How to Evaluate and Adjust the Recommended Level of Physical Activity in Patients with Congenital Heart Diseases? A Practical Approach
Abstract
1. Introduction
2. Material and Methods
3. Before Recommendations Can Be Made—Patients’ Condition Assessment
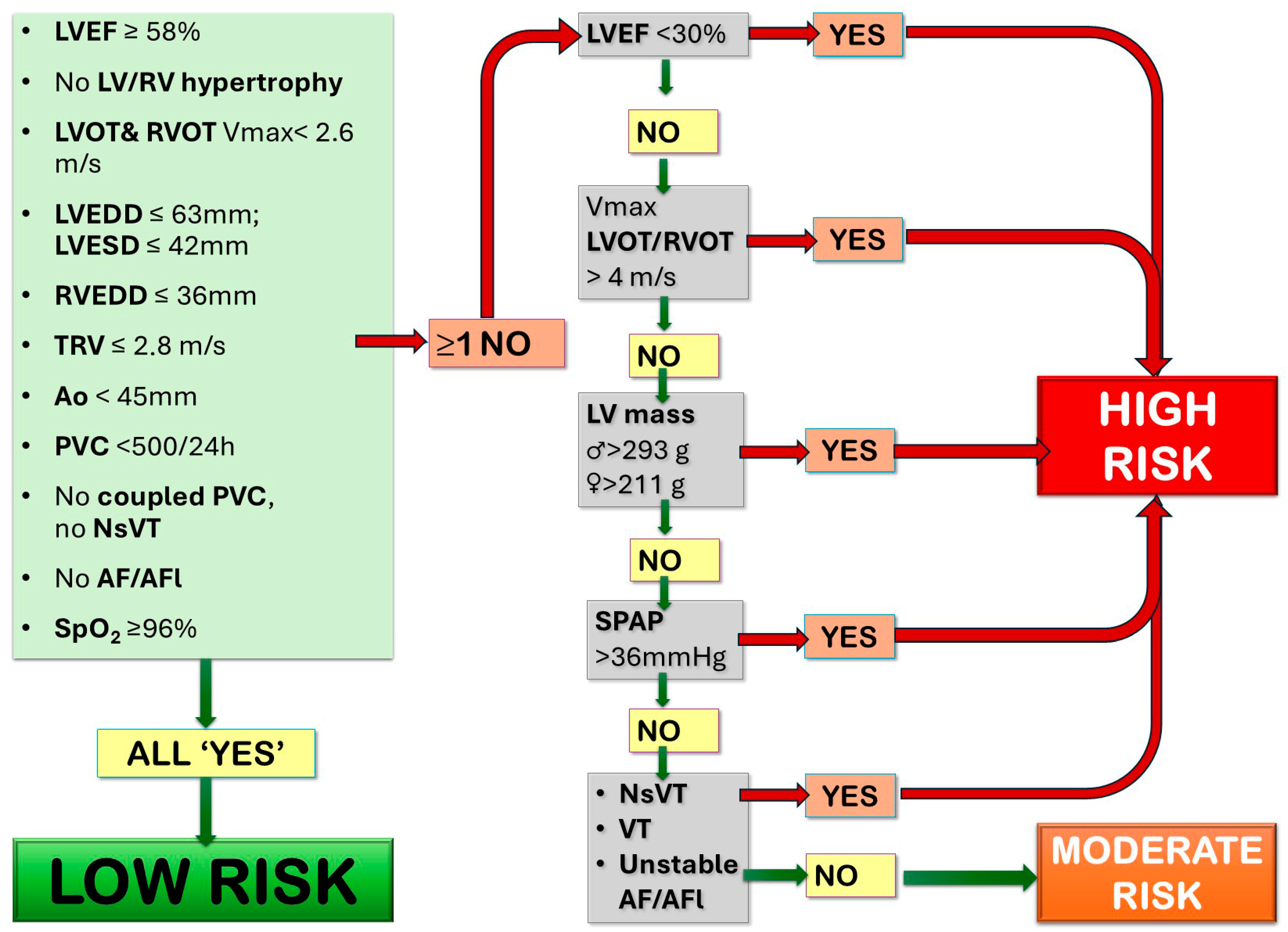
3.1. At Rest—Basic Methods
3.1.1. Echocardiography
- Ventricle systolic function: left ventricle ejection fraction (LVEF), tricuspid annular plane systolic excursion (TAPSE), and fractional area change (FAC) for RV function, 3D echocardiography when feasible;
- LV/RV pressure load: left/right ventricular outflow tract (LVOT/RVOT) peak systolic velocity to assess the LVOT/RVOT gradient;
- LV/RV volume load: the presence of valve regurgitation or shunt, the presence of RV or LV dilatation (LV end diastolic volume—LV EDV, RV end diastolic area—RV EDA)
- Pulmonary pressure estimation; in most risk stratification scale tricuspid valve regurgitation velocity (TRV) was used; however, in certain clinical scenarios (e.g., excentric jet) where reliable TRV measurement is not possible, other parameters, such as acceleration time of the pulmonary valve or mid-systolic notching, early diastolic pulmonary regurgitation velocity, or pulmonary artery dilatation, should be taken into consideration. In particularly complicated cases, right heart catheterization should be considered a golden standard.
