Sex and Age Differences in Clinicopathological Characteristics of Gastric Cancer
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Source
2.3. Study Population
2.4. Variables
2.5. Statistical Methods
3. Results
3.1. Sex and Age Differences in Patient Characteristics
3.2. Sex and Age Differences in Tumor Characteristics and Distant Metastasis
3.3. Sex Differences in Patient and Tumor Characteristics by Age Group
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.C.; Goto, Y.; Zabaleta, J.; Morgan, D.R.; Correa, P.; Rabkin, C.S. Sex hormones, hormonal interventions, and gastric cancer risk: A meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2012, 21, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Lope, V.; Fernández de Larrea, N.; Perez-Gomez, B.; Martín, V.; Moreno, V.; Costas, L.; Longo, F.; Jiménez-Moleón, J.J.; Llorca, J.; Ascunce, N.; et al. Menstrual and Reproductive Factors and Risk of Gastric and Colorectal Cancer in Spain. PLoS ONE 2016, 11, e0164620. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Butler, L.M.; Wu, A.H.; Koh, W.P.; Jin, A.; Wang, R.; Yuan, J.M. Reproductive factors, hormone use and gastric cancer risk: The Singapore Chinese Health Study. Int. J. Cancer 2016, 138, 2837–2845. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Chow, W.H.; Gao, Y.T.; Shu, X.O.; Ji, B.T.; Yang, G.; Lubin, J.H.; Li, H.L.; Rothman, N.; Zheng, W.; et al. Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut 2007, 56, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, Z.; Wang, C.; Chen, W.; He, Y.; Zhang, C. Gender Differences in Gastric Cancer Survival: 99,922 Cases Based on the SEER Database. J. Gastrointest. Surg. 2020, 24, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.H.; Zhao, X.K.; Song, X.; Lei, L.L.; Zhong, K.; Han, W.L.; Wang, R.; De Bao, Q.; Hu, J.F.; Wei, M.X.; et al. Survival influence of gender on 42,345 patients with gastric cardia adenocarcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 5205–5217. [Google Scholar] [CrossRef] [PubMed]
- Kalff, M.C.; Wagner, A.D.; Verhoeven, R.H.; Lemmens, V.E.; van Laarhoven, H.W.; Gisbertz, S.S.; van Berge Henegouwen, M.I.; Dutch Upper GI Cancer Audit group. Sex differences in tumor characteristics, treatment, and outcomes of gastric and esophageal cancer surgery: Nationwide cohort data from the Dutch Upper GI Cancer Audit. Gastric Cancer 2022, 25, 22–32. