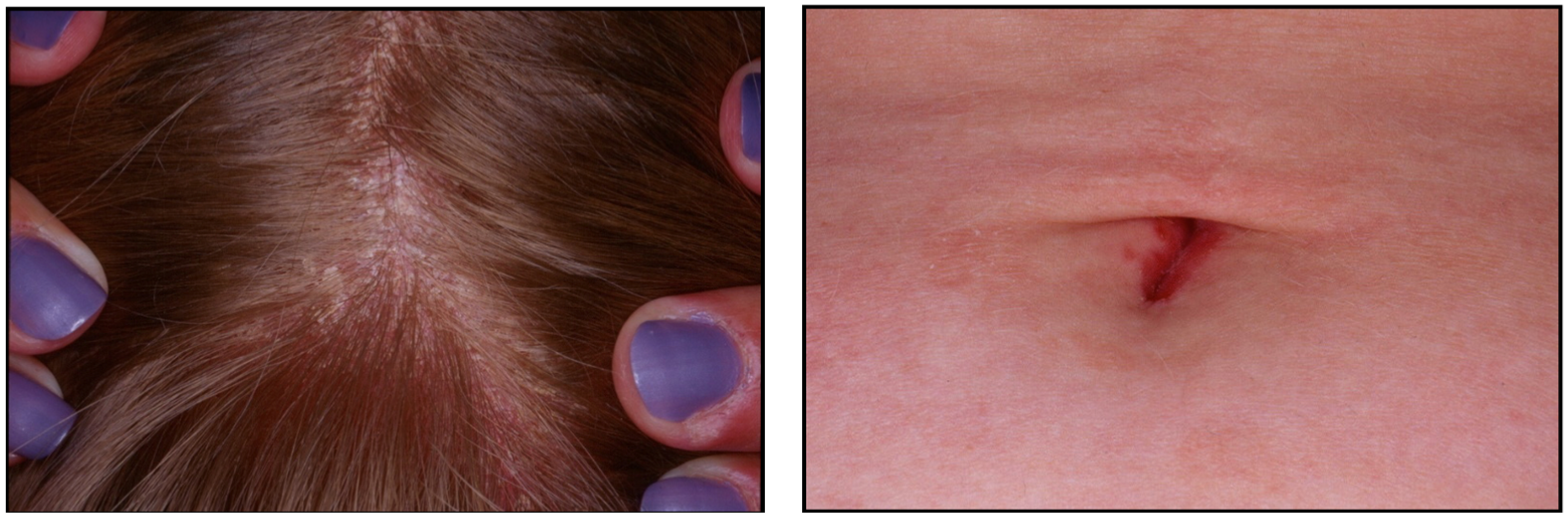
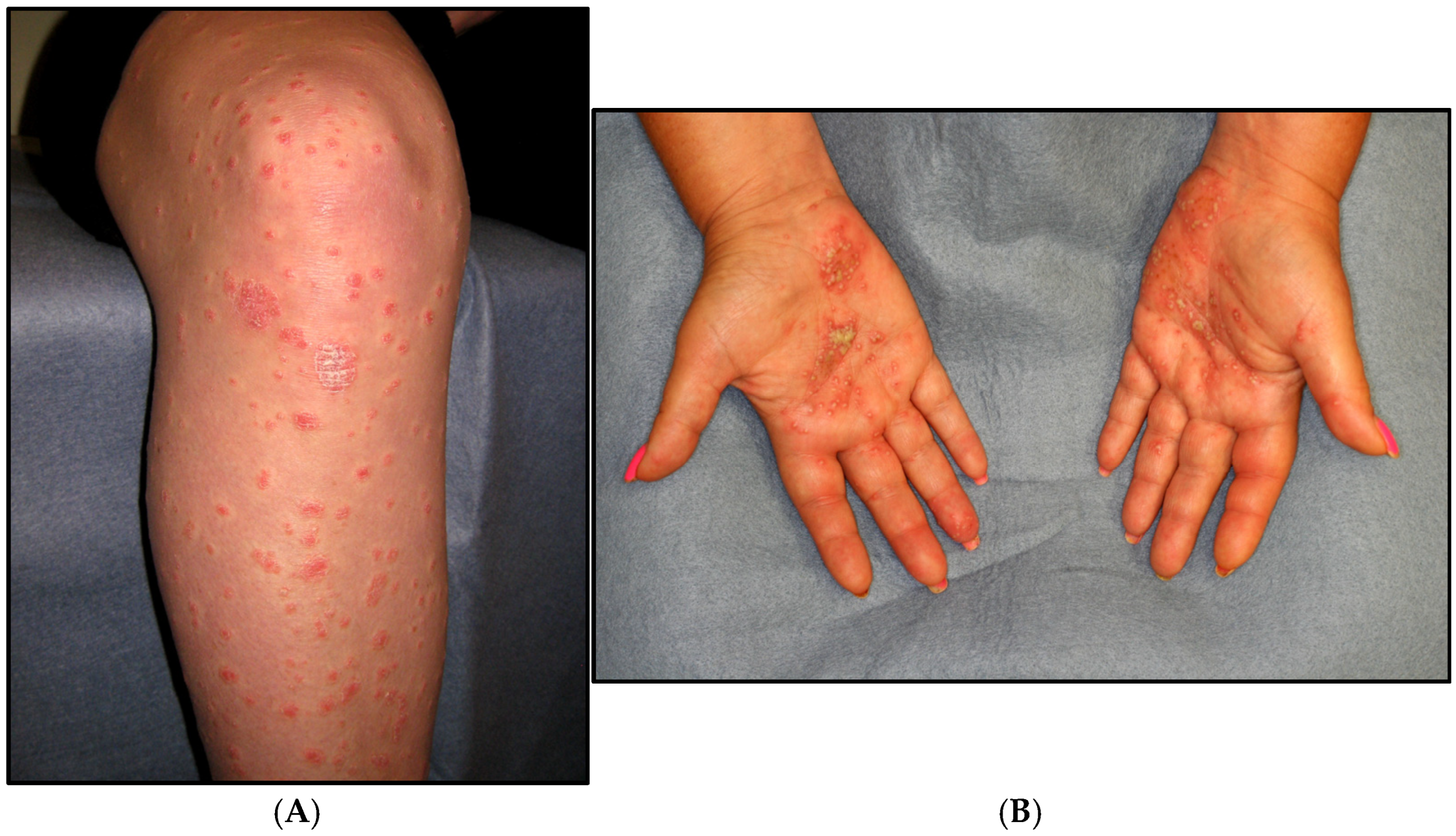
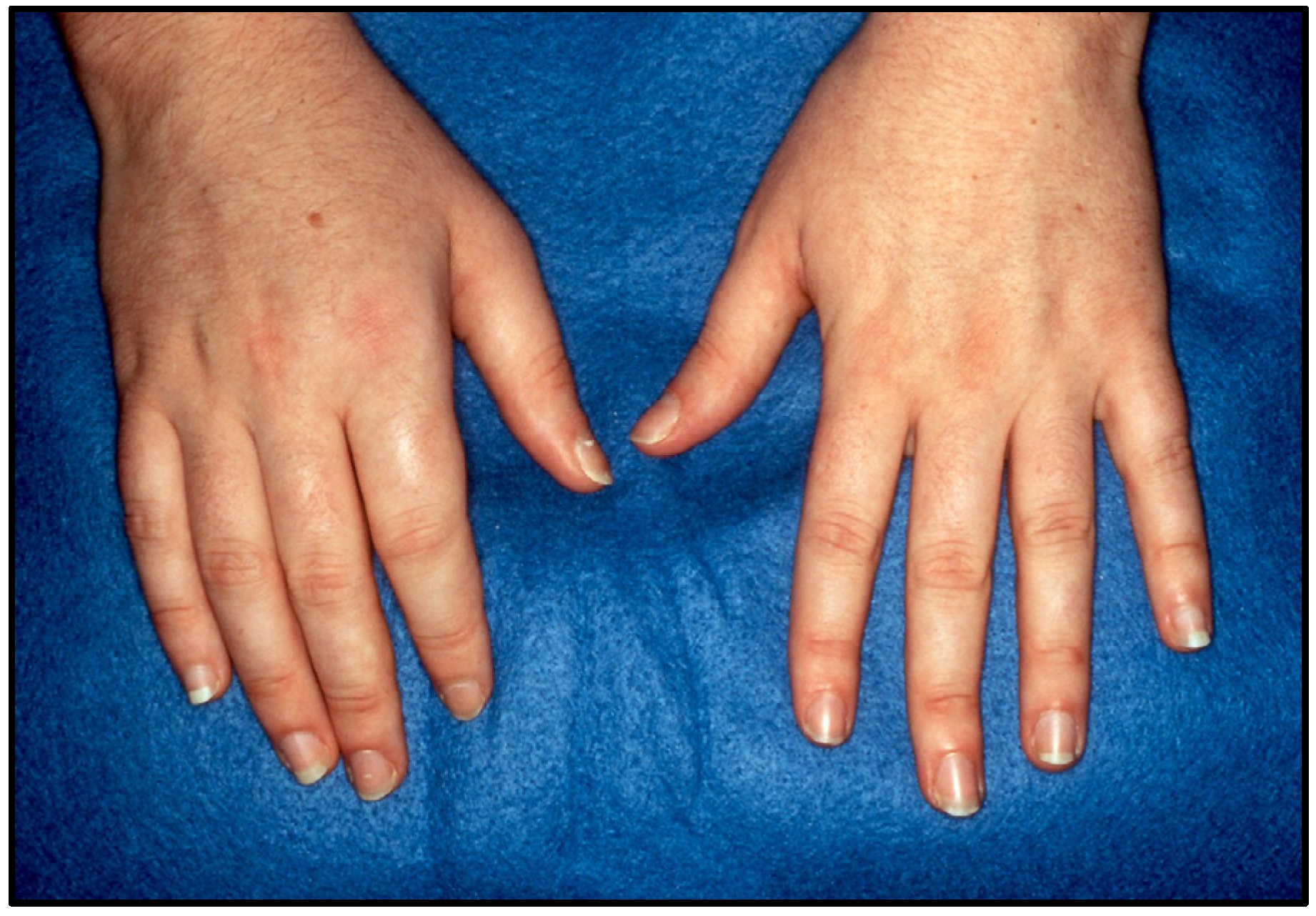
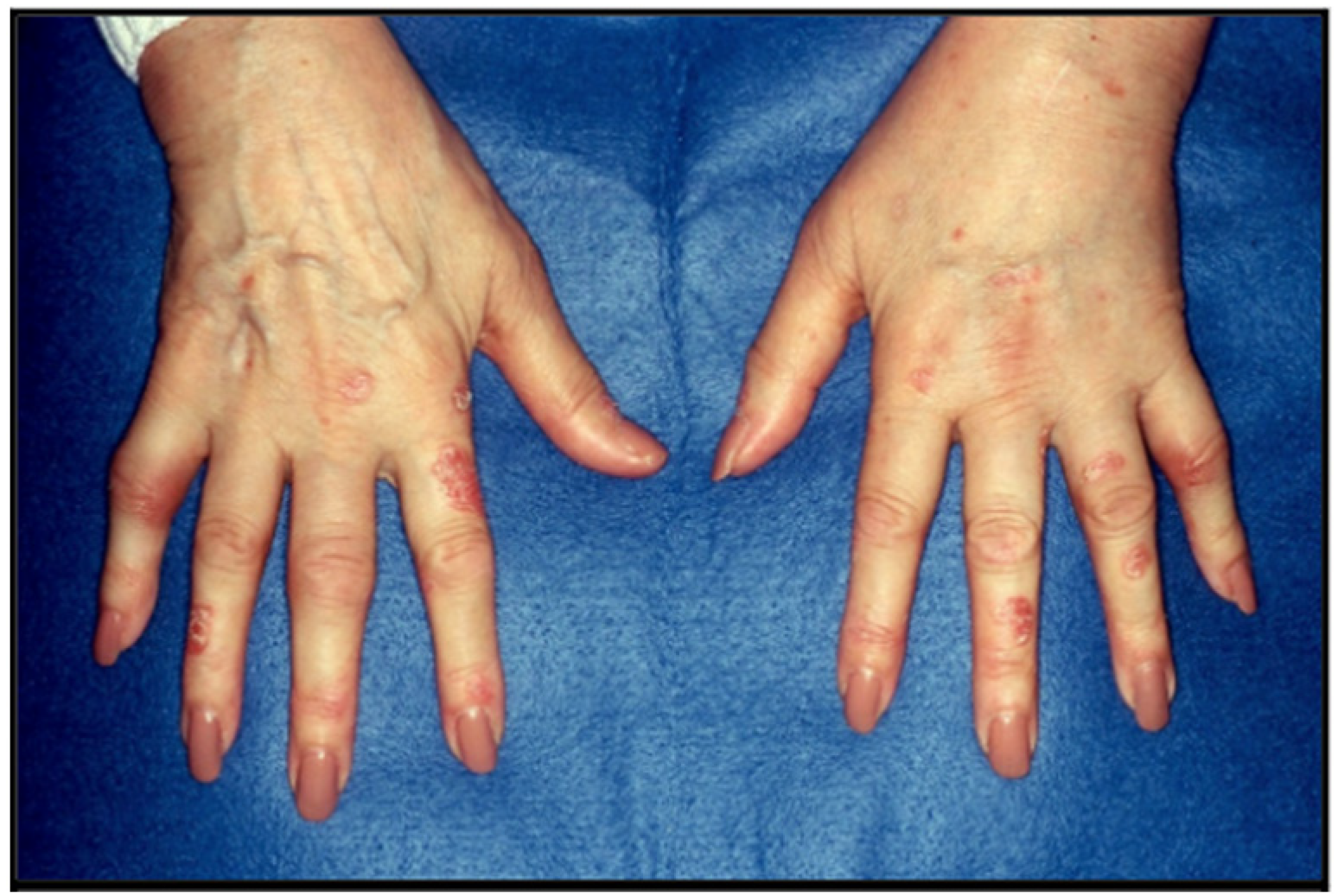
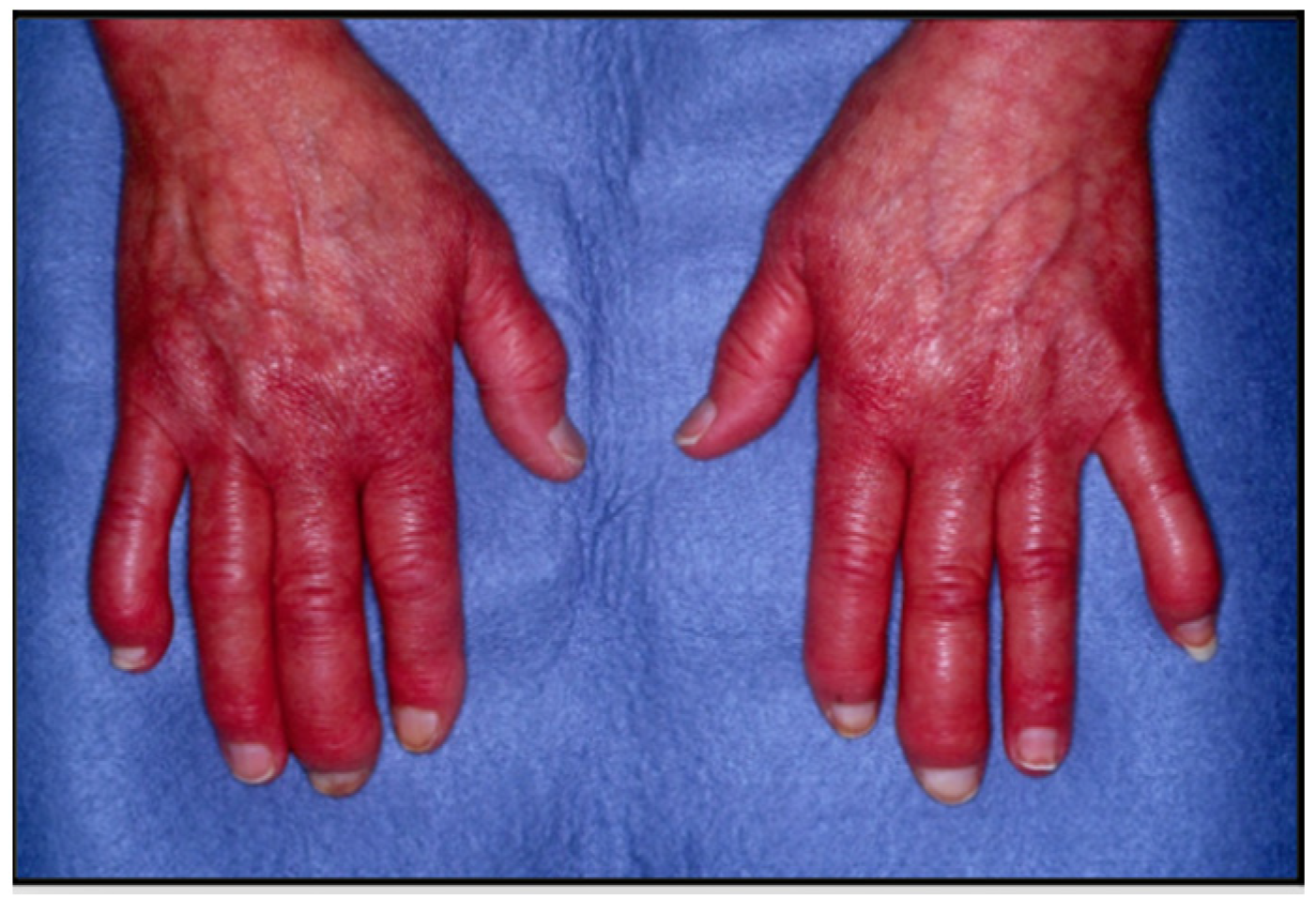
5. Clinical Features
6. Classification Criteria
7. Screening Tools
7.1. Questionnaires
7.2. Imaging
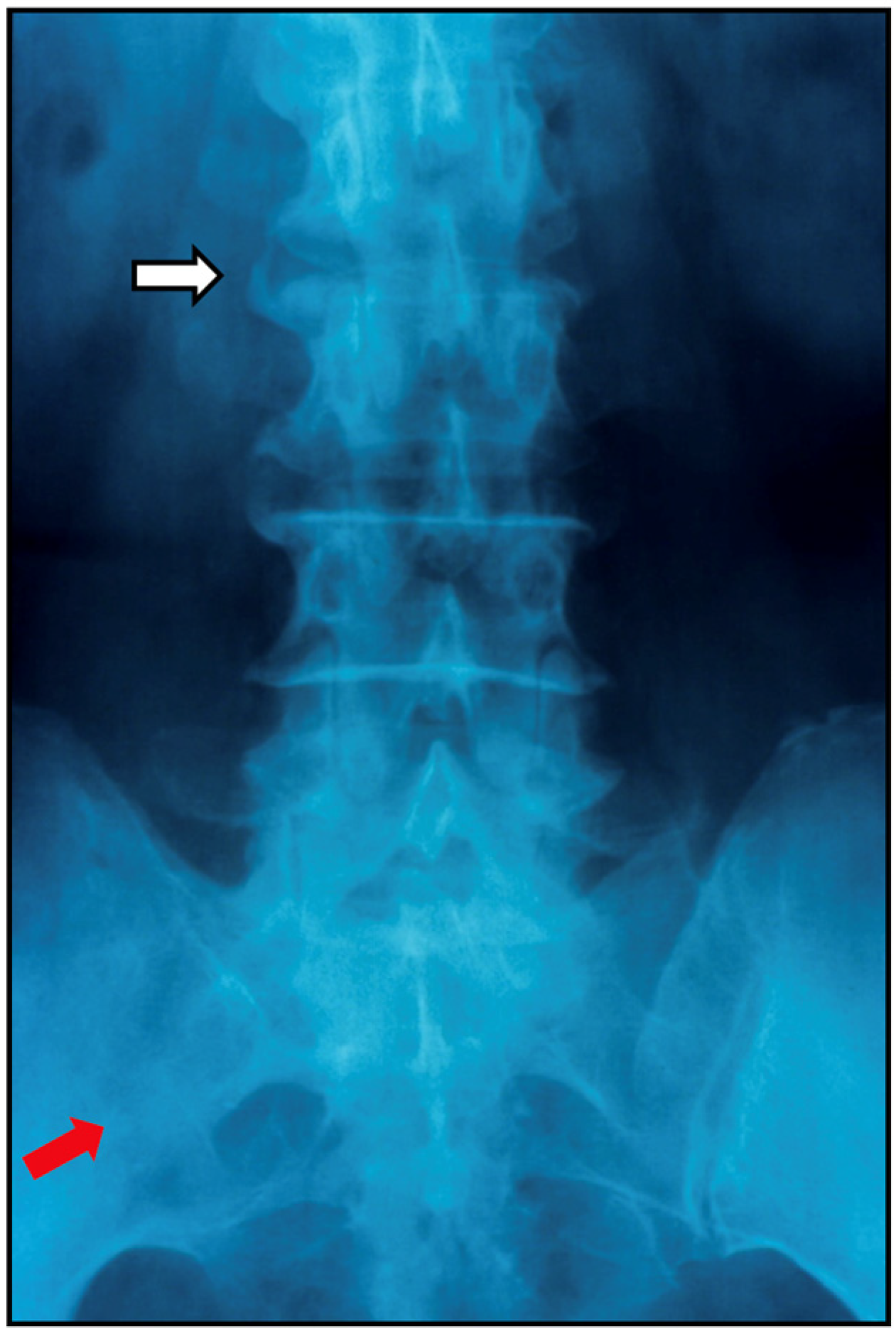
7.2.1. Plain Radiographs
7.2.2. Sonography
7.2.3. MRI
7.3. Biomarkers
8. Management
8.1. NSAIDs and Glucocorticoids
8.2. Conventional Synthetic Disease-Modifying Antirheumatic Drugs (csDMARDs)
8.2.1. Methotrexate
8.2.2. Sulfasalazine
8.2.3. Leflunomide
8.2.4. Cyclosporine
8.3. Biological Disease-Modifying Anti-Rheumatic Drugs (bDMARDs)
8.3.1. Tumor Necrosis Factor Inhibitors (TNFi)
8.3.2. IL-17 Inhibitors
8.3.3. IL-23 Inhibitors
8.3.4. Abatacept
8.4. Targeted Disease-Modifying Anti-Rheumatic Drugs (tsDMARDs)
8.4.1. Phosphodiesterase 4 (PDE4) Inhibitors
8.4.2. Janus Kinase Inhibitors
8.5. Preventing PsA in Patients with PsO
9. Future Directions
10. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef]
- Winchester, R.; FitzGerald, O. The many faces of psoriatic arthritis: Their genetic determinism. Rheumatology 2020, 59 (Suppl. S1), i4–i9. [Google Scholar] [CrossRef]
- Siegel, E.L.; Orbai, A.M.; Ritchlin, C.T. Targeting extra-articular manifestations in PsA: A closer look at enthesitis and dactylitis. Curr. Opin. Rheumatol. 2015, 27, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Gossec, L.; Kerschbaumer, A.; Ferreira, R.J.; Aletaha, D.; Baraliakos, X.; Bertheussen, H.; Boehncke, W.H.; Esbensen, B.A.; McInnes, I.B.; McGonagle, D.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann. Rheum. Dis. 2024, 83, 706–719. [Google Scholar] [CrossRef]
- Coates, L.C.; Moverley, A.R.; McParland, L.; Brown, S.; Navarro-Coy, N.; O’Dwyer, J.L.; Meads, D.M.; Emery, P.; Conaghan, P.G.; Helliwell, P.S. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): A UK multicentre, open-label, randomised controlled trial. Lancet 2015, 386, 2489–2498. [Google Scholar] [CrossRef]
- Menter, A.; Gottlieb, A.; Feldman, S.R.; Van Voorhees, A.S.; Leonardi, C.L.; Gordon, K.B.; Lebwohl, M.; Koo, J.Y.; Elmets, C.A.; Korman, N.J.; et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 2008, 58, 826–850. [Google Scholar] [CrossRef] [PubMed]
- Karmacharya, P.; Chakradhar, R.; Ogdie, A. The epidemiology of psoriatic arthritis: A literature review. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101692. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, C.; van Onna, M.; Boonen, A.; van Tubergen, A. Global Prevalence of Spondyloarthritis: A Systematic Review and Meta-Regression Analysis. Arthritis Care Res 2016, 68, 1320–1331. [Google Scholar] [CrossRef]
- Alinaghi, F.; Calov, M.; Kristensen, L.E.; Gladman, D.D.; Coates, L.C.; Jullien, D.; Gottlieb, A.B.; Gisondi, P.; Wu, J.J.; Thyssen, J.P.; et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J. Am. Acad. Dermatol. 2019, 80, 251–265.e19. [Google Scholar] [CrossRef]
- Gladman, D.D.; Shuckett, R.; Russell, M.L.; Thorne, J.C.; Schachter, R.K. Psoriatic arthritis (PSA)—An analysis of 220 patients. QJM Int. J. Med. 1987, 62, 127–141. [Google Scholar]
- Christophers, E.; Barker, J.; Griffiths, C.; Daudén, E.; Milligan, G.; Molta, C.; Sato, R.; Boggs, R. The risk of psoriatic arthritis remains constant following initial diagnosis of psoriasis among patients seen in European dermatology clinics. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 548–554. [Google Scholar] [CrossRef]
- Eder, L.; Chandran, V.; Shen, H.; Cook, R.J.; Shanmugarajah, S.; Rosen, C.F.; Gladman, D.D. Incidence of arthritis in a prospective cohort of psoriasis patients. Arthritis Care Res. 2011, 63, 619–622. [Google Scholar] [CrossRef]
- Eder, L.; Haddad, A.; Rosen, C.F.; Lee, K.; Chandran, V.; Cook, R.; Gladman, D.D. The Incidence and Risk Factors for Psoriatic Arthritis in Patients With Psoriasis: A Prospective Cohort Study. Arthritis Rheumatol. 2016, 68, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Belman, S.; Walsh, J.A.; Carroll, C.; Milliken, M.; Haaland, B.; Duffin, K.C.; Krueger, G.G.; Feng, B.-J. Psoriasis Characteristics for the Early Detection of Psoriatic Arthritis. J. Rheumatol. 2021, 48, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Eder, L.; Law, T.; Chandran, V.; Shanmugarajah, S.; Shen, H.; Rosen, C.F.; Cook, R.J.; Gladman, D.D. Association between environmental factors and onset of psoriatic arthritis in patients with psoriasis. Arthritis Care Res. 2011, 63, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Watad, A.; Zabotti, A.; Patt, Y.S.; Gendelman, O.; Dotan, A.; Ben-Shabat, N.; Fisher, L.; McGonagle, D.; Amital, H. From Psoriasis to Psoriatic Arthritis: Decoding the Impact of Treatment Modalities on the Prevention of Psoriatic Arthritis. Rheumatol. Ther. 2024, 11, 963–976. [Google Scholar] [CrossRef]
- Exarchou, S.; Di Giuseppe, D.; Klingberg, E.; Sigurdardottir, V.; Wedrén, S.; Lindström, U.; Turesson, C.; Jacobsson, L.T.H.; Askling, J.; Wallman, J.K. Mortality in patients with psoriatic arthritis in Sweden: A nationwide, population-based cohort study. Ann. Rheum. Dis. 2024, 83, 446–456. [Google Scholar] [CrossRef]
- FitzGerald, O.; Haroon, M.; Giles, J.T.; Winchester, R. Concepts of pathogenesis in psoriatic arthritis: Genotype determines clinical phenotype. Arthritis Res. Ther. 2015, 17, 115. [Google Scholar] [CrossRef]
- Lee, B.W.; Moon, S.J. Inflammatory Cytokines in Psoriatic Arthritis: Understanding Pathogenesis and Implications for Treatment. Int. J. Mol. Sci. 2023, 24, 11662. [Google Scholar] [CrossRef]