- Aortic diameters: the aortic root diameter, measured in the parasternal long axis view (as the short axis may underestimate the diameter due to possible plane obliquity); the ascending aorta diameter, measured in end-diastole in the parasternal long axis view, often 1–2 intercostal spaces up [15,19,20].
3.1.2. Arrythmia Detection: Resting ECG and 24-h (or Prolonged) ECG Monitoring
3.1.3. Assessment of Oxygen Saturation
3.2. Exercise Testing—Cardiopulmonary Exercise Test (CPET)
- Cardiopulmonary indices:
3.3. Additional Assessment Methods—To Be Used in Specific Circumstances
3.3.1. Cardiovascular Magnetic Resonance
3.3.2. Computed Tomography
3.3.3. Right Heart Catheterization
3.3.4. Spirometry
3.3.5. Implantable Loop Recorder and an Electrophysiology Study
3.3.6. Coronary Artery Disease Diagnostic Methods–Imaging, Exercise Test and Coronarography
4. Physical Activity Prescription
4.1. Types of Physical Effort Activities
4.1.1. Aerobic Training
4.1.2. Resistance Training
4.2. How to Assess the Recommended Exercise Intensity?
5. Special Needs Patient Population
5.1. Pulmonary Hypertension
5.2. Desaturation
5.3. Pathologies of the Aorta
5.4. Cardiac Implantable Electronic Devices
5.5. Fontan Circulation
6. Conclusions
- For the vast majority of patients, physical activity is not only safe but essential and should be strongly promoted.
- Activity restrictions are now reserved for a very small subset of patients with specific high-risk conditions.
- The contemporary role of the healthcare professional has expanded beyond simply permitting activity.
- Physical activity counseling is considered an essential component of every patient interaction, regardless of whether the patient’s clinical status warrants any restrictions.