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Heidari, S.; Babor, T.F.; De Castro, P.; Tort, S.; Curno, M. Sex and Gender Equity in Research: Rationale for the SAGER guidelines and recommended use. Res. Integr. Peer Rev. 2016, 1, 2, Erratum in Res. Integr. Peer Rev. 2024, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, A.; Halber, M.; Meyer, M.; Pflüger, M.; Eberle, A.; Nennecke, A.; Kim-Wanner, S.Z.; Hartz, T.; Weitmann, K.; Stang, A.; et al. Population-Based Clinical Cancer Registration in Germany. Cancers 2023, 15, 3934. [Google Scholar] [CrossRef] [PubMed]
- Wittekind, C. The development of the TNM classification of gastric cancer. Pathol. Int. 2015, 65, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Niu, P.; Wang, W.; Zhao, L.; Zhang, X.; Zhao, D.; Chen, Y. Sex Disparity in Patients with Gastric Cancer: A Systematic Review and Meta-Analysis. J. Oncol. 2022, 2022, 1269435. [Google Scholar] [CrossRef] [PubMed]
- Loew, A.; von Ruesten, A.; Schneider, C.; Mantke, R.; Weylandt, K.H.; Gretschel, S. Gastric Cancer in the Countryside or in the City: Does the Prognosis Change? An Analysis from the German States of Brandenburg and Berlin. Curr. Oncol. 2025, 32, 228. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lin, A.; Luo, P. Revisiting Gender Differences in Gastric Cancer Prevention with Helicobacter pylori Eradication Therapy. Gastroenterology 2025. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Plum, P.S.; Mönig, S.P.; Gockel, I.; Keller, G.; Ott, K. Gendermedizin bei Erkrankungen des oberen Gastrointestinaltrakts [Gender medicine in diseases of the upper gastrointestinal tract]. Chirurgie 2024, 95, 685–695. (In German) [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Hosaka, H.; Moki, F.; Tomaru, S.; Itoi, Y.; Sato, K.; Hashimoto, Y.; Tanaka, H.; Kuribayashi, S.; Takeuchi, Y.; et al. Gender Differences in Patients with Gastric Adenocarcinoma. J. Clin. Med. 2024, 13, 2524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
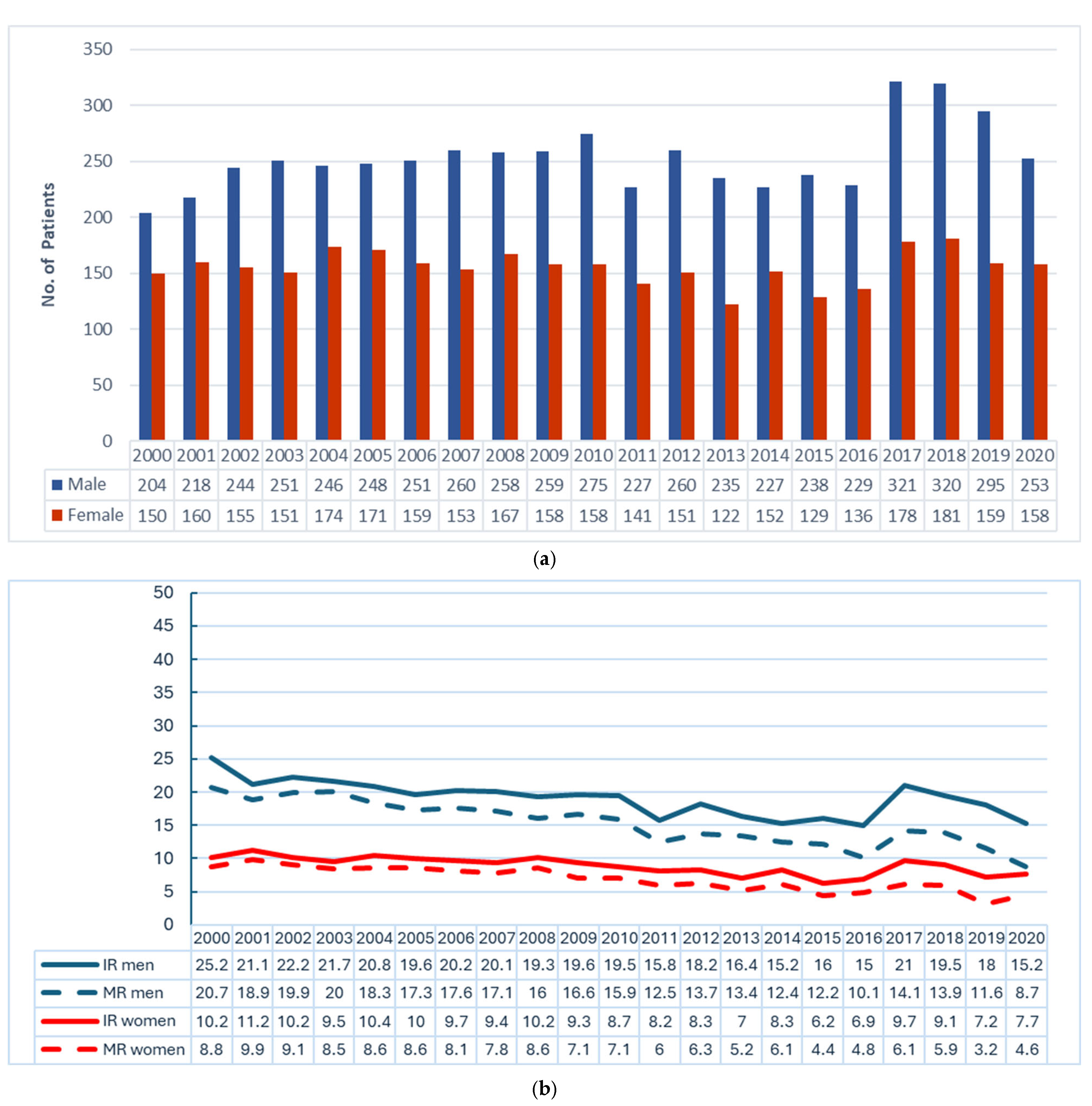

| Characteristic | Total (n = 8582) | By Sex | By Age Group (Year) | |||||
|---|---|---|---|---|---|---|---|---|
| Males (n = 5319) | Females (n = 3263) | p-Value | <45 (n = 279) | 45–60 (n = 1604) | >60 (n = 6699) | p Value | ||
| no. (%) | no. (%) | no. (%) | ||||||
| Age | <0.001 | <0.001 | ||||||