- FitzGerald, O.; Ogdie, A.; Chandran, V.; Coates, L.C.; Kavanaugh, A.; Tillett, W.; Leung, Y.Y.; Dewit, M.; Scher, J.U.; Mease, P.J. Psoriatic arthritis. Nat. Rev. Dis. Primers 2021, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Jadon, D.R.; Stober, C.; Pennington, S.R.; FitzGerald, O. Applying precision medicine to unmet clinical needs in psoriatic disease. Nat. Rev. Rheumatol. 2020, 16, 609–627. [Google Scholar] [CrossRef]
- Feld, J.; Chandran, V.; Haroon, N.; Inman, R.; Gladman, D. Axial disease in psoriatic arthritis and ankylosing spondylitis: A critical comparison. Nat. Rev. Rheumatol. 2018, 14, 363–371. [Google Scholar] [CrossRef]
- Kavanaugh, A.; Baraliakos, X.; Gao, S.; Chen, W.; Sweet, K.; Chakravarty, S.D.; Song, Q.; Shawi, M.; Rahman, P. Genetic and Molecular Distinctions Between Axial Psoriatic Arthritis and Radiographic Axial Spondyloarthritis: Post Hoc Analyses from Four Phase 3 Clinical Trials. Adv. Ther. 2023, 40, 2439–2456. [Google Scholar] [CrossRef]
- Stober, C. Pathogenesis of psoriatic arthritis. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101694. [Google Scholar] [CrossRef]
- Lories, R.J.; McInnes, I.B. Primed for inflammation: Enthesis-resident T cells. Nat. Med. 2012, 18, 1018–1019. [Google Scholar] [CrossRef]
- Kishimoto, M.; Deshpande, G.A.; Fukuoka, K.; Kawakami, T.; Ikegaya, N.; Kawashima, S.; Komagata, Y.; Kaname, S. Clinical features of psoriatic arthritis. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101670. [Google Scholar] [CrossRef]
- Moll, J.M.; Wright, V. Psoriatic arthritis. Semin. Arthritis Rheum. 1973, 3, 55–78. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Ye, J.Y.; Chandran, V.; Lee, K.-A.; Cook, R.J. Oligoarticular vs Polyarticular Psoriatic Arthritis: A Longitudinal Study Showing Similar Characteristics. J. Rheumatol. 2021, 48, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.; Johnson, S.R.; Somaily, M.; Fazelzad, R.; Kron, A.T.; Chau, C.; Chandran, V. Psoriatic Arthritis Mutilans: Clinical and Radiographic Criteria. A Systematic Review. J. Rheumatol. 2015, 42, 1432–1438. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Merola, J.F. Axial psoriatic arthritis: An update for dermatologists. J. Am. Acad. Dermatol. 2021, 84, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Poddubnyy, D.; Jadon, D.R.; Bosch, F.V.D.; Mease, P.J.; Gladman, D.D. Axial involvement in psoriatic arthritis: An update for rheumatologists. Semin. Arthritis Rheum. 2021, 51, 880–887. [Google Scholar] [CrossRef]
- Gladman, D.D.; Ziouzina, O.; Thavaneswaran, A.; Chandran, V. Dactylitis in psoriatic arthritis: Prevalence and response to therapy in the biologic era. J. Rheumatol. 2013, 40, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Kehl, A.S.; Corr, M.; Weisman, M.H. Review: Enthesitis: New Insights Into Pathogenesis, Diagnostic Modalities, and Treatment. Arthritis Rheumatol. 2016, 68, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Dubash, S.; Alabas, O.A.; Michelena, X.; Garcia-Montoya, L.; Wakefield, R.J.; Helliwell, P.S.; Emery, P.; McGonagle, D.G.; Tan, A.L.; Marzo-Ortega, H. Dactylitis is an indicator of a more severe phenotype independently associated with greater SJC, CRP, ultrasound synovitis and erosive damage in DMARD-naive early psoriatic arthritis. Ann. Rheum. Dis. 2022, 81, 490–495. [Google Scholar] [CrossRef]
- Aguado Casanova, V.; Ventas, B.; Palomo, J.A.; Alcubierre, F.J.H.; Sánchez, L.V.; Martínez, M.R.; Gonzalez-Lopez, J.J. Epidemiology and clinical characteristics of psoriatic arthritis-related uveitis in Madrid, Spain. Int. Ophthalmol. 2023, 43, 771–777. [Google Scholar] [CrossRef]
- Ball, G.V. Arthritis and allied conditions. Arthritis Rheumatol. 1979, 22, 645. [Google Scholar] [CrossRef]
- Dougados, M.; Van Der Linden, S.; Juhlin, R.; Huitfeldt, B.; Amor, B.; Calin, A.; Cats, A.; Dijkmans, B.; Olivieri, I.; Pasero, G.; et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991, 34, 1218–1227. [Google Scholar] [CrossRef]
- Vasey, F.; Espinoza, L.R. Psoriatic Arthropathy. In Spondyloarthropathies; Calin, A., Ed.; Grune and Stratton: New York, NY, USA, 1984; pp. 166–167. [Google Scholar]
- Fournié, B.; Crognier, L.; Arnaud, C.; Zabraniecki, L.; Lascaux-Lefebvre, V.; Marc, V.; Ginesty, E.; Andrieu, V.; Dromer, C.; Fournié, A. Proposed classification criteria of psoriatic arthritis. A preliminary study in 260 patients. Rev. Rhum. 1999, 66, 446–456. [Google Scholar]
- McGonagle, D.; Conaghan, P.G.; Emery, P. Psoriatic arthritis: A unified concept twenty years on. Arthritis Rheumatol. 1999, 42, 1080–1086. [Google Scholar] [CrossRef]
- Helliwell, P.S.; Taylor, W.J. Classification and diagnostic criteria for psoriatic arthritis. Ann. Rheum. Dis. 2005, 64 (Suppl. S2), ii3–ii8. [Google Scholar] [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheumatol. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Coates, L.C.; Conaghan, P.G.; Emery, P.; Green, M.J.; Ibrahim, G.; MacIver, H.; Helliwell, P.S. Sensitivity and specificity of the classification of psoriatic arthritis criteria in early psoriatic arthritis. Arthritis Rheum. 2012, 64, 3150–3155. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Goek, O.; Singh-Grewal, D.; Vlad, S.C.; Feldman, B.M.; Felson, D.T.; Hawker, G.A.; Singh, J.A.; Solomon, D.H. Classification criteria in rheumatic diseases: A review of methodologic properties. Arthritis Care Res. 2007, 57, 1119–1133. [Google Scholar] [CrossRef]