- The management of physical activity is a dynamic, lifelong process. The initial prescription is not static but must be subject to ongoing surveillance and adjustment
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 6MWT | 6-min walk test |
| AF | Atrial fibrillation |
| AFl | Atrial fluttering |
| Ao | Aorta |
| ccTGA | Congenitally corrected transposition of the great arteries |
| CHD | Congenital heart diseases |
| CMR | Cardiovascular magnetic resonance |
| CPET | Cardiopulmonary exercise test |
| CRF | Cardiorespiratory fitness |
| CT | Computed tomography |
| ECG | Electrocardiogram |
| EDA | End diastolic area |
| EDV | End diastolic volume |
| ET | Exercise training |
| FAC | Fractional area change |
| HITT | High-intensity interval training |
| HRR | Heart rate reserve |
| LV | Left ventricle |
| LVEDD | Left ventricular end diastolic diameter |
| LVEF | Left ventricular ejection fraction |
| LVESD | Left ventricular end systolic diameter |
| LVOT | Left ventricular outflow tract |
| NsVT | Non-sustained ventricular tachycardia |
| PAH | Pulmonary arterial hypertension |
| PASP | Pulmonary artery systolic pressure |
| PVC | Premature ventricular complex |
| RPE | Rate of perceived exertion |
| RV | Right ventricle |
| RVEDD | Right ventricular end diastolic diameter |
| RVOT | Right ventricular outflow tract |
| SPAP | Systolic pulmonary artery pressure |
| SpO2 | Oxygen saturation |
| TAPSE | Tricuspid annular plane systolic excursion |
| TRV | Tricuspid regurgitant velocity |
| VAT | Ventilatory anaerobic threshold |
| VE/VCO2 | Ventilation to carbon dioxide output |
| VO2 | Oxygen uptake |
| VT | Ventricular tachycardia |
References
- Van Der Linde, D.; Konings, E.E.M.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.M.; Roos-Hesselink, J.W. Birth Prevalence of Congenital Heart Disease Worldwide. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef]
- Van Der Bom, T.; Bouma, B.J.; Meijboom, F.J.; Zwinderman, A.H.; Mulder, B.J.M. The Prevalence of Adult Congenital Heart Disease, Results from a Systematic Review and Evidence Based Calculation. Am. Heart J. 2012, 164, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Moons, P.; Marelli, A. Born to Age: When Adult Congenital Heart Disease Converges with Geroscience. JACC Adv. 2022, 1, 100012. [Google Scholar] [CrossRef]
- Dellborg, M. Adult Congenital Heart Disease. Circulation 2024, 149, 1397–1399. [Google Scholar] [CrossRef]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the Management of Adult Congenital Heart Disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Reybrouck, T.; Mertens, L. Physical Performance and Physical Activity in Grown-up Congenital Heart Disease. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Van Deutekom, A.W.; Lewandowski, A.J. Physical Activity Modification in Youth with Congenital Heart Disease: A Comprehensive Narrative Review. Pediatr. Res. 2021, 89, 1650–1658. [Google Scholar] [CrossRef]
- Moons, P.; Deyk, K.V.; Dedroog, D.; Troost, E.; Budts, W. Prevalence of Cardiovascular Risk Factors in Adults with Congenital Heart Disease. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Liu, P.-H.; Wu, L.-S.; Chen, Y.-M.; Chang, C.-J.; Chu, P.-H. Major Adverse Cardiovascular Events in Adult Congenital Heart Disease: A Population-Based Follow-up Study from Taiwan. BMC Cardiovasc. Disord. 2014, 14, 38. [Google Scholar] [CrossRef]
- Afilalo, J.; Therrien, J.; Pilote, L.; Ionescu-Ittu, R.; Martucci, G.; Marelli, A.J. Geriatric Congenital Heart Disease. J. Am. Coll. Cardiol. 2011, 58, 1509–1515. [Google Scholar] [CrossRef]