| Median (IQR) | 71.0 (62.0–78.0) | 70.0 (61.0–77.0) | 73.0 (64.0–80.0) | 40.0 (36.0–43.0) | 55.0 (50.0–58.0) | 74.0 (68.0–80.0) | ||
| Age Group (Year) | <0.001 | |||||||
| <45 | 279 (3.3) | 145 (2.7) | 134 (4.1) | - | - | - | ||
| 45–60 | 1604 (18.7) | 1100 (20.7) | 504 (15.4) | - | - | - | ||
| >60 | 6699 (78.0) | 4074 (76.6) | 2625 (80.5) | - | - | - | ||
| Previous Tumors | 0.011 | <0.001 | ||||||
| yes | 1012 (11.8) | 674 (12.7) | 338 (10.4) | 6 (2.2) | 99 (6.2) | 907 (13.5) | ||
| no | 7570 (88.2) | 4645 (87.3) | 2925 (89.6) | 273 (97.8) | 1505 (93.8) | 5792 (86.5) | ||
| ECOG | <0.001 | <0.001 | ||||||
| 0 | 1144 (22.9) | 752 (24.5) | 392 (20.2) | 59 (38.1) | 345 (36.8) | 740 (18.9) | ||
| 1 | 1614 (32.3) | 1007 (32.9) | 607 (31.3) | 63 (40.6) | 318 (33.9) | 1233 (31.5) | ||
| 2 | 1494 (29.9) | 905 (29.5) | 589 (30.4) | 26 (16.8) | 225 (24.0) | 1243 (31.8) | ||
| 3 | 599 (12.0) | 327 (10.7) | 272 (14.0) | 6 (3.9) | 41 (4.4) | 552 (14.1) | ||
| 4 | 153 (3.1) | 73 (2.4) | 80 (4.1) | 1 (0.6) | 8 (0.9) | 144 (3.7) | ||
| missing | 3578 | 2255 | 1323 | 124 | 667 | 2787 | ||
| Characteristic | Total (n = 8582) | By Sex | By Age Group (Year) | |||||
|---|---|---|---|---|---|---|---|---|
| Males (n = 5319) | Females (n = 3263) | p-Value | <45 (n = 279) | 45–60 (n = 1604) | >60 (n = 6699) | p Value | ||
| no. (%) | no. (%) | no. (%) | ||||||
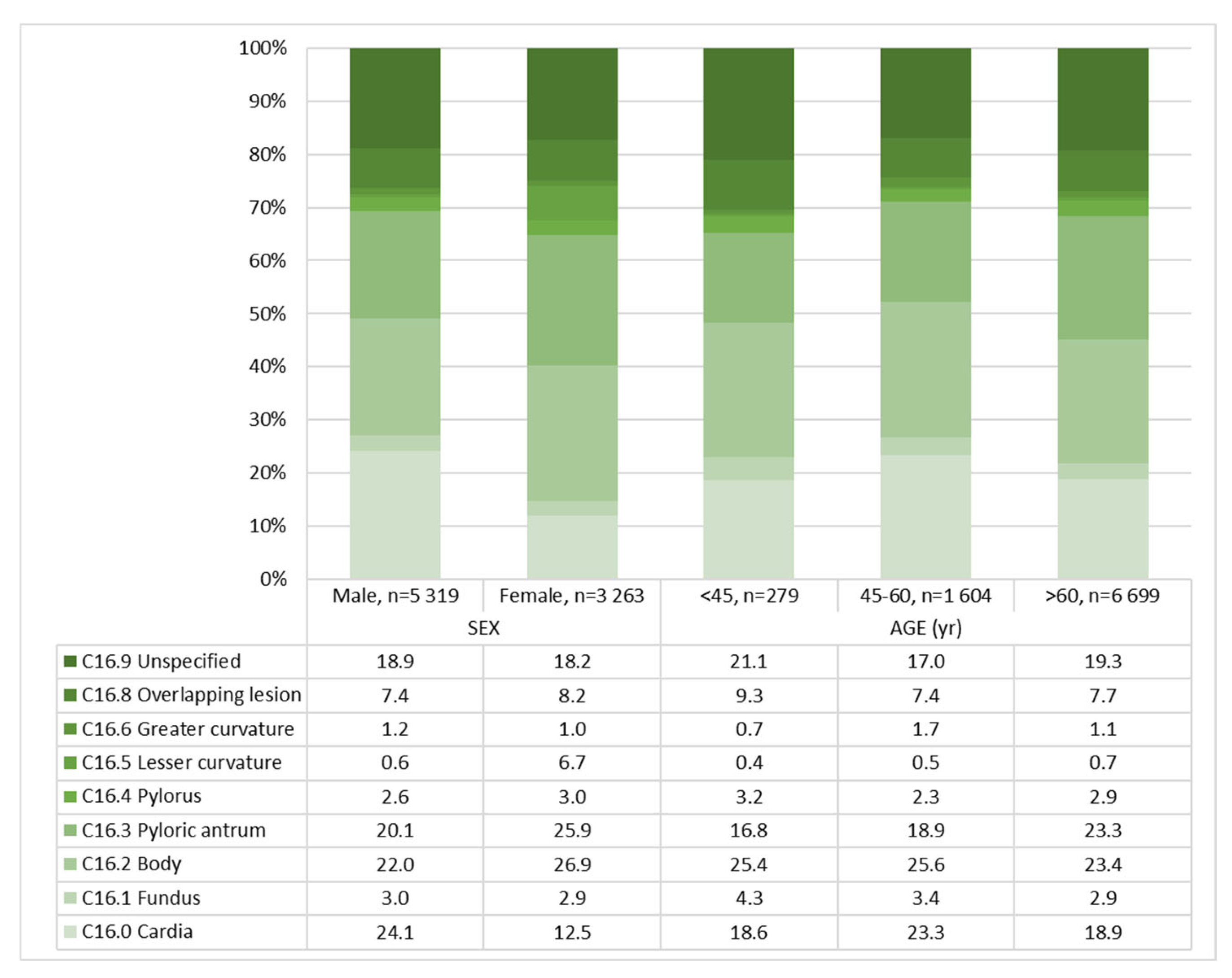
| Location | <0.001 | <0.001 | ||||||
| C16.0 Cardia | 1692 (19.7) | 1284 (24.1) | 408 (12.5) | 52 (18.6) | 374 (23.3) | 1266 (18.9) | ||
| C16.1 Fundus | 257 (3.0) | 162 (3.0) | 95 (2.9) | 12 (4.3) | 54 (3.4) | 191 (2.9) | ||
| C16.2 Body | 2048 (23.9) | 1170 (22.0) | 878 (26.9) | 71 (25.4) | 411 (25.6) | 1566 (23.4) | ||
| C16.3 Pyloric antrum | 1913 (22.2) | 1069 (20.1) | 844 (25.9) | 47 (16.8) | 303 (18.9) | 1563 (23.3) | ||
| C16.4 Pylorus | 238 (2.8) | 139 (2.6) | 99 (3.0) | 9 (3.2) | 37 (2.3) | 192 (2.9) | ||
| C16.5 Lesser curvature | 55 (0.6) | 33 (0.6) | 22 (6.7) | 1 (0.4) | 8 (0.5) | 46 (0.7) | ||
| C16.6 Greater curvature | 100 (1.2) | 66 (1.2) | 34 (1.0) | 2 (0.7) | 27 (1.7) | 71 (1.1) | ||
| C16.8 Overlapping lesion | 658 (7.7) | 392 (7.4) | 266 (8.2) | 26 (9.3) | 118 (7.4) | 514 (7.7) | ||
| C16.9 Unspecified | 1621 (18.9) | 1004 (18.9) | 616 (18.2) | 59 (21.1) | 272 (17.0) | 1290 (19.3) | ||
| Histology | <0.001 | <0.001 | ||||||