- Chandran, V.; Schentag, C.T.; Gladman, D.D. Sensitivity of the classification of psoriatic arthritis criteria in early psoriatic arthritis. Arthritis Care Res. 2007, 57, 1560–1563. [Google Scholar] [CrossRef]
- Dominguez, P.; Gladman, D.D.; Helliwell, P.; Mease, P.J.; Husni, M.E.; Qureshi, A.A. Development of screening tools to identify psoriatic arthritis. Curr. Rheumatol. Rep. 2010, 12, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Schentag, C.T.; Tom, B.D.M.; Chandran, V.; Brockbank, J.; Rosen, C.; Farewell, V.T. Development and initial validation of a screening questionnaire for psoriatic arthritis: The Toronto Psoriatic Arthritis Screen (ToPAS). Ann. Rheum. Dis. 2009, 68, 497–501. [Google Scholar] [CrossRef]
- Husni, M.E.; Meyer, K.H.; Cohen, D.S.; Mody, E.; Qureshi, A.A. The PASE questionnaire: Pilot-testing a psoriatic arthritis screening and evaluation tool. J. Am. Acad. Dermatol. 2007, 57, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, P.L.; Husni, M.E.; Holt, E.W.; Tyler, S.; Qureshi, A.A. Validity, reliability, and sensitivity-to-change properties of the psoriatic arthritis screening and evaluation questionnaire. Arch. Dermatol. Res. 2009, 301, 573–579. [Google Scholar] [CrossRef]
- Tom, B.D.; Chandran, V.; Farewell, V.T.; Rosen, C.F.; Gladman, D.D. Validation of the Toronto Psoriatic Arthritis Screen Version 2 (ToPAS 2). J. Rheumatol. 2015, 42, 841–846. [Google Scholar] [CrossRef]
- Ibrahim, G.H.; Buch, M.H.; Lawson, C.; Waxman, R.; Helliwell, P.S. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: The Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin. Exp. Rheumatol. 2009, 27, 469–474. [Google Scholar]
- Iragorri, N.; Hazlewood, G.; Manns, B.; Danthurebandara, V.; Spackman, E. Psoriatic arthritis screening: A systematic review and meta-analysis. Rheumatology 2019, 58, 692–707. [Google Scholar] [CrossRef]
- Mishra, S.; Kancharla, H.; Dogra, S.; Sharma, A. Comparison of four validated psoriatic arthritis screening tools in diagnosing psoriatic arthritis in patients with psoriasis (COMPAQ Study). Br. J. Dermatol. 2017, 176, 765–770. [Google Scholar] [CrossRef]
- Salaffi, F.; Di Carlo, M.; Luchetti, M.M.; Di Donato, E.; Campanati, A.; Benfaremo, D.; Nicolini, M.; Carotti, M.; Giacchetti, A.; Ganzetti, G.; et al. A validation study of the Simple Psoriatic Arthritis Screening (SiPAS) questionnaire to screen psoriasis patients for psoriatic arthritis. Clin. Exp. Rheumatol. 2018, 36, 127–135. [Google Scholar] [PubMed]
- Mathew, A.J.; Østergaard, M.; Eder, L. Imaging in psoriatic arthritis: Status and recent advances. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101690. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.J.; Zhou, A.L.; Østergaard, M.; Koppikar, S. Imaging in the diagnosis and management of peripheral psoriatic arthritis: Update and recent advances. Best. Pract. Res. Clin. Rheumatol. 2025, 39, 102061. [Google Scholar] [CrossRef] [PubMed]
- Baraliakos, X.; Coates, L.C.; Braun, J. The involvement of the spine in psoriatic arthritis. Clin. Exp. Rheumatol. 2015, 33 (Suppl. S93), S31–S35. [Google Scholar]
- Bonfiglioli, K.R.; Lopes, F.O.d.A.; de Figueiredo, L.Q.; Ferrari, L.F.F.; Guedes, L. Ultrasonographic Insights into Peripheral Psoriatic Arthritis: Updates in Diagnosis and Monitoring. J. Pers. Med. 2024, 14, 550. [Google Scholar] [CrossRef]
- Gouze, H.; Backhaus, M.; Balint, P.; Di Matteo, A.; Grassi, W.; Iagnocco, A.; Naredo, E.; Wakefield, R.J.; Østergaard, M.; Emery, P.; et al. Ultrasound in the Management of Patients With Psoriatic Arthritis: Systematic Literature Review and Novel Algorithms for Pragmatic Use. J. Rheumatol. 2023, 51, 50–60. [Google Scholar] [CrossRef]
- Jin, Y.; Cheng, I.T.; Wu, D.; Yan, X.; Lau, S.-L.; Wong, N.S.; Hung, V.W.; Qin, L.; Lee, R.K.L.; Griffith, J.F.; et al. Imaging in psoriatic arthritis: Established methods and emerging techniques. Ther. Adv. Musculoskelet. Dis. 2024, 16, 1759720x241288060. [Google Scholar] [CrossRef]
- Zabotti, A.; Bandinelli, F.; Batticciotto, A.; Scire, C.A.; Iagnocco, A.; Sakellariou, G.; Musculoskeletal Ultrasound Study Group of the Italian Society of Rheumatology. Musculoskeletal ultrasonography for psoriatic arthritis and psoriasis patients: A systematic literature review. Rheumatology 2017, 56, 1518–1532. [Google Scholar] [CrossRef]
- Fassio, A.; Matzneller, P.; Idolazzi, L. Recent Advances in Imaging for Diagnosis, Monitoring, and Prognosis of Psoriatic Arthritis. Front. Med. 2020, 7, 551684. [Google Scholar] [CrossRef]
- Maldonado-Ficco, H.; Sheane, B.J.M.; Thavaneswaran, A.M.; Chandran, V.M.; Gladman, D.D.M. Magnetic Resonance Imaging in Psoriatic Arthritis: A Descriptive Study of Indications, Features and Effect on Treatment Change. J. Clin. Rheumatol. 2017, 23, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Pennington, S.R.; FitzGerald, O. Early Origins of Psoriatic Arthritis: Clinical, Genetic and Molecular Biomarkers of Progression From Psoriasis to Psoriatic Arthritis. Front. Med. 2021, 8, 723944. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Cook, R.J.; Edwin, J.; Shen, H.; Pellett, F.J.; Shanmugarajah, S.; Rosen, C.F.; Gladman, D.D. Soluble biomarkers differentiate patients with psoriatic arthritis from those with psoriasis without arthritis. Rheumatology 2010, 49, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Kar, B.R.; Sathishkumar, D.; Tahiliani, S.; Parthasarathi, A.; Neema, S.; Ganguly, S.; Venkatachalam, K.; Parasramani, S.G.; Komeravelli, H.; Thomas, J. Biomarkers in Psoriasis: The Future of Personalised Treatment. Indian J. Dermatol. 2024, 69, 256–263. [Google Scholar] [CrossRef]