- Zomer, A.C.; Vaartjes, I.; Uiterwaal, C.S.P.; Van Der Velde, E.T.; Sieswerda, G.-J.T.; Wajon, E.M.C.; Plomp, K.; Van Bergen, P.F.M.; Verheugt, C.L.; Krivka, E.; et al. Social Burden and Lifestyle in Adults with Congenital Heart Disease. Am. J. Cardiol. 2012, 109, 1657–1663. [Google Scholar] [CrossRef]
- Lui, G.K.; Rogers, I.S.; Ding, V.Y.; Hedlin, H.K.; MacMillen, K.; Maron, D.J.; Sillman, C.; Romfh, A.; Dade, T.C.; Haeffele, C.; et al. Risk Estimates for Atherosclerotic Cardiovascular Disease in Adults with Congenital Heart Disease. Am. J. Cardiol. 2017, 119, 112–118. [Google Scholar] [CrossRef]
- Tutarel, O. Acquired Heart Conditions in Adults with Congenital Heart Disease: A Growing Problem. Heart 2014, 100, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.A.; Lavie, C.J.; Squires, R.W.; Milani, R.V. Exercise-Based Cardiac Rehabilitation and Improvements in Cardiorespiratory Fitness: Implications Regarding Patient Benefit. Mayo Clin. Proc. 2013, 88, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Budts, W.; Borjesson, M.; Chessa, M.; Van Buuren, F.; Trigo Trindade, P.; Corrado, D.; Heidbuchel, H.; Webb, G.; Holm, J.; Papadakis, M. Physical Activity in Adolescents and Adults with Congenital Heart Defects: Individualized Exercise Prescription. Eur. Heart J. 2013, 34, 3669–3674. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of Adult Transthoracic Echocardiography Reporting in Agreement with Recent Chamber Quantification, Diastolic Function, and Heart Valve Disease Recommendations: An Expert Consensus Document of the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Di Salvo, G.; Miller, O.; Babu Narayan, S.; Li, W.; Budts, W.; Valsangiacomo Buechel, E.R.; Frigiola, A.; Van Den Bosch, A.E.; Bonello, B.; Mertens, L.; et al. Imaging the Adult with Congenital Heart Disease: A Multimodality Imaging Approach—Position Paper from the EACVI. Eur. Heart J.-Cardiovasc. Imaging 2018, 19, 1077–1098. [Google Scholar] [CrossRef]
- Budts, W.; Pieles, G.E.; Roos-Hesselink, J.W.; Sanz De La Garza, M.; D’Ascenzi, F.; Giannakoulas, G.; Müller, J.; Oberhoffer, R.; Ehringer-Schetitska, D.; Herceg-Cavrak, V.; et al. Recommendations for Participation in Competitive Sport in Adolescent and Adult Athletes with Congenital Heart Disease (CHD): Position Statement of the Sports Cardiology & Exercise Section of the European Association of Preventive Cardiology (EAPC), the European Society of Cardiology (ESC) Working Group on Adult Congenital Heart Disease and the Sports Cardiology, Physical Activity and Prevention Working Group of the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2020, 41, 4191–4199. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Khairy, P. Arrhythmias and Sudden Cardiac Death in Adult Congenital Heart Disease. In Clinical Approach to Sudden Cardiac Death Syndromes; Brugada, R., Ed.; Springer: London, UK, 2010; pp. 37–56. ISBN 978-1-84882-926-8. [Google Scholar]
- Krieger, E.V.; Zeppenfeld, K.; DeWitt, E.S.; Duarte, V.E.; Egbe, A.C.; Haeffele, C.; Lin, K.Y.; Robinson, M.R.; Sillman, C.; Upadhyay, S.; et al. Arrhythmias in Repaired Tetralogy of Fallot: A Scientific Statement from the American Heart Association. Circ. Arrhythmia Electrophysiol. 2022, 15, e000084. [Google Scholar] [CrossRef]
- Small, A.J.; Dai, M.; Halpern, D.G.; Tan, R.B. Updates in Arrhythmia Management in Adult Congenital Heart Disease. J. Clin. Med. 2024, 13, 4314. [Google Scholar] [CrossRef]