| Adenocarcinoma, n. specified | 4041 (47.1) | 2677 (50.3) | 1364 (41.8) | 81 (29.0) | 681 (42.5) | 3279 (48.9) | ||
| Intestinal | 1547 (18.0) | 1028 (19.3) | 519 (15.9) | 31 (11.1) | 236 (14.7) | 1280 (19.1) | ||
| Diffuse | 845 (9.8) | 456 (8.6) | 389 (11.9) | 53 (19.0) | 200 (12.5) | 592 (8.8) | ||
| Tubular | 384 (4.5) | 257 (4.8) | 127 (3.9) | 9 (3.2) | 36 (2.2) | 339 (5.1) | ||
| Papillary | 26 (0.3) | 18 (0.3) | 8 (0.2) | 0 (0.0) | 6 (0.4) | 20 (0.3) | ||
| Signet cell carcinoma | 1440 (16.8) | 700 (13.2) | 744 (22.8) | 90 (32.3) | 387 (24.1) | 967 (14.4) | ||
| Mixed adenocarcinoma | 53 (0.6) | 32 (0.6) | 21 (0.6) | 0 (0.0) | 11 (0.7) | 42 (0.6) | ||
| Other | 242 (2.8) | 151 (2.8) | 91 (2.8) | 15 (5.4) | 47 (2.9) | 180 (2.7) | ||
| Grading | <0.001 | <0.001 | ||||||
| G1 well differentiated | 415 (5.2) | 280 (5.6) | 135 (4.4) | 4 (1.5) | 50 (3.4) | 361 (5.8) | ||
| G2 moderately differentiated | 2461 (30.7) | 1666 (33.5) | 795 (26.2) | 43 (16.5) | 345 (23.2) | 2073 (33.1) | ||
| G3 poorly differentiated | 4671 (58.3) | 2757 (55.4) | 1914 (63.1) | 186 (71.3) | 987 (66.4) | 3498 (55.8) | ||
| G4 undifferentiated | 134 (1.7) | 66 (1.3) | 68 (2.2) | 10 (3.8) | 37 (2.5) | 87 (1.4) | ||
| GX cannot be assessed | 330 (4.1) | 208 (4.2) | 122 (4.0) | 18 (6.9) | 67 (4.5) | 245 (3.9) | ||
| missing | 571 | 342 | 229 | 18 | 118 | 435 | ||
| cUICC | 0.001 | <0.001 | ||||||
| I | 789 (16.8) | 490 (16.6) | 299 (17.2) | 21 (14.2) | 112 (11.2) | 656 (18.5) | ||
| II | 893 (19.0) | 568 (19.2) | 325 (18.7) | 26 (17.6) | 202 (20.2) | 665 (18.8) | ||
| III | 763 (16.3) | 526 (17.8) | 237 (13.7) | 23 (15.5) | 185 (18.5) | 555 (15.7) | ||
| IV | 2246 (47.9) | 1371 (46.4) | 875 (50.4) | 78 (52.7) | 502 (50.1) | 1666 (47.0) | ||
| Missing | 3891 | 2364 | 1527 | 131 | 603 | 3157 | ||
| Characteristic | Total (n = 3656) | By Sex | By Age Group (Year) | |||||
|---|---|---|---|---|---|---|---|---|
| Males (n = 2278) | Females (n = 1378) | p-Value | <45 (n = 167) | 45–60 (n = 859) | >60 (n = 2630) | p Value | ||
| no. (%) | no. (%) | no. (%) | ||||||
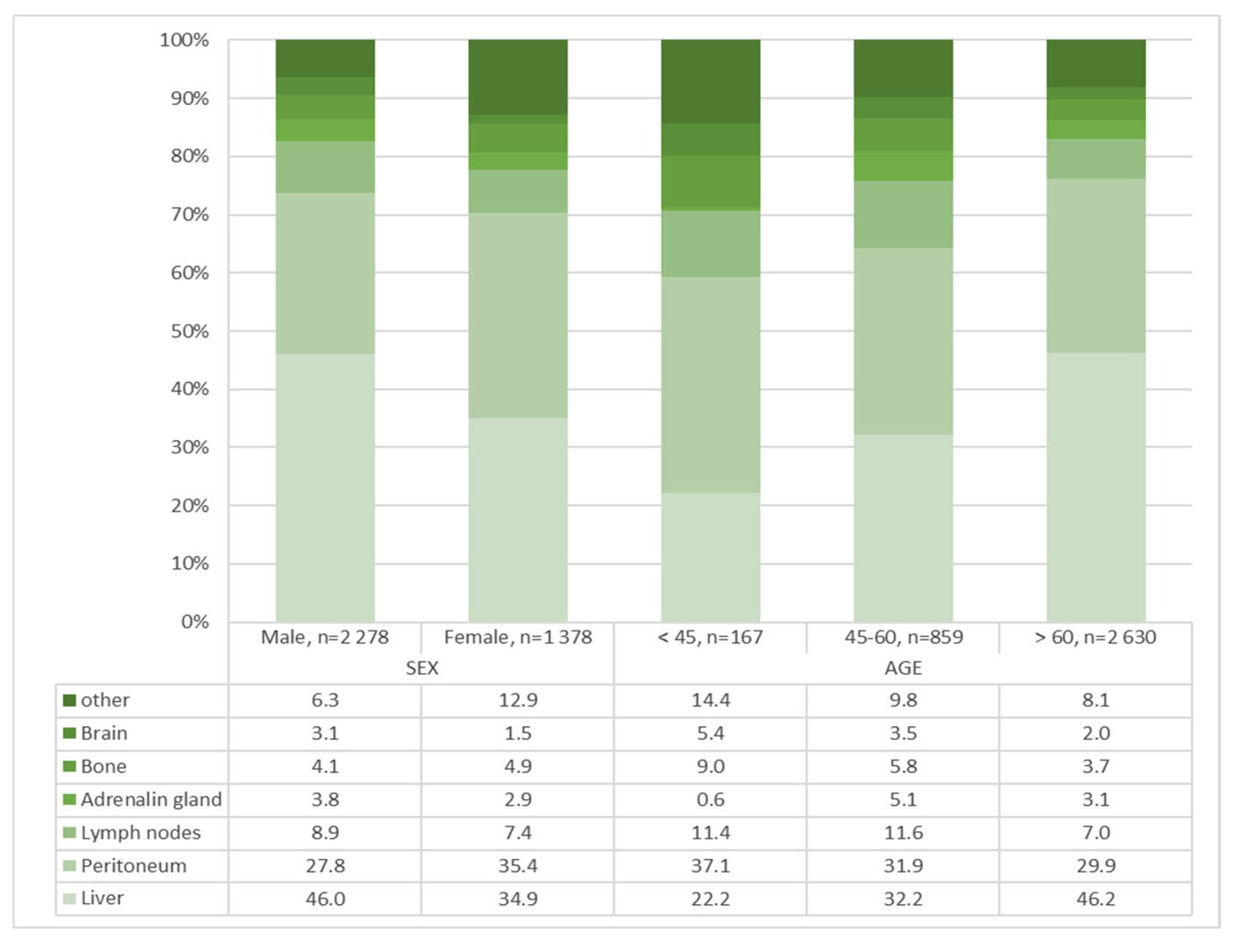
| Distant metastasis | <0.001 | <0.001 | ||||||
| Liver | 1530 (41.8) | 1049 (46.0) | 481 (34.9) | 37 (22.2) | 277 (32.2) | 1216 (46.2) | ||
| Peritoneum | 1122 (30.7) | 634 (27.8) | 488 (35.4) | 62 (37.1) | 274 (31.9) | 786 (29.9) | ||
| Lymph node | 304 (8.3) | 202 (8.9) | 102 (7.4 | 19 (11.4) | 100 (11.6) | 185 (7.0) | ||