- Mahendran, S.M.; Chandran, V. Exploring the Psoriatic Arthritis Proteome in Search of Novel Biomarkers. Proteomes 2018, 6, 5. [Google Scholar] [CrossRef]
- Koussiouris, J.; Looby, N.; Kotlyar, M.; Kulasingam, V.; Jurisica, I.; Chandran, V. Classifying patients with psoriatic arthritis according to their disease activity status using serum metabolites and machine learning. Metabolomics 2024, 20, 17. [Google Scholar] [CrossRef]
- Ogdie, A.; Coates, L.C.; Gladman, D.D. Treatment guidelines in psoriatic arthritis. Rheumatology 2020, 59 (Suppl. S1), i37–i46. [Google Scholar] [CrossRef]
- Coates, L.C.; Soriano, E.R.; Corp, N.; Bertheussen, H.; Duffin, K.C.; Campanholo, C.B.; Chau, J.; Eder, L.; Fernández-Ávila, D.G.; Garg, A.; et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): Updated treatment recommendations for psoriatic arthritis 2021. Nat. Rev. Rheumatol. 2022, 18, 465–479. [Google Scholar] [CrossRef]
- Kivitz, A.J.; Espinoza, L.R.; Sherrer, Y.R.; Liu-Dumaw, M.; West, C.R. A comparison of the efficacy and safety of celecoxib 200 mg and celecoxib 400 mg once daily in treating the signs and symptoms of psoriatic arthritis. Semin. Arthritis Rheum. 2007, 37, 164–173. [Google Scholar] [CrossRef]
- Sewerin, P.; Baraliakos, X. 2023 EULAR Recommendations for the Treatment of PsA: Advances and Pending Issues. Mediterr. J. Rheumatol. 2024, 35, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, G.H.; Kowalczyk, A.; Taylor, H.; Ibrahim, F.; Packham, J.C.; McHugh, N.J.; Mulherin, D.M.; Kitas, G.D.; Chakravarty, K.; Tom, B.D.M.; et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology 2012, 51, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Gladman, D.D.; Collier, D.H.; Ritchlin, C.T.; Helliwell, P.S.; Liu, L.; Kricorian, G.; Chung, J.B. Etanercept and Methotrexate as Monotherapy or in Combination for Psoriatic Arthritis: Primary Results From a Randomized, Controlled Phase III Trial. Arthritis Rheumatol. 2019, 71, 1112–1124. [Google Scholar] [CrossRef]
- Ceponis, A.; Kavanaugh, A. Use of methotrexate in patients with psoriatic arthritis. Clin. Exp. Rheumatol. 2010, 28 (Suppl. S61), S132–S137. [Google Scholar]
- Clegg, D.O.; Reda, D.J.; Mejias, E.; Cannon, G.W.; Weisman, M.H.; Taylor, T.; Budiman-Mak, E.; Blackburn, W.D.; Vasey, F.B.; Mahowald, M.L.; et al. Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis. A Department of Veterans Affairs Cooperative Study. Arthritis Rheumatol. 1996, 39, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Kaltwasser, J.P.; Nash, P.; Gladman, D.; Rosen, C.F.; Behrens, F.; Jones, P.; Wollenhaupt, J.; Falk, F.G.; Mease, P. Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: A multinational, double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheumatol. 2004, 50, 1939–1950. [Google Scholar] [CrossRef]
- Mease, P.J.; Armstrong, A.W. Managing patients with psoriatic disease: The diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs 2014, 74, 423–441. [Google Scholar] [CrossRef]
- Azuaga, A.B.; Ramírez, J.; Cañete, J.D. Psoriatic Arthritis: Pathogenesis and Targeted Therapies. Int. J. Mol. Sci. 2023, 24, 4901. [Google Scholar] [CrossRef]
- Lemos, L.L.; de Oliveira Costa, J.; Almeida, A.M.; Junior, H.O.; Barbosa, M.M.; Kakehasi, A.M.; Acurcio, F.A. Treatment of psoriatic arthritis with anti-TNF agents: A systematic review and meta-analysis of efficacy, effectiveness and safety. Rheumatol. Int. 2014, 34, 1345–1360. [Google Scholar] [CrossRef]
- Mease, P.J.; Goffe, B.S.; Metz, J.; VanderStoep, A.; Finck, B.; Burge, D.J. Etanercept in the treatment of psoriatic arthritis and psoriasis: A randomised trial. Lancet 2000, 356, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Antoni, C.E.; Kavanaugh, A.; Kirkham, B.; Tutuncu, Z.; Burmester, G.R.; Schneider, U.; Furst, D.E.; Molitor, J.; Keystone, E.; Gladman, D.; et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: Results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheumatol. 2005, 52, 1227–1236. [Google Scholar] [CrossRef]
- Fénix-Caballero, S.; Rey, E.J.A.-D.; Castaño-Lara, R.; Puigventós-Latorre, F.; Borrero-Rubio, J.M.; López-Vallejo, J.F. Direct and indirect comparison of the efficacy and safety of adalimumab, etanercept, infliximab and golimumab in psoriatic arthritis. J. Clin. Pharm. Ther. 2013, 38, 286–293. [Google Scholar] [CrossRef]
- Levin, A.D.; Wildenberg, M.E.; van den Brink, G.R. Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 989–997. [Google Scholar] [CrossRef]
- Wendling, D.; Joshi, A.; Reilly, P.; Jalundhwala, Y.J.; Mittal, M.; Bao, Y. Comparing the risk of developing uveitis in patients initiating anti-tumor necrosis factor therapy for ankylosing spondylitis: An analysis of a large US claims database. Curr. Med. Res. Opin. 2014, 30, 2515–2521. [Google Scholar] [CrossRef]
- Behrens, F.; Koehm, M.; Arndt, U.; Wittig, B.M.; Greger, G.; Thaçi, D.; Scharbatke, E.; Tony, H.-P.; Burkhardt, H. Does Concomitant Methotrexate with Adalimumab Influence Treatment Outcomes in Patients with Psoriatic Arthritis? Data from a Large Observational Study. J. Rheumatol. 2016, 43, 632–639. [Google Scholar] [CrossRef]
- Combe, B.; Behrens, F.; McHugh, N.; Brock, F.; Kerkmann, U.; Kola, B.; Gallo, G. Comparison of Etanercept Monotherapy and Combination Therapy with Methotrexate in Psoriatic Arthritis: Results from 2 Clinical Trials. J. Rheumatol. 2016, 43, 1063–1067. [Google Scholar] [CrossRef]