- Cifra, B.; Cordina, R.L.; Gauthier, N.; Murphy, L.C.; Pham, T.D.; Veldtman, G.R.; Ward, K.; White, D.A.; Paridon, S.M.; Powell, A.W.; et al. Cardiopulmonary Exercise Test Interpretation Across the Lifespan in Congenital Heart Disease: A Scientific Statement From the American Heart Association. JAHA 2025, 14, e038200. [Google Scholar] [CrossRef]
- Guglielmi, G.; Moscatelli, S.; Rocchetti, G.; Agostoni, P.; Chessa, M.; Mapelli, M. Cardiopulmonary Exercise Testing in Congenital Heart Disease: A Never-Ending Story from Paediatrics to Adult Life. Children 2025, 12, 1175. [Google Scholar] [CrossRef]
- Inuzuka, R.; Diller, G.-P.; Borgia, F.; Benson, L.; Tay, E.L.W.; Alonso-Gonzalez, R.; Silva, M.; Charalambides, M.; Swan, L.; Dimopoulos, K.; et al. Comprehensive Use of Cardiopulmonary Exercise Testing Identifies Adults With Congenital Heart Disease at Increased Mortality Risk in the Medium Term. Circulation 2012, 125, 250–259. [Google Scholar] [CrossRef]
- Giardini, A.; Specchia, S.; Tacy, T.A.; Coutsoumbas, G.; Gargiulo, G.; Donti, A.; Formigari, R.; Bonvicini, M.; Picchio, F.M. Usefulness of Cardiopulmonary Exercise to Predict Long-Term Prognosis in Adults with Repaired Tetralogy of Fallot. Am. J. Cardiol. 2007, 99, 1462–1467. [Google Scholar] [CrossRef]
- Udholm, S.; Aldweib, N.; Hjortdal, V.E.; Veldtman, G.R. Prognostic Power of Cardiopulmonary Exercise Testing in Fontan Patients: A Systematic Review. Open Heart 2018, 5, e000812. [Google Scholar] [CrossRef]
- Kempny, A.; Dimopoulos, K.; Uebing, A.; Moceri, P.; Swan, L.; Gatzoulis, M.A.; Diller, G.-P. Reference Values for Exercise Limitations among Adults with Congenital Heart Disease. Relation to Activities of Daily Life--Single Centre Experience and Review of Published Data. Eur. Heart J. 2012, 33, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Milani, J.G.P.O.; Milani, M.; Verboven, K.; Cipriano, G.; Hansen, D. Exercise Intensity Prescription in Cardiovascular Rehabilitation: Bridging the Gap between Best Evidence and Clinical Practice. Front. Cardiovasc. Med. 2024, 11, 1380639. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Abreu, A.; Ambrosetti, M.; Cornelissen, V.; Gevaert, A.; Kemps, H.; Laukkanen, J.A.; Pedretti, R.; Simonenko, M.; Wilhelm, M.; et al. Exercise Intensity Assessment and Prescription in Cardiovascular Rehabilitation and beyond: Why and How: A Position Statement from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2022, 29, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Koyak, Z.; Harris, L.; De Groot, J.R.; Silversides, C.K.; Oechslin, E.N.; Bouma, B.J.; Budts, W.; Zwinderman, A.H.; Van Gelder, I.C.; Mulder, B.J.M. Sudden Cardiac Death in Adult Congenital Heart Disease. Circulation 2012, 126, 1944–1954. [Google Scholar] [CrossRef]
- Norozi, K.; Wessel, A.; Alpers, V.; Arnhold, J.O.; Binder, L.; Geyer, S.; Zoege, M.; Buchhorn, R. Chronotropic Incompetence in Adolescents and Adults with Congenital Heart Disease After Cardiac Surgery. J. Card. Fail. 2007, 13, 263–268. [Google Scholar] [CrossRef]
- Gatzoulis, M.A.; Beghetti, M.; Landzberg, M.J.; Galiè, N. Pulmonary Arterial Hypertension Associated with Congenital Heart Disease: Recent Advances and Future Directions. Int. J. Cardiol. 2014, 177, 340–347. [Google Scholar] [CrossRef]
- Miranda, W.R.; Aboulhosn, J.A.; Hagler, D.J. Catheterization in Adults with Congenital Heart Disease. JACC Cardiovasc. Interv. 2022, 15, 907–921. [Google Scholar] [CrossRef]
- Jokinen, E. Coronary Artery Disease in Patients with Congenital Heart Defects. J. Intern. Med. 2020, 288, 383–389. [Google Scholar] [CrossRef]