| Bone | 162 (4.4) | 94 (4.1) | 68 (4.9) | 15 (9.0) | 50 (5.8) | 97 (3.7) | ||
| Adrenal gland | 126 (3.4) | 86 (3.8) | 40 (2.9) | 1 (0.6) | 44 (5.1) | 81 (3.1) | ||
| Brain | 91 (2.5) | 70 (3.1) | 21 (1.5) | 9 (5.4) | 30 (3.5) | 52 (2.0) | ||
| Other | 321 (8.8) | 143 (6.3) | 178 (12.9) | 24 (14.4) | 84 (9.8) | 213 (8.1) | ||
| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Total (n = 4037) | By Sex | By Age Group (Year) | |||||
| Males (n = 2439) | Females (n = 1598) | p-Value | <45 (n = 174) | 45–60 (n = 755) | >60 (n = 3108) | p Value | ||
| no. (%) | no. (%) | no. (%) | ||||||
| cT Category | 0.304 | 0.156 | ||||||
| T0: No evidence of tumor | 1 (0.1) | 0 (0.0) | 1 (0.2) | 0 (0) | 1 (0.3) | 0 (0) | ||
| Tis: In situ | 3 (0.2) | 2 (0.2) | 1 (0.2) | 0 (0.) | 1 (0.3) | 2 (0.2) | ||
| T1: Lamina propria or submucosa | 183 (11.3) | 110 (11.2) | 73 (11.4) | 7 (10.0) | 23 (6.8) | 153 (12.6) | ||
| T2: Muscularis propria or subserosa | 242 (14.9) | 146 (14.9) | 96 (14.9) | 8 (11.4) | 52 (15.3) | 182 (15.0) | ||
| T3: Serosa | 466 (28.7) | 301 (30.7) | 165 (25.7) | 22 (31.4) | 106 (31.2) | 338 (27.8) | ||
| T4: Adjacent structures | 334 (20.6) | 190 (19.4) | 144 (22.4) | 11 (15.7) | 75 (22.1) | 248 (20.4) | ||
| TX: Cannot be assessed | 395 (24.3) | 232 (23.6) | 163 (25.3) | 22 (31.4) | 82 (24.1) | 291 (24.0) | ||
| Missing | 2413 | 1458 | 955 | 104 | 415 | 1894 | ||
| cN Category | 0.2 | 0.019 | ||||||
| N0: No met. in regional lymph nodes | 307 (18.9) | 195 (19.9) | 112 (17.4) | 11 (15.9) | 60 (17.6) | 236 (19.4) | ||
| N1: 1 to 6 regional lymph nodes | 407 (25.1) | 255 (26.0) | 152 (23.6) | 16 (23.2) | 100 (29.3) | 291 (24.0) | ||
| N2: 7 to 15 regional lymph nodes | 170 (10.5) | 106 (10.8) | 64 (10.0) | 4 (5.8) | 40 (11.7) | 126 (10.4) | ||
| N3: More than 15 regional lymph nodes | 61 (3.8) | 31 (3.2) | 30 (4.7) | 7 (10.1) | 16 (4.7) | 38 (3.1) | ||
| NX: Cannot be assessed | 679 (41.8) | 394 (40.2) | 285 (44.3) | 31 (44.9) | 125 (36.7) | 523 (43.1) | ||
| Missing | 2413 | 1458 | 955 | 105 | 414 | 1894 | ||
| cM Category | 0.002 | <0.015 | ||||||
| M0: No distant metastasis | 568 (34.6) | 375 (37.9) | 193 (29.6) | 20 (27.8) | 133 (38.8) | 415 (33.8) | ||
| M1: Distant metastasis | 843 (51.3) | 487 (49.2) | 356 (54.6) | 44 (61.1) | 178 (51.9) | 621 (50.6) | ||
| MX: Cannot be assessed | 231 (14.1) | 128 (12.9) | 103 (15.8) | 8 (11.1) | 32. (9.3) | 191 (15.6) | ||