- Fagerli, K.M.; Lie, E.; van der Heijde, D.; Heiberg, M.S.; Lexberg, Å.S.; Rødevand, E.; Kalstad, S.; Mikkelsen, K.; Kvien, T.K. The role of methotrexate co-medication in TNF-inhibitor treatment in patients with psoriatic arthritis: Results from 440 patients included in the NOR-DMARD study. Ann. Rheum. Dis. 2014, 73, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Baddley, J.W.; Winthrop, K.L.; Chen, L.; Liu, L.; Grijalva, C.G.; Delzell, E.; Beukelman, T.; Patkar, N.M.; Xie, F.; Saag, K.G.; et al. Non-viral opportunistic infections in new users of tumour necrosis factor inhibitor therapy: Results of the SAfety Assessment of Biologic ThERapy (SABER) study. Ann. Rheum. Dis. 2014, 73, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Salt, E.; Wiggins, A.T.; Rayens, M.K.; Huaman, M.A.; Mannino, D.; Schwieterman, P.; Merkley, S.A.; Jones, A.R.; Crofford, L.J. Risk Factors for Targeted Fungal and Mycobacterial Infections in Patients Taking Tumor Necrosis Factor Inhibitors. Arthritis Rheumatol. 2016, 68, 597–603. [Google Scholar] [CrossRef]
- Tubach, F.; Salmon, D.; Ravaud, P.; Allanore, Y.; Goupille, P.; Bréban, M.; Pallot-Prades, B.; Pouplin, S.; Sacchi, A.; Chichemanian, R.M.; et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: The three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheumatol. 2009, 60, 1884–1894. [Google Scholar] [CrossRef]
- Dreyer, L.; Mellemkjær, L.; Andersen, A.R.; Bennett, P.; Poulsen, U.E.; Ellingsen, T.J.; Hansen, T.H.; Jensen, D.V.; Linde, L.; Lindegaard, H.M.; et al. Incidences of overall and site specific cancers in TNFα inhibitor treated patients with rheumatoid arthritis and other arthritides—A follow-up study from the DANBIO Registry. Ann. Rheum. Dis. 2013, 72, 79–82. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- McInnes, I.B.; Mease, P.J.; Kirkham, B.; Kavanaugh, A.; Ritchlin, C.T.; Rahman, P.; van der Heijde, D.; Landewé, R.; Conaghan, P.G.; Gottlieb, A.B.; et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.; van der Heijde, D.; Landewé, R.; Mpofu, S.; Rahman, P.; Tahir, H.; Singhal, A.; Boettcher, E.; Navarra, S.; Meiser, K.; et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: Primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann. Rheum. Dis. 2018, 77, 890–897. [Google Scholar] [CrossRef]
- Mease, P.J.; McInnes, I.B.; Kirkham, B.; Kavanaugh, A.; Rahman, P.; van der Heijde, D.; Landewé, R.; Nash, P.; Pricop, L.; Yuan, J.; et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N. Engl. J. Med. 2015, 373, 1329–1339. [Google Scholar] [CrossRef]
- Nash, P.; Mease, P.J.; McInnes, I.B.; Rahman, P.; Ritchlin, C.T.; Blanco, R.; Dokoupilova, E.; Andersson, M.; Kajekar, R.; Mpofu, S.; et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: Results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res. Ther. 2018, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Kivitz, A.J.; Nash, P.; Tahir, H.; Everding, A.; Mann, H.; Kaszuba, A.; Pellet, P.; Widmer, A.; Pricop, L.; Abrams, K. Efficacy and Safety of Subcutaneous Secukinumab 150 mg with or Without Loading Regimen in Psoriatic Arthritis: Results from the FUTURE 4 Study. Rheumatol. Ther. 2019, 6, 393–407. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Behrens, F.; Mease, P.J.; Kavanaugh, A.; Ritchlin, C.; Nash, P.; Masmitja, J.G.; Goupille, P.; Korotaeva, T.; Gottlieb, A.B.; et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): A double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet 2020, 395, 1496–1505. [Google Scholar] [CrossRef]
- Mease, P.J.; Smolen, J.S.; Behrens, F.; Nash, P.; Leage, S.L.; Li, L.; Tahir, H.; Gooderham, M.; Krishnan, E.; Liu-Seifert, H.; et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann. Rheum. Dis. 2020, 79, 123–131. [Google Scholar] [CrossRef]
- Mease, P.J.; van der Heijde, D.; Ritchlin, C.T.; Okada, M.; Cuchacovich, R.S.; Shuler, C.L.; Lin, C.-Y.; Braun, D.K.; Lee, C.H.; Gladman, D.D. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: Results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 2017, 76, 79–87. [Google Scholar] [CrossRef]
- Merola, J.F.; Landewé, R.; McInnes, I.B.; Mease, P.J.; Ritchlin, C.T.; Tanaka, Y.; Asahina, A.; Behrens, F.; Gladman, D.D.; Gossec, L.; et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: A randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet 2023, 401, 38–48. [Google Scholar] [CrossRef]
- McInnes, I.B.; Asahina, A.; Coates, L.C.; Landewé, R.; Merola, J.F.; Ritchlin, C.T.; Tanaka, Y.; Gossec, L.; Gottlieb, A.B.; Warren, R.B.; et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: A randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet 2023, 401, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; van der Heijde, D.; Fleischmann, R.M.; Lespessailles, E.; Helliwell, P.S.; Kameda, H.; Burgos-Vargas, R.; Erickson, J.S.; Rathmann, S.S.; Sprabery, A.T.; et al. Ixekizumab treatment of biologic-naïve patients with active psoriatic arthritis: 3-year results from a phase III clinical trial (SPIRIT-P1). Rheumatology 2020, 59, 2774–2784. [Google Scholar] [CrossRef]