- Shah, S.S.; Mohanty, S.; Karande, T.; Maheshwari, S.; Kulkarni, S.; Saxena, A. Guidelines for Physical Activity in Children with Heart Disease. Ann. Pediatr. Cardiol. 2022, 15, 467–488. [Google Scholar] [CrossRef]
- Takken, T.; Giardini, A.; Reybrouck, T.; Gewillig, M.; Hövels-Gürich, H.; Longmuir, P.; McCrindle, B.; Paridon, S.; Hager, A. Recommendations for Physical Activity, Recreation Sport, and Exercise Training in Paediatric Patients with Congenital Heart Disease: A Report from the Exercise, Basic & Translational Research Section of the European Association of Cardiovascular Prevention and Rehabilitation, the European Congenital Heart and Lung Exercise Group, and the Association for European Paediatric Cardiology. Eur. J. Prev. Cardiol. 2012, 19, 1034–1065. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P. Physical Activity Guidelines for Americans from the US Department of Health and Human Services: Cardiovascular Benefits and Recommendations. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e005263. [Google Scholar] [CrossRef]
- Longmuir, P.E.; Brothers, J.A.; De Ferranti, S.D.; Hayman, L.L.; Van Hare, G.F.; Matherne, G.P.; Davis, C.K.; Joy, E.A.; McCrindle, B.W. Promotion of Physical Activity for Children and Adults with Congenital Heart Disease: A Scientific Statement From the American Heart Association. Circulation 2013, 127, 2147–2159. [Google Scholar] [CrossRef]
- Becker-Grünig, T.; Klose, H.; Ehlken, N.; Lichtblau, M.; Nagel, C.; Fischer, C.; Gorenflo, M.; Tiede, H.; Schranz, D.; Hager, A.; et al. Efficacy of Exercise Training in Pulmonary Arterial Hypertension Associated with Congenital Heart Disease. Int. J. Cardiol. 2013, 168, 375–381. [Google Scholar] [CrossRef]
- Tran, D.; Maiorana, A.; Ayer, J.; Lubans, D.R.; Davis, G.M.; Celermajer, D.S.; d’Udekem, Y.; Cordina, R. Recommendations for Exercise in Adolescents and Adults with Congenital Heart Disease. Prog. Cardiovasc. Dis. 2020, 63, 350–366. [Google Scholar] [CrossRef]
- Opocher, F.; Varnier, M.; Sanders, S.P.; Tosoni, A.; Zaccaria, M.; Stellin, G.; Milanesi, O. Effects of Aerobic Exercise Training in Children after the Fontan Operation. Am. J. Cardiol. 2005, 95, 150–152. [Google Scholar] [CrossRef]
- Bhasipol, A.; Sanjaroensuttikul, N.; Pornsuriyasak, P.; Yamwong, S.; Tangcharoen, T. Efficiency of the Home Cardiac Rehabilitation Program for Adults with Complex Congenital Heart Disease. Congenit. Heart Dis. 2018, 13, 952–958. [Google Scholar] [CrossRef]
- Rocha Conceição, L.S.; Gauthier, N.; Andrade Guimarães, A.L.; Gois, C.O.; Oliveira, I.K.; Souza, D.S.; Carvalho, V.O. High-Intensity Interval Training in Adults with Congenital Heart Disease: A Systematic Review. Heart Lung Circ. 2025, 34, 16–24. [Google Scholar] [CrossRef]
- Novaković, M.; Prokšelj, K.; Rajkovič, U.; Vižintin Cuderman, T.; Janša Trontelj, K.; Fras, Z.; Jug, B. Exercise Training in Adults with Repaired Tetralogy of Fallot: A Randomized Controlled Pilot Study of Continuous versus Interval Training. Int. J. Cardiol. 2018, 255, 37–44. [Google Scholar] [CrossRef]
- Hao, K.; Wadey, C.A.; Barker, A.R.; Williams, C.A. The Effect of Strength Training Interventions on People with Congenital Heart Disease: A Systematic Review. Open Heart 2025, 12, e003091. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Cordina, R.L.; O’Meagher, S.; Karmali, A.; Rae, C.L.; Liess, C.; Kemp, G.J.; Puranik, R.; Singh, N.; Celermajer, D.S. Resistance Training Improves Cardiac Output, Exercise Capacity and Tolerance to Positive Airway Pressure in Fontan Physiology. Int. J. Cardiol. 2013, 168, 780–788. [Google Scholar] [CrossRef]