| Missing | 2395 | 1449 | 946 | 102 | 412 | 1881 | ||
| (b) | ||||||||
| Characteristic | Total (n = 4545) | By Sex | By Age Group (Year) | |||||
| Males (n = 2880) | Females (n = 1665) | p-Value | <45 (n = 105) | 45–60 (n = 849) | >60 (n = 3591) | pValue | ||
| no. (%) | no. (%) | no. (%) | ||||||
| cT Category | 0.9 | 0.010 | ||||||
| T0: No evidence of tumor | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Tis: In situ | 8 (0.3) | 5 (0.2) | 3 (0.3) | 0 (0) | 0 (0) | 8 (0.3) | ||
| T1: Lamina propria or submucosa | 320 (10.1) | 205 (10.1) | 115 (10.1) | 6 (7.3) | 38 (5.7) | 276 (11.5) | ||
| T2: Muscularis propria or subserosa | 427 (13.5) | 271 (13.4) | 156 (13.8) | 9 (11.0) | 97 (14.5) | 321 (13.3) | ||
| T3: Serosa | 1228 (38.9) | 802 (39.6) | 426 (37.6) | 37 (45.1) | 278 (41.4) | 913 (37.9) | ||
| T4: Adjacent structures | 619 (19.6) | 393 (19.4) | 226 (19.9) | 17 (20.7) | 151 (22.5) | 451 (18.7) | ||
| TX: Cannot be assessed | 557 (17.6) | 349 (17.2) | 208 (18.3) | 13 (15.9) | 107 (15.9) | 437 (18.2) | ||
| Missing | 1386 | 855 | 531 | 23 | 178 | 1185 | ||
| cN Category | 0.014 | <0.001 | ||||||
| N0: No met. in regional lymph nodes | 865 (27.7) | 554 (27.6) | 311 (27.8) | 21 (26.6) | 139 (20.9) | 705 (29.6) | ||
| N1: 1 to 6 regional lymph nodes | 1086 (34.8) | 714 (35.6) | 372 (33.3) | 30 (38.0) | 272 (41.0) | 784 (32.9) | ||
| N2: 7 to 15 regional lymph nodes | 340 (10.9) | 235 (11.7) | 105 (9.4) | 10 (12.7) | 79 (11.9) | 251 (10.5) | ||
| N3: More than 15 regional lymph nodes | 187 (6.0) | 122 (6.1) | 65 (5.8) | 5 (6.3) | 44(6.6) | 138(5.8) | ||
| NX: Cannot be assessed | 646 (20.7) | 381 (19.0) | 265 (23.7) | 13 (16.5) | 130 (19.6) | 503 (21.1) | ||
| Missing | 1421 | 874 | 547 | 26 | 185 | 1210 | ||
| cM Category | 0.5 | 0.663 | ||||||
| M0: No distant metastasis | 1782 (55.9) | 1158.0 (56.5) | 624 (54.6) | 47 (56.6) | 358 (53.2) | 1377 (56.6) | ||
| M1: Distant metastasis | 1278 (40.1) | 806 (39.4) | 472 (41.3) | 33 (39.8) | 287 (42.6) | 958 (39.4) | ||
| MX: Cannot be assessed | 130 (4.1) | 84 (4.1) | 46 (4.0) | 3 (3.6) | 28 (4.2) | 99 (4.1) | ||
| Missing | 1355 | 832 | 523 | 22 | 176 | 1157 | ||
| Characteristic | Age < 45 Years | Age 45 to 59 Years | Age 60 Years and Older | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Males (n = 145) | Females (n = 134) | p-Value | Males (n = 1100) | Females (n = 504) | p-Value | Males (n = 4074) | Females (n = 2625) | p-Value | |