- van der Heijde, D.; Mease, P.J.; Landewé, R.B.M.; Rahman, P.; Tahir, H.; Singhal, A.; Boettcher, E.; Navarra, S.; Zhu, X.; Ligozio, G.; et al. Secukinumab provides sustained low rates of radiographic progression in psoriatic arthritis: 52-week results from a phase 3 study, FUTURE 5. Rheumatology 2020, 59, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Helliwell, P.S.; Hjuler, K.F.; Raymond, K.; McInnes, I. Brodalumab in psoriatic arthritis: Results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann. Rheum. Dis. 2021, 80, 185–193. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sendid, B.; Jouault, T.; Poulain, D. Secukinumab failure in Crohn’s disease: The yeast connection? Gut 2013, 62, 800–801. [Google Scholar] [CrossRef]
- Hueber, W.; Sands, B.E.; Lewitzky, S.; Vandemeulebroecke, M.; Reinisch, W.; Higgins, P.D.R.; Wehkamp, J.; Feagan, B.G.; Yao, M.D.; Karczewski, M.; et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: Unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012, 61, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Targan, S.R.; Feagan, B.; Vermeire, S.; Panaccione, R.; Melmed, G.Y.; Landers, C.; Li, D.; Russell, C.; Newmark, R.; Zhang, N.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study of Brodalumab in Patients With Moderate-to-Severe Crohn’s Disease. Am. J. Gastroenterol. 2016, 111, 1599–1607. [Google Scholar] [CrossRef]
- Łukasik, Z.; Gracey, E.; Venken, K.; Ritchlin, C.; Elewaut, D. Crossing the boundaries: IL-23 and its role in linking inflammation of the skin, gut and joints. Rheumatology 2021, 60 (Suppl. S4), iv16–iv27. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Chen, Y.; Tato, C.M.; Laurence, A.; Joyce-Shaikh, B.; Blumenschein, W.M.; McClanahan, T.K.; O’Shea, J.J.; Cua, D.J. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 2009, 10, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Deodhar, A.; Helliwell, P.S.; Boehncke, W.-H.; Kollmeier, A.P.; Hsia, E.C.; Subramanian, R.A.; Xu, X.L.; Sheng, S.; Agarwal, P.; Zhou, B.; et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1115–1125. [Google Scholar] [CrossRef]
- Azuaga, A.B.; Frade-Sosa, B.; Laiz, A.; Estrada, P.; Prior-Español, A.; Horcada, L.; Polino, L.; Moreno, M.; Moragues, C.; Urruticoechea-Arana, A.; et al. Effectiveness of ustekinumab in patients with psoriatic arthritis in a real-world, multicenter study. Clin. Rheumatol. 2020, 39, 2963–2971. [Google Scholar] [CrossRef]
- McInnes, I.B.; Kavanaugh, A.; Gottlieb, A.B.; Puig, L.; Rahman, P.; Ritchlin, C.; Brodmerkel, C.; Li, S.; Wang, Y.; Mendelsohn, A.M.; et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013, 382, 780–789. [Google Scholar] [CrossRef]
- Ritchlin, C.; Rahman, P.; Kavanaugh, A.; McInnes, I.B.; Puig, L.; Li, S.; Wang, Y.; Shen, Y.-K.; Doyle, M.K.; Mendelsohn, A.M.; et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann. Rheum. Dis. 2014, 73, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, A.; Ritchlin, C.; Rahman, P.; Puig, L.; Gottlieb, A.B.; Li, S.; Wang, Y.; Noonan, L.; Brodmerkel, C.; Song, M.; et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: Results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann. Rheum. Dis. 2014, 73, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Östör, A.; Bosch, F.V.D.; Papp, K.; Asnal, C.; Blanco, R.; Aelion, J.; Alperovich, G.; Lu, W.; Wang, Z.; Soliman, A.M.; et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial. Ann. Rheum. Dis. 2022, 81, 351–358. [Google Scholar] [CrossRef]
- Mease, P.J.; Chohan, S.; Fructuoso, F.J.G.; Luggen, M.E.; Rahman, P.; Raychaudhuri, S.P.; Chou, R.C.; Mendelsohn, A.M.; Rozzo, S.J.; Gottlieb, A. Efficacy and safety of tildrakizumab in patients with active psoriatic arthritis: Results of a randomised, double-blind, placebo-controlled, multiple-dose, 52-week phase IIb study. Ann. Rheum. Dis. 2021, 80, 1147–1157. [Google Scholar] [CrossRef]
- Baeten, D.; Østergaard, M.; Wei, J.C.-C.; Sieper, J.; Järvinen, P.; Tam, L.-S.; Salvarani, C.; Kim, T.-H.; Solinger, A.; Datsenko, Y.; et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: Results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis. 2018, 77, 1295–1302. [Google Scholar] [CrossRef]
- Deodhar, A.; Gensler, L.S.; Sieper, J.; Clark, M.; Calderon, C.; Wang, Y.; Zhou, Y.; Leu, J.H.; Campbell, K.; Sweet, K.; et al. Three Multicenter, Randomized, Double-Blind, Placebo-Controlled Studies Evaluating the Efficacy and Safety of Ustekinumab in Axial Spondyloarthritis. Arthritis Rheumatol. 2019, 71, 258–270. [Google Scholar] [CrossRef]
- Baeten, D.; Sieper, J.; Braun, J.; Baraliakos, X.; Dougados, M.; Emery, P.; Deodhar, A.; Porter, B.; Martin, R.; Andersson, M.; et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 2015, 373, 2534–2548. [Google Scholar] [CrossRef]
- Mease, P.J.; Gottlieb, A.B.; van der Heijde, D.; FitzGerald, O.; Johnsen, A.; Nys, M.; Banerjee, S.; Gladman, D.D. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann. Rheum. Dis. 2017, 76, 1550–1558. [Google Scholar] [CrossRef]
- Eigler, A.; Siegmund, B.; Emmerich, U.; Baumann, K.H.; Hartmann, G.; Endres, S. Anti-inflammatory activities of cAMP-elevating agents: Enhancement of IL-10 synthesis and concurrent suppression of TNF production. J. Leukoc. Biol. 1998, 63, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Schafer, P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem. Pharmacol. 2012, 83, 1583–1590. [Google Scholar] [CrossRef]