- Avitabile, C.M.; Goldberg, D.J.; Leonard, M.B.; Wei, Z.A.; Tang, E.; Paridon, S.M.; Yoganathan, A.P.; Fogel, M.A.; Whitehead, K.K. Leg Lean Mass Correlates with Exercise Systemic Output in Young Fontan Patients. Heart 2018, 104, 680–684. [Google Scholar] [CrossRef]
- Vanhees, L.; Stevens, A. Exercise Intensity: A Matter of Measuring or of Talking? J. Cardiopulm. Rehabil. 2006, 26, 78–79. [Google Scholar] [CrossRef]
- Matteucci, A.; Bonanni, M.; Centioni, M.; Zanin, F.; Geuna, F.; Massaro, G.; Sangiorgi, G. Home Management of Heart Failure and Arrhythmias in Patients with Cardiac Devices during Pandemic. J. Clin. Med. 2021, 10, 1618. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, H.; Ruan, H.; Ye, X.; Li, J.; Hong, C. Effectiveness and Safety of Exercise Training and Rehabilitation in Pulmonary Hypertension: A Systematic Review and Meta-Analysis. J. Thorac. Dis. 2020, 12, 2691–2705. [Google Scholar] [CrossRef]
- Albanaqi, A.L.; Rahimi, G.R.M.; Smart, N.A. Exercise Training for Pulmonary Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biol. Res. Nurs. 2021, 23, 442–454. [Google Scholar] [CrossRef]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e637–e697. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Nicholson, M.R.; Barratt-Boyes, B.G.; Neutze, J.M.; Whitlock, R.M. Results after Repair of Coarctation of the Aorta beyond Infancy: A 10 to 28 Year Follow-up with Particular Reference to Late Systemic Hypertension. Am. J. Cardiol. 1983, 51, 1481–1488. [Google Scholar] [CrossRef]
- Gati, S.; Malhotra, A.; Sharma, S. Exercise Recommendations in Patients with Valvular Heart Disease. Heart 2019, 105, 106–110. [Google Scholar] [CrossRef]
- Kiuchi, V. Lifestyle Advice for Patients with ICDs: Physical Activity—What Is Healthy and What Is Contraindicated. e-J. Cardiol. Pract. 2019, 17. [Google Scholar]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise Standards for Testing and Training: A Scientific Statement From the American Heart Association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- Steinhaus, D.A.; Lubitz, S.A.; Noseworthy, P.A.; Kramer, D.B. Exercise Interventions in Patients With Implantable Cardioverter-Defibrillators and Cardiac Resynchronization Therapy: A Systematic Review and Meta-Analysis. J. Cardiopulm. Rehabil. Prev. 2019, 39, 308–317. [Google Scholar] [CrossRef]
- Gewillig, M. The Fontan Circulation. Heart 2005, 91, 839–846. [Google Scholar] [CrossRef]
- Ven, L.V.D.; Félix, A.C.; Suarez, J.; Dias, J.; Pinto, F.F.; Laranjo, S. Cardiac Rehabilitation for Fontan Circulation Patients: A Systematic Review, and Meta-Analysis. Medicina 2024, 60, 1817. [Google Scholar] [CrossRef] [PubMed]
- Turquetto, A.L.R.; Dos Santos, M.R.; Agostinho, D.R.; Sayegh, A.L.C.; De Souza, F.R.; Amato, L.P.; Barnabe, M.S.R.; De Oliveira, P.A.; Liberato, G.; Binotto, M.A.; et al. Aerobic Exercise and Inspiratory Muscle Training Increase Functional Capacity in Patients with Univentricular Physiology after Fontan Operation: A Randomized Controlled Trial. Int. J. Cardiol. 2021, 330, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kim, S.-J.; Lee, D.W.; Gwon, S.H.; Son, N.-H.; Cho, M.J.; Oh, K.J.; Lee, J.S.; Na, J.Y.; Seol, J.H. Effects of Exercise Training in Patients with Fontan Circulation: A Systematic Review and Meta-Analysis. Korean Circ. J. 2025, 55, e63. [Google Scholar] [CrossRef] [PubMed]
- Scheffers, L.E.; Helbing, W.A.; Pereira, T.; Utens, E.M.W.J.; Dulfer, K.; Hirsch, A.; Koopman, L.P.; Van Den Berg, L.E. Leg-Focused High-Weight Resistance Training Improves Ventricular Stroke Volume, Exercise Capacity and Strength in Young Patients with a Fontan Circulation. Eur. J. Prev. Cardiol. 2024, 31, 389–399. [Google Scholar] [CrossRef]