| Location | <0.001 | <0.001 | <0.001 | ||||||
| C16 Cardia | 41 (28.3) | 11 (8.2) | 317 (28.8) | 57 (11.3) | 926 (22.7) | 340 (13.0) | |||
| C16.1 Fundus | 6 (4.1) | 6 (4.5) | 38 (3.5) | 16 (3.2) | 118 (2.9) | 73 (2.8) | |||
| C16.2 Body | 33 (22.8) | 38 (28.4) | 241 (21.9) | 170 (33.7) | 896 (22) | 670 (25.5) | |||
| C16.3 Pyloric antrum | 22 (15.2) | 25 (18.7) | 200 (18.2) | 103 (20.4) | 847 (20.8) | 716 (27.3) | |||
| C16.4 Pylorus | 6 (4.1) | 3 (2.2) | 29 (2.6) | 8 (1.6) | 104 (2.6) | 88 (3.4) | |||
| C16.5 Lesser curvature | 0 (0) | 1 (0.7) | 5 (0.5) | 3 (0.6) | 28 (0.7) | 18 (0.7) | |||
| C16.6 Greater curvature | 0 (0) | 2 (1.5) | 21 (1.9) | 6 (1.2) | 45 (1.1) | 26 (1.0) | |||
| C16.8 Overlapping lesion | 11 (7.6) | 15 (11.2) | 76 (6.9) | 42 (8.3) | 305 (7.5) | 209 (8.0) | |||
| C16.9 Unspecified | 26 (17.9) | 33 (24.6) | 173 (15.7) | 99 (19.6) | 805 (19.8) | 485 (18.5) | |||
| Histology | 0.018 | <0.001 | <0.001 | ||||||
| Adenocarcinoma, n. specified | 45 (31) | 36 (26.9) | 506 (46) | 175 (34.7) | 2126 (52.2) | 1153 (43.9) | |||
| Intestinal | 21 (14.5) | 10 (7.5) | 194 (17.6) | 42 (8.3) | 813 (20.0) | 467 (17.8) | |||
| Diffuse | 23 (15.9) | 30 (22.4) | 120 (10.9) | 80 (15.9) | 313 (7.7) | 279 (10.6) | |||
| Tubular | 7 (4.8) | 2 (1.5) | 31 (2.8) | 5 (1.0) | 219 (5.4) | 120 (4.6) | |||
| Papillary | 0 (0) | 0 (0) | 5 (0.5) | 1 (0.2) | 13 (0.3) | 7 (0.3) | |||
| Signet cell carcinoma | 38 (26.2) | 52 (38.8) | 205 (18.6) | 182 (36.1) | 457 (11.2) | 510 (19.4) | |||
| Mixed adenocarcinoma | 0 (0) | 0 (0) | 6 (0.5) | 5 (1.0) | 26 (0.6) | 16 (0.6) | |||
| Other | 11 (7.6) | 4 (3.0) | 33 (3.0) | 14 (2.8) | 107 (2.6) | 73 (2.8) | |||
| Grading | <0.001 | <0.001 | <0.001 | ||||||
| G1 well differentiated | 4 (2.9) | 0 (0) | 42 (4.1) | 8 (1.7) | 234 (6.1) | 127 (5.2) | |||
| G2 moderately differentiated | 33 (24.1) | 10 (8.1) | 280 (27.3) | 65 (14.1) | 1353 (35.5) | 720 (29.4) | |||
| G3 poorly differentiated | 84 (61.3) | 102 (82.3) | 635 (62.0) | 352 (76.2) | 2038 (53.4) | 1460 (59.6) | |||
| G4 undifferentiated | 5 (3.6) | 5 (4.0) | 21 (2.1) | 16 (3.5) | 40 (1.0) | 47 (1.9) | |||
| GX cannot be assessed | 11 (8.0) | 7 (5.6) | 46 (4.5) | 21 (4.5) | 151 (4.0) | 94 (3.8) | |||
| Missing | 8 | 10 | 76 | 42 | 258 | 177 | |||
| cUICC | 0.2 | 0.2 | 0.033 | ||||||