- Claveau, D.; Chen, S.L.; O’keefe, S.; Zaller, D.M.; Styhler, A.; Liu, S.; Huang, Z.; Nicholson, D.W.; Mancini, J.A. Preferential inhibition of T helper 1, but not T helper 2, cytokines in vitro by L-826,141 [4-[2-(3,4-Bisdifluromethoxyphenyl)-2-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-phenyl]-ethyl]3-methylpyridine-1-oxide], a potent and selective phosphodiesterase 4 inhibitor. J. Pharmacol. Exp. Ther. 2004, 310, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Myerson, G.E.; Fleischmann, R.M.; Lioté, F.; Díaz-González, F.; Bosch, F.V.D.; Marzo-Ortega, H.; Feist, E.; Shah, K.; Hu, C.; et al. A Phase III, Randomized, Controlled Trial of Apremilast in Patients with Psoriatic Arthritis: Results of the PALACE 2 Trial. J. Rheumatol. 2016, 43, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.J.; Blanco, F.J.; Crowley, J.; Birbara, C.A.; Jaworski, J.; Aelion, J.; Stevens, R.M.; Vessey, A.; Zhan, X.; Bird, P. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: A phase III, randomised, controlled trial (PALACE 3). Ann. Rheum. Dis. 2016, 75, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, A.; Mease, P.J.; Gomez-Reino, J.J.; Adebajo, A.O.; Wollenhaupt, J.; Gladman, D.D.; Lespessailles, E.; Hall, S.; Hochfeld, M.; Hu, C.; et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann. Rheum. Dis. 2014, 73, 1020–1026. [Google Scholar] [CrossRef]
- Wells, A.F.; Edwards, C.J.; Kivitz, A.J.; Bird, P.; Nguyen, D.; Paris, M.; Teng, L.; Aelion, J.A. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: Results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology 2018, 57, 1253–1263. [Google Scholar] [CrossRef]
- Traves, P.G.; Murray, B.; Campigotto, F.; Galien, R.; Meng, A.; Di Paolo, J.A. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann. Rheum. Dis. 2021, 80, 865–875. [Google Scholar] [CrossRef]
- Gladman, D.; Rigby, W.; Azevedo, V.F.; Behrens, F.; Blanco, R.; Kaszuba, A.; Kudlacz, E.; Wang, C.; Menon, S.; Hendrikx, T.; et al. Tofacitinib for Psoriatic Arthritis in Patients with an Inadequate Response to TNF Inhibitors. N. Engl. J. Med. 2017, 377, 1525–1536. [Google Scholar] [CrossRef]
- Mease, P.; Hall, S.; FitzGerald, O.; van der Heijde, D.; Merola, J.F.; Avila-Zapata, F.; Cieślak, D.; Graham, D.; Wang, C.; Menon, S.; et al. Tofacitinib or Adalimumab versus Placebo for Psoriatic Arthritis. N. Engl. J. Med. 2017, 377, 1537–1550. [Google Scholar] [CrossRef]
- McInnes, I.B.; Anderson, J.K.; Magrey, M.; Merola, J.F.; Liu, Y.; Kishimoto, M.; Jeka, S.; Pacheco-Tena, C.; Wang, X.; Chen, L.; et al. Trial of Upadacitinib and Adalimumab for Psoriatic Arthritis. N. Engl. J. Med. 2021, 384, 1227–1239. [Google Scholar] [CrossRef]
- Mease, P.J.; Lertratanakul, A.; Anderson, J.K.; Papp, K.; Bosch, F.V.D.; Tsuji, S.; Dokoupilova, E.; Keiserman, M.; Wang, X.; Zhong, S.; et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann. Rheum. Dis. 2021, 80, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Baraliakos, X.; Ranza, R.; Östör, A.; Ciccia, F.; Coates, L.C.; Rednic, S.; Walsh, J.A.; Douglas, K.; Gao, T.; Kato, K.; et al. Efficacy and safety of upadacitinib in patients with active psoriatic arthritis and axial involvement: Results from two phase 3 studies. Arthritis Res. Ther. 2023, 25, 56. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Deodhar, A.A.; van der Heijde, D.; Behrens, F.; Kivitz, A.J.; Neal, J.; Kim, J.; Singhal, S.; Nowak, M.; Banerjee, S. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann. Rheum. Dis. 2022, 81, 815–822. [Google Scholar] [CrossRef]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Bar, D.; Lidar, M.; Baum, S.; Barzilai, A.; Pavlotsky, F.; Druyan, A. Early methotrexate treatment for psoriatic arthritis prevention in psoriasis patients—A retrospective cohort study. Jt. Bone Spine 2025, 92, 105833. [Google Scholar] [CrossRef]
- Floris, A.; Mugheddu, C.; Sichi, L.; Anedda, J.; Frau, A.; Sorgia, J.; Volsi, L.L.; Paladino, M.T.; Congia, M.; Chessa, E.; et al. Treatment of psoriasis with different classes of biologics reduces the likelihood of peripheral and axial psoriatic arthritis development. Rheumatology 2025, 64, 1131–1137. [Google Scholar] [CrossRef]
- Poddubnyy, D.; Parikh, B.; Elewaut, D.; Navarro-Compán, V.; Siebert, S.; Paley, M.; Coombs, D.; Lagunes, I.; Biljan, A.; Nakasato, P.; et al. Development of Extramusculoskeletal Manifestations in Upadacitinib-Treated Patients With Psoriatic Arthritis or Axial Spondyloarthritis. Arthritis Rheumatol. 2025, 77, 536–546. [Google Scholar] [CrossRef]
- Carmona-Rocha, E.; Rusiñol, L.; Puig, L. New and Emerging Oral/Topical Small-Molecule Treatments for Psoriasis. Pharmaceutics 2024, 16, 239. [Google Scholar] [CrossRef]
- Yi, R.C.; Akbik, M.; Smith, L.R.; Klionsky, Y.; Feldman, S.R. Therapeutic Advancements in Psoriasis and Psoriatic Arthritis. J. Clin. Med. 2025, 14, 1312. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Mease, P.J.; de Vlam, K.; Mpofu, S.; Wetzel, D.; Stevens, A.M.; Wiens, B.; Koskinen, L.O.; Ohlman, S.; Feldwisch, J.; et al. Efficacy and safety of izokibep in patients with active psoriatic arthritis: A randomised, double-blind, placebo-controlled, phase 2 study. Ann. Rheum. Dis. 2025, 84, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Iznardo, H.; Puig, L. Dual inhibition of IL-17A and IL-17F in psoriatic disease. Ther. Adv. Chronic. Dis. 2021, 12, 20406223211037846. [Google Scholar] [CrossRef]
- Bissonnette, R.; Pinter, A.; Ferris, L.K.; Gerdes, S.; Rich, P.; Vender, R.; Miller, M.; Shen, Y.-K.; Kannan, A.; Li, S.; et al. An Oral Interleukin-23-Receptor Antagonist Peptide for Plaque Psoriasis. N. Engl. J. Med. 2024, 390, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, A.; Coates, L.C.; Mease, P.J.; Nowak, M.; Hippeli, L.; Lehman, T.; Banerjee, S.; Merola, J.F. Deucravacitinib, a selective, TYK2 inhibitor, in psoriatic arthritis: Achievement of minimal disease activity components in a phase 2 trial. Rheumatology 2025, 64, 2557–2564. [Google Scholar] [CrossRef]