| CHD Type | Peak VO2 | VE/VCO2 Slope | VAT | HRR |
|---|---|---|---|---|
| Biventricular circulation with systemic LV | ~71% predicted; | Normal/mildly elevated | Often reduced | Frequently reduced. |
| Biventricular circulation with systemic RV | ~63–67% predicted | Mildly elevated | Reduced | Frequently reduced; |
| Fontan circulation | ~59% predicted | Mildly elevated | Reduced | Frequently reduced |
| Eisenmenger syndrome | ~42% predicted | Significantly elevated | Markedly reduced | Frequently reduced |
| FITT-VP Component | Low Risk | Moderate Risk | High Risk (Medically Supervised) |
|---|---|---|---|
| Frequency | 5–7 days/week | 3–5 days/week | 3–5 days/week |
| Intensity (Aerobic) | Moderate to vigorous. RPE 13–17. Can progress to activity above VAT/VT1 or even VT2. | Low to Moderate. RPE 11–14. Generally maintain activity between VAT/VT1 and VT2. | Low. RPE 9–11. Activity must remain below VAT/VT1. Continuous monitoring may be required. |
| Time (Duration) | 30–60 min per session. | 20–40 min per session. May start with 10–15 min bouts. | 15–30 min per session. Start with short bouts (5–10 min) with frequent rest periods. |
| Type | Wide variety of aerobic and recreational sports. Competitive sports often permissible after evaluation. | Rhythmic, large muscle group activities (e.g., walking, cycling, swimming). Avoid high-intensity competitive sports. | Low-impact, low-intensity activities (e.g., slow walking, light cycling, water aerobics). |
| Volume | Aim for ≥150 min moderate or ≥75 min vigorous activity/week. | Gradually work toward 150 min moderate activity/week. | Volume is secondary to safety; focus on consistency and gradual progression of duration at low intensity. |
| Progression | Gradual increase in intensity and duration as tolerated. | Slow and gradual progression. Increase duration before intensity. Re-evaluate before progressing to vigorous activity. | Very slow progression under medical guidance. Any change requires re-evaluation. |
| Resistance Training | 2–3 days/week. Moderate intensity. | 2 days/week. Low intensity, higher repetitions (12–15). Avoid Valsalva maneuver. | 1 to 2 days/week. Very low intensity, focusing on activities of daily living. May require supervision. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipiak-Strzecka, D.; Bytyçi, I.; Bielecka-Dabrowa, A. How to Evaluate and Adjust the Recommended Level of Physical Activity in Patients with Congenital Heart Diseases? A Practical Approach. J. Clin. Med. 2025, 14, 8126. https://doi.org/10.3390/jcm14228126
Filipiak-Strzecka D, Bytyçi I, Bielecka-Dabrowa A. How to Evaluate and Adjust the Recommended Level of Physical Activity in Patients with Congenital Heart Diseases? A Practical Approach. Journal of Clinical Medicine. 2025; 14(22):8126. https://doi.org/10.3390/jcm14228126
Chicago/Turabian StyleFilipiak-Strzecka, Dominika, Ibadete Bytyçi, and Agata Bielecka-Dabrowa. 2025. "How to Evaluate and Adjust the Recommended Level of Physical Activity in Patients with Congenital Heart Diseases? A Practical Approach" Journal of Clinical Medicine 14, no. 22: 8126. https://doi.org/10.3390/jcm14228126
APA StyleFilipiak-Strzecka, D., Bytyçi, I., & Bielecka-Dabrowa, A. (2025). How to Evaluate and Adjust the Recommended Level of Physical Activity in Patients with Congenital Heart Diseases? A Practical Approach. Journal of Clinical Medicine, 14(22), 8126. https://doi.org/10.3390/jcm14228126