| I | 9 (11.8) | 12 (16.7) | 77 (11.2) | 35 (11.2) | 404 (18.4) | 252 (18.6) | |||
| II | 14 (18.4) | 12 (16.7) | 140 (20.3) | 62 (19.9) | 414 (18.9) | 251 (18.6) | |||
| III | 16 (21.1) | 7 (9.7) | 138 (20.0) | 47 (15.1) | 372 (17.0) | 183 (13.5) | |||
| IV | 37 (48.7) | 41 (56.9) | 334 (48.5) | 168 (53.8) | 1000 (45.7) | 666 (49.3) | |||
| Missing | 69 | 62 | 411 | 192 | 1884 | 1273 | |||
| Characteristic | Age < 45 Years | Age 45 to 59 Years | Age 60 Years and Older | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Males (n = 79) | Females (n = 88) | p-Value | Males (n = 576) | Females (n = 283) | p-Value | Males (n = 1623) | Females (n = 1007) | p-Value | |
| no. (%) | no. (%) | no. (%) | |||||||
| Distant Metastasis | 0.072 | <0.001 | <0.001 | ||||||
| Liver | 20 (25.3) | 17 (19.3) | 220 (38.2) | 57 (20.1) | 809 (49.8) | 407 (40.4) | |||
| Peritoneum | 24 (30.4) | 38 (43.2) | 171 (29.7) | 103 (36.4) | 439 (27.0) | 347 (34.5) | |||
| Lymph node | 14 (17.7) | 5 (5.7) | 70 (12.2) | 30 (10.6) | 118 (7.3) | 67 (6.7) | |||
| Bone | 7 (8.9) | 8 (9.1) | 28 (4.9) | 22 (7.8) | 59 (3.6) | 38 (3.8) | |||
| Adrenal gland | 1 (1.3) | 0 (0.0) | 31 (5.4) | 13 (4.6) | 54 (3.3) | 27 (2.7) | |||
| Brain | 5 (6.3) | 4 (4.5) | 21 (3.6) | 9 (3.2) | 44 (2.7) | 8 (0.8) | |||
| Other | 8 10.1) | 16 (18.2) | 35 (6.1) | 49 (17.3) | 100 (6.2) | 113 (11.2) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schildberg, C.; Weber, U.; Dietrich, N.; Seeland, U.; Mantke, R. Sex and Age Differences in Clinicopathological Characteristics of Gastric Cancer. J. Clin. Med. 2025, 14, 7894. https://doi.org/10.3390/jcm14227894
Schildberg C, Weber U, Dietrich N, Seeland U, Mantke R. Sex and Age Differences in Clinicopathological Characteristics of Gastric Cancer. Journal of Clinical Medicine. 2025; 14(22):7894. https://doi.org/10.3390/jcm14227894
Chicago/Turabian StyleSchildberg, Claus, Ulrike Weber, Nina Dietrich, Ute Seeland, and René Mantke. 2025. "Sex and Age Differences in Clinicopathological Characteristics of Gastric Cancer" Journal of Clinical Medicine 14, no. 22: 7894. https://doi.org/10.3390/jcm14227894
APA StyleSchildberg, C., Weber, U., Dietrich, N., Seeland, U., & Mantke, R. (2025). Sex and Age Differences in Clinicopathological Characteristics of Gastric Cancer. Journal of Clinical Medicine, 14(22), 7894. https://doi.org/10.3390/jcm14227894