- Kivitz, A.; Baraliakos, X.; Muensterman, E.T.; Kavanaugh, A.; van der Heijde, D.; Klimiuk, P.A.; Valenzuela, G.; Dokoupilova, E.; Poirier, G.; Srivastava, B.; et al. Highly selective tyrosine kinase 2 inhibition with zasocitinib (TAK-279) improves outcomes in patients with active psoriatic arthritis: A randomised phase 2b study. Ann. Rheum. Dis. 2025, 84, 1660–1674. [Google Scholar] [CrossRef]
- Mease, P.; Helliwell, P.; Silwinska-Stanczyk, P.; Miakisz, M.; Ostor, A.; Peeva, E.; Vincent, M.S.; Sun, Q.; Sikirica, V.; Winnette, R.; et al. Efficacy and Safety of the TYK2/JAK1 Inhibitor Brepocitinib for Active Psoriatic Arthritis: A Phase IIb Randomized Controlled Trial. Arthritis Rheumatol. 2023, 75, 1370–1380. [Google Scholar] [CrossRef]
- Papp, K.A.; Helliwell, P.; Silwinska-Stanczyk, P.; Miakisz, M.; Ostor, A.; Peeva, E.; Vincent, M.S.; Sun, Q.; Sikirica, V.; Winnette, R.; et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) as a therapeutic target in psoriasis: Randomized, controlled investigation using namilumab, a specific human anti-GM-CSF monoclonal antibody. Br. J. Dermatol. 2019, 180, 1352–1360. [Google Scholar] [CrossRef]
- Mortier, C.; Gracey, E.; Coudenys, J.; Manuello, T.; Decruy, T.; Maelegheer, M.; Stappers, F.; Gilis, E.; Gaublomme, D.; Van Hoorebeke, L.; et al. RORγt inhibition ameliorates IL-23 driven experimental psoriatic arthritis by predominantly modulating γδ-T cells. Rheumatology 2023, 62, 3169–3178. [Google Scholar] [CrossRef]


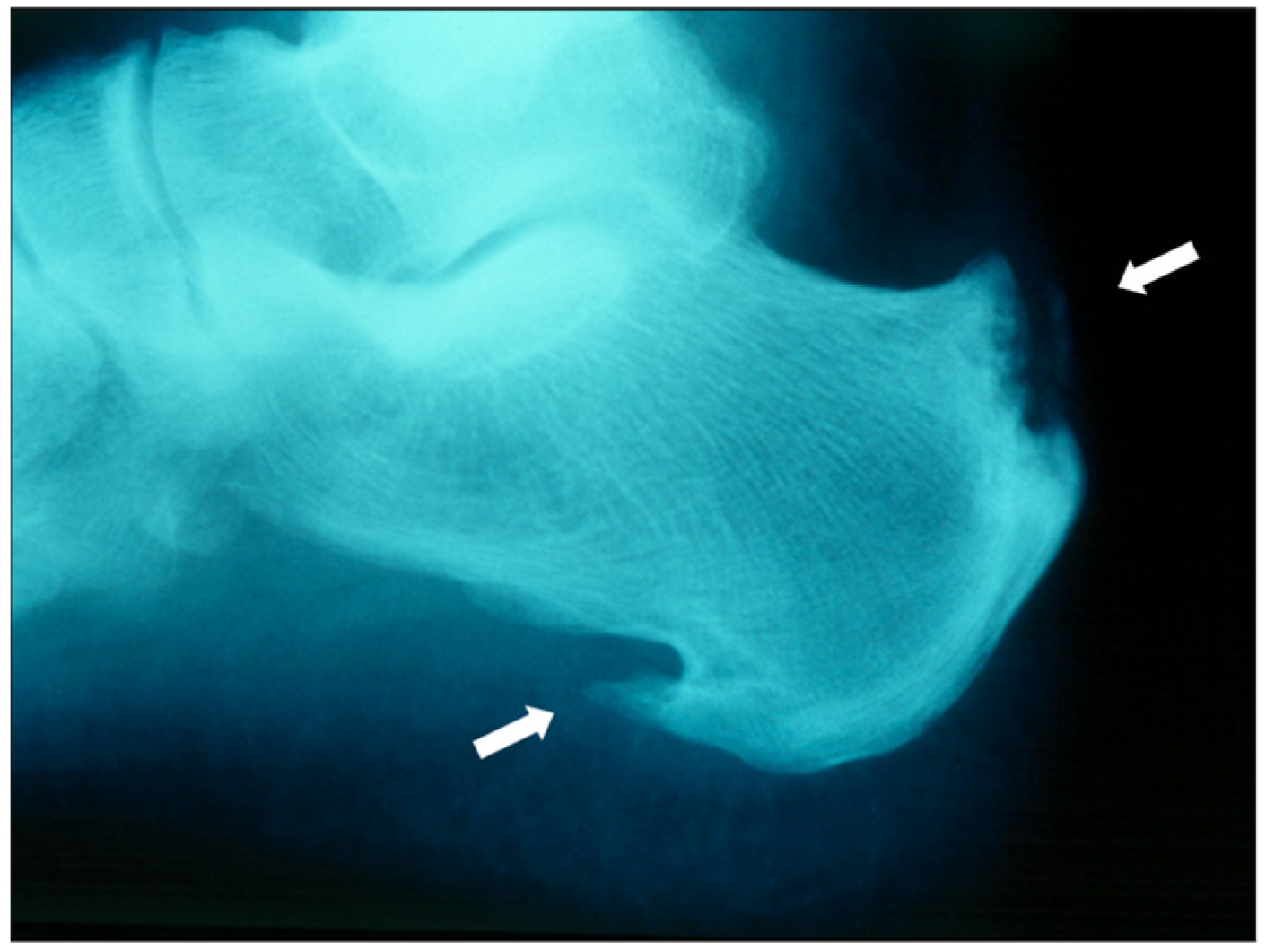
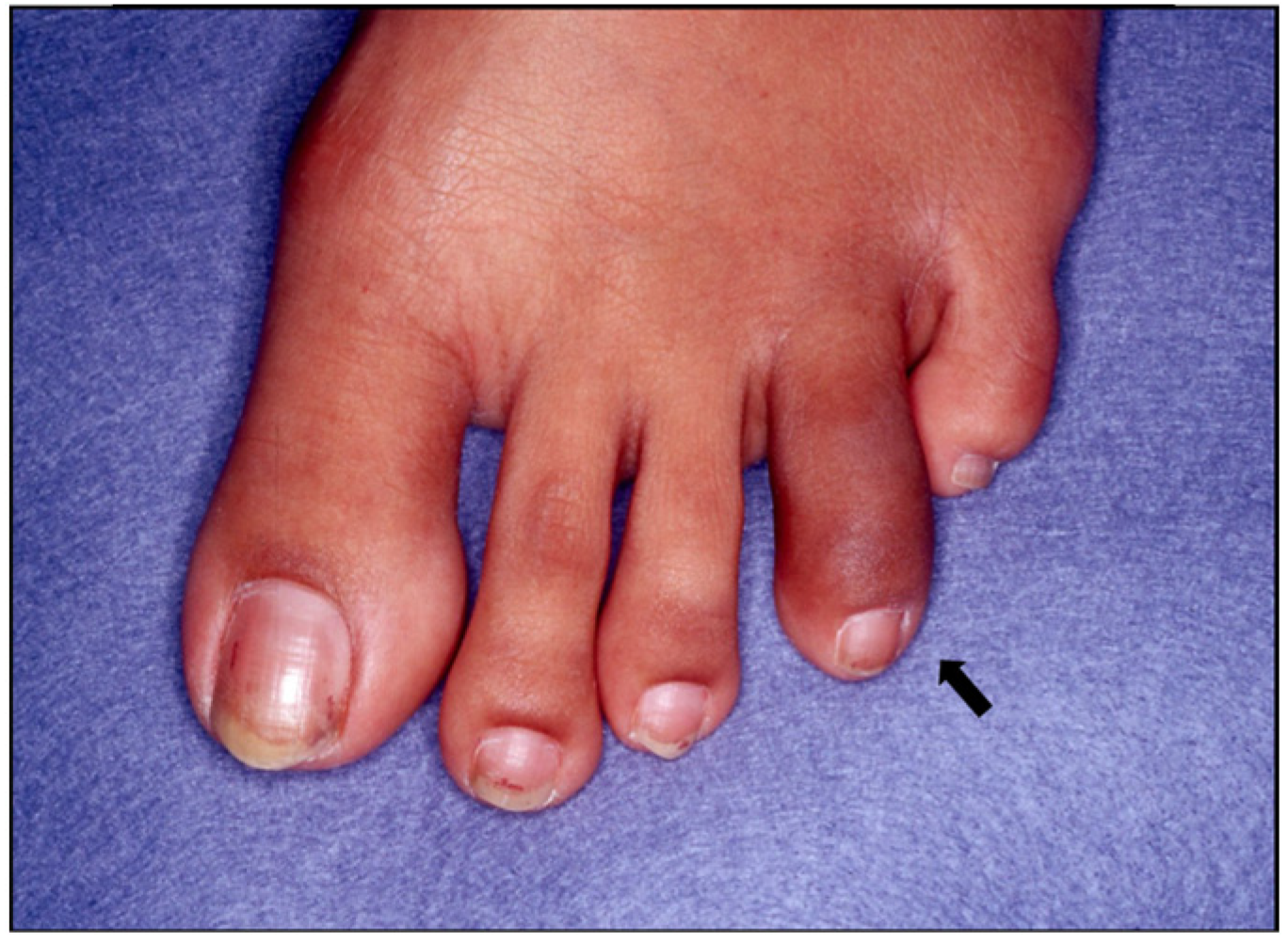
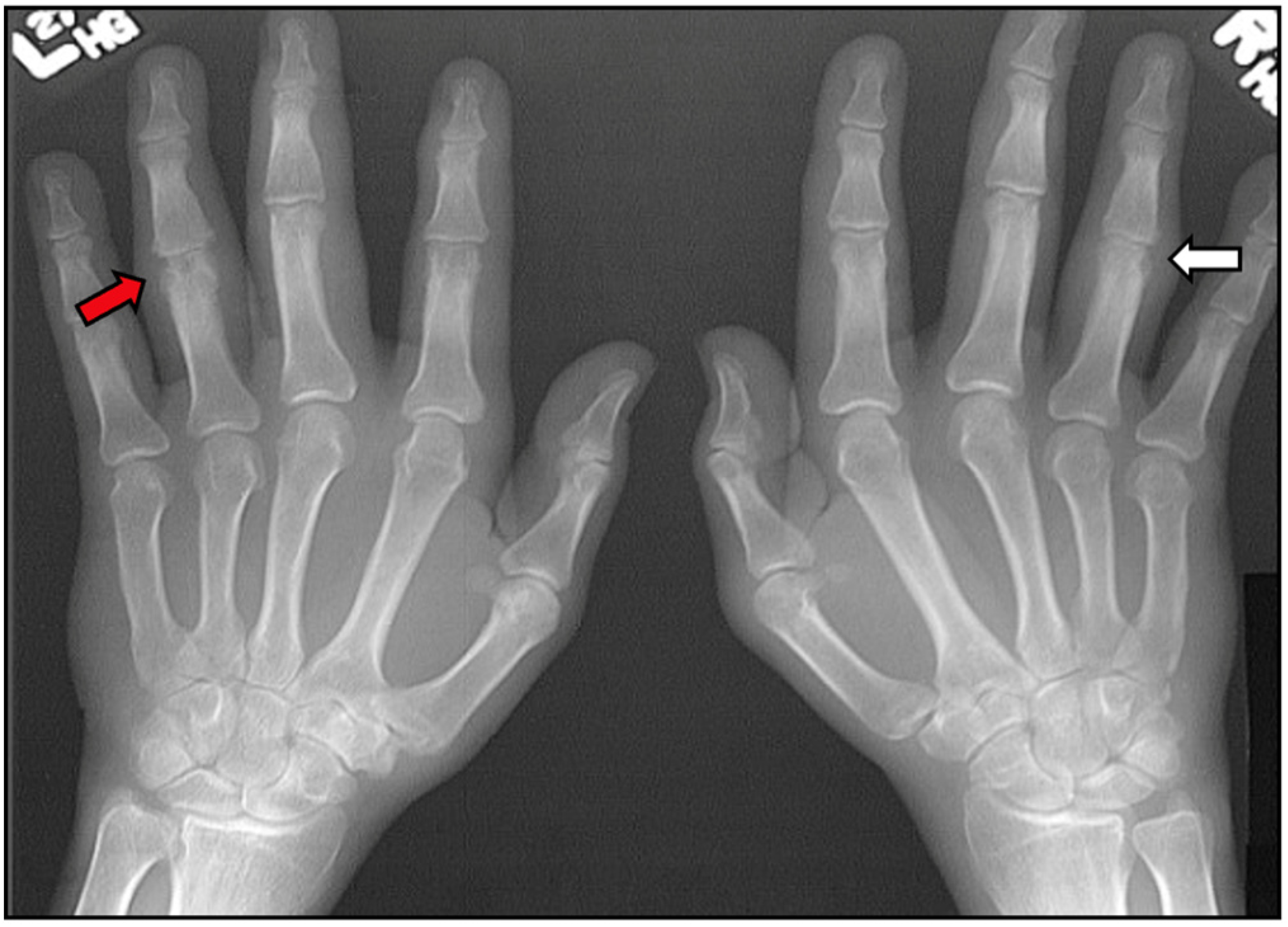
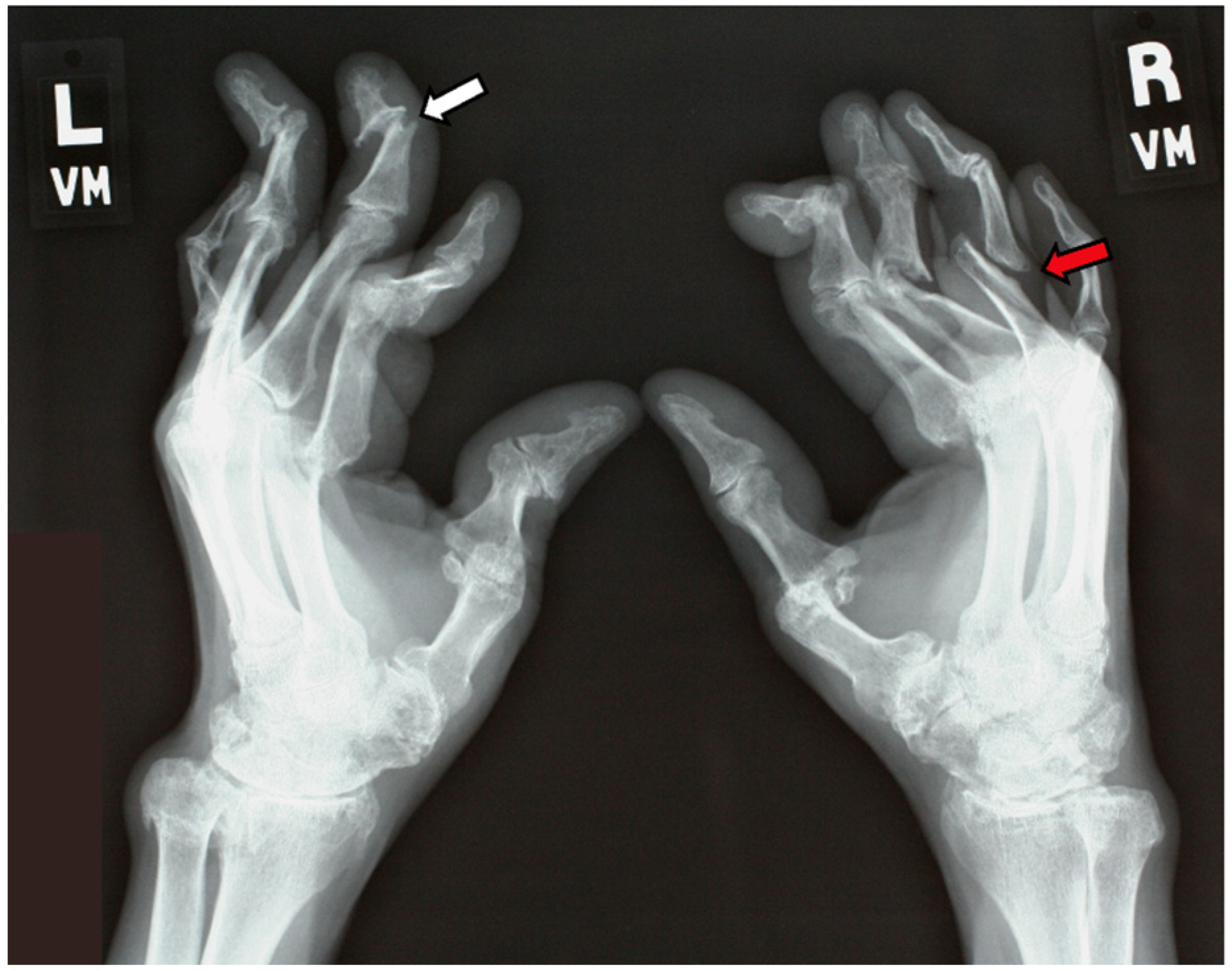
| Inflammatory Articular Disease (Joint, Spine, or Entheseal) with ≥3 of the Following: | ||
|---|---|---|
|
| Psoriasis is present as per rheumatologist or dermatologist |
| History of psoriasis obtained from patient, family, or qualified health care professional | |
| History of psoriasis in a first- or second-degree relative according to the patient | |
| Psoriatic nail dystrophy, including onycholysis, pitting, and hyperkeratosis observed | |
| By any method except latex, but preferably by ELISA or nephelometry, according to the local laboratory reference range and negative CCP | |
|
| |
| Ill-defined ossification near joint margins (but excluding osteophyte formation) on plain X-rays of hands or feet | |
| Questionnaire Name | Description | Scoring | Sensitivity (%)/Specificity (%) |
|---|---|---|---|
| PASE | 15 questions divided into 2 subscales: 7 questions that assess symptoms and 8 questions that assess function | Each question is scored from 1 to 5; Max score of 75 | 93%/80% (Dominguez) |
| ToPAS | 12 questions regarding skin, nail, and joint involvement | Each question is 1 point; cut off score of 8 | 93.1%/86.8% (Gladman) |
| ToPAS 2 | 13 questions with focus on axial and enthesitis domains | Each question is 1 point; cut off score of 7 or 8 | 92.0%/77.2% (Tom) |
| PEST | 5 yes/no items designed for easy use in dermatology or primary care | Score out of 5; cut-off ≥3 suggests PsA referral | 68%/73% (Iragorri) |
| EARP | 10-item questionnaire optimized for early PsA detection in dermatology settings | Score ≥3 suggests PsA | 91%/88% (Mishra) |
| SiPAS | 5 yes/no items for rapid dermatology use | Score ≥3 suggests PsA referral | 79%/87% (Salaffi) |
| Drug Class (Examples) | Disease Domains | ||||
|---|---|---|---|---|---|
| Peripheral Arthritis | Axial Disease | Enthesitis | Dactylitis | Skin | |
| csDMARDs (Methotrexate, Sulfasalazine, Cyclosporine, Leflunomide) | Moderate (Methotrexate preferred first line) | Limited | Limited | Limited | Moderate |
| TNF inhibitors (Etanercept, Infliximab, Adalimumab, Golimumab, Certolizumab pegol) | Strong | Strong | Strong | Strong | Moderate |
| IL-17 inhibitors (Secukinumab, Ixekizumab, Bimekizumab, Brodalumab) | Strong | Moderate | Strong | Strong | Strong |
| IL-12/IL-23 inhibitors (Ustekinumab) | Moderate | Limited | Moderate | Moderate | Strong |
| IL-23 inhibitors (Guselkumab, Risankizumab, Tildrakizumab) | Moderate | Limited | Moderate | Moderate | Strong |
| CTLA-4 Ig (Abatacept) | Moderate | Limited | Limited | Limited | Limited |
| PDE-4 inhibitors (Apremilast) | Moderate | Limited | Limited | Limited | Moderate |
| JAK/STAT inhibitors (Tofacitinib, Upadacitinib, Filgotinib, Deucravacitinib, Brepocitinib) | Strong | Limited | Moderate | Moderate | Strong |
| Symptomatic therapies (NSAIDs, glucocorticoids) | |||||
| Non-pharmacological therapies (Physical therapy, occupational therapy, smoking cessation, weight loss, massage therapy, exercise) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kannappan, R.; Kim, S.; Lau, A.; Brent, L.H. Psoriatic Arthritis: From Diagnosis to Treatment. J. Clin. Med. 2025, 14, 8151. https://doi.org/10.3390/jcm14228151
Kannappan R, Kim S, Lau A, Brent LH. Psoriatic Arthritis: From Diagnosis to Treatment. Journal of Clinical Medicine. 2025; 14(22):8151. https://doi.org/10.3390/jcm14228151
Chicago/Turabian StyleKannappan, Renuka, Sarah Kim, Arthur Lau, and Lawrence H. Brent. 2025. "Psoriatic Arthritis: From Diagnosis to Treatment" Journal of Clinical Medicine 14, no. 22: 8151. https://doi.org/10.3390/jcm14228151
APA StyleKannappan, R., Kim, S., Lau, A., & Brent, L. H. (2025). Psoriatic Arthritis: From Diagnosis to Treatment. Journal of Clinical Medicine, 14(22), 8151. https://doi.org/10.3390/jcm14228151






