Abstract
Background/Objectives: Children with cerebral palsy (CP) commonly present impairments in gross motor function and limitations in activities of daily living (ADLs), which negatively impact independence and quality of life. Identifying effective rehabilitation strategies is essential to promote functional development. To evaluate the effectiveness of occupational therapy (OT) interventions on gross motor function and independence in ADLs among children with CP. Methods: Seven electronic databases were searched through August 2025. The review protocol was registered in PROSPERO (CRD42025634706) and conducted in accordance with PRISMA guidelines. Methodological quality and certainty of evidence were assessed using the Oxford Centre for Evidence-Based Medicine scale, the Risk of Bias 2 (RoB 2) tool, and GRADEpro. Randomized controlled trials reporting OT interventions targeting gross motor and ADL outcomes were included. Results: Of 594 identified records, 14 studies met the inclusion criteria. Meta-analysis indicated that OT interventions significantly improved gross motor function (GMFM-66; ES = 0.32 [0.01–0.63], p = 0.04), mobility (PEDI-Mobility; ES = 0.46 [0.05–0.87], p = 0.02), and occupational performance (COPM-Performance; ES = 2.63 [1.14–4.11], p = 0.001) and satisfaction (COPM-Satisfaction; ES = 2.17 [0.82–3.51], p = 0.002). No significant changes were observed in self-care (PEDI-Self-Care; ES = 0.19 [−0.14–0.53], p = 0.26). Conclusions: Evidence suggests that OT interventions effectively enhance gross motor function, mobility, and occupational performance in children with CP. These results support the integration of OT within pediatric rehabilitation programs to optimize functional outcomes.
1. Introduction
Cerebral palsy (CP) represents the leading cause of physical disability in childhood, with a global prevalence estimated at roughly 2–3 cases per 1000 live births [1]. It is characterized by impairments in movement and postural control, often accompanied by disturbances in balance [2]. The earlier belief that cerebral asphyxia was the predominant cause of CP has been replaced by evidence supporting a complex, multifactorial origin that includes prenatal, perinatal, and postnatal risk factors [3].
Advancements in the classification of CP now allow for categorization based on motor type, functional severity, topographical distribution, and underlying etiology [4,5]. Clinical manifestations evolve over time but are typically evident between the ages of 3 and 5 years [5]. Management requires a multidisciplinary approach, integrating neurological rehabilitation with the treatment of comorbidities such as epilepsy, and sensory, cognitive, and gastrointestinal disorders, necessitating the involvement of a wide range of healthcare professionals [6].
Given that CP is categorized by impairments in gross motor performance, postural control, hand dexterity, communication, and progressive musculoskeletal limitations, these deficits lead to functional dependence and restrictions in activities of daily living (ADLs) [6]. Intervention outcomes are frequently evaluated through standardized classification frameworks such as the Manual Ability Classification System (MACS), the Gross Motor Function Classification System (GMFCS), and the Communication Function Classification System (CFCS) [7]. These tools demonstrate strong psychometric properties. The GMFCS demonstrates high inter- and intra-rater reliability [8], while the MACS reports intra-rater reliability of 0.97 and inter-rater reliability of 0.94 (95% CI: 0.89–0.97) [9,10]. Evidence of construct validity has been demonstrated through strong inter-scale correlations, specifically between GMFCS and MACS, and between MACS and CFCS; indicating a consistent relationship among motor, manual, and communication domains [11,12].
Measurement stability is confirmed by low standard error of measurement (SEM) values for the GMFCS and MACS, although the small effect sizes suggest limited sensitivity to detect clinically significant changes post-intervention [11,12]. Moreover, results from the Egger’s test suggested the presence of publication bias in studies involving the MACS (p = 0.041) [13]. Gross motor performance is often quantified with the Gross Motor Function Measure-88 (GMFM-88) [14], and self-care or daily activity independence is typically examined through the WeeFIM scale [15].
Early diagnosis of CP is fundamental to assessing therapeutic effectiveness and fostering favorable developmental outcomes [16]. Evidence-based motor interventions, such as constraint-induced movement therapy (CIMT), have resulted in measurable improvements in unimanual and bimanual motor control [17]. Additionally, enriched environments have been shown to facilitate motor and cognitive development [18]. Targeted therapies like hippotherapy enhance gross motor skills and functional independence [19], while neurodevelopmental therapy supports both neural and motor maturation [20]. Moreover, virtual reality-based interventions have shown promising results compared with conventional rehabilitation methods in improving motor outcomes [21]. However, few reviews have examined these variables comprehensively [22].
Occupational therapy (OT) contributes significantly to the management of CP by encouraging participation in meaningful daily activities that promote overall health and well-being [23]. In children with CP, OT focuses on enhancing skills necessary for ADLs, promoting independence, and improving quality of life [24]. OT is defined as a health profession aimed at enabling participation in activities that support health and well-being. It addresses physical, emotional, social, and environmental factors that influence occupational performance [25]. In pediatric populations, evidence-based OT interventions include bimanual training, CIMT, goal-directed programs, early intervention, assistive technology, and virtual rehabilitation [16]. These strategies prioritize functional outcomes and emphasize collaboration with caregivers to enhance the child’s autonomy and engagement in meaningful activities [26].
Therefore, the objective of this systematic review and meta-analysis was to evaluate the effectiveness of occupational therapy-based intervention on gross motor function and ADL independence in children with cerebral palsy.
2. Materials and Methods
2.1. Protocol and Registration
The present systematic review with meta-analysis adhered to Cochrane methodological standards [27] and complied with PRISMA reporting requirements [28] (Table S1). The protocol was registered in PROSPERO (CRD42025634706) on 17 January 2025.
2.2. Eligibility Criteria
Eligible studies comprised randomized controlled trials (RCTs) published from January 2024 through August 2025, without restrictions regarding language or publication status. Non-randomized designs, descriptive reports, reviews, and non-peer-reviewed sources were excluded. The inclusion process was structured using the PICOS framework (Population, Intervention, Comparator, Outcome, Study Design), as presented in Table 1.

Table 1.
Selection criteria used in the systematic review.
2.3. Information Search Process and Database
Data sources included seven databases: PubMed (National Center for Biotechnology Information), Web of Science Core Collection, Scopus, EBSCOhost, ProQuest, Cochrane Library, and CINAHL. The search terms included: (“Cerebral Palsy” OR “Ataxic” OR “Cerebral Palsy Spastic” OR “Diplegic Cerebral Palsy” OR “Spastic Quadriplegic” OR “dystonic cerebral palsy”) AND (“Motor skills” OR “Gross Motor Skills” OR “Motor Coordination” OR “Large Muscle Movement” OR “Gross Motor Abilities” OR “Motor Control” OR “Body Movement Coordination” OR “Postural Control” OR “Physical Coordination” OR “Movement Efficiency” OR “Motor Proficiency” OR “Locomotor Skills”) AND (“Occupational Therapy” OR “Occupational Therapy Interventions” OR “Occupational Therapist”). Validation of the study selection and eligibility criteria was performed by an independent expert to confirm the relevance of the included publications. The independent expert met two criteria: holding a PhD in health sciences and having published peer-reviewed research in Journal Citation Reports®-indexed journals on occupational therapy-based intervention addressing motor function and ADL performance. To minimize bias, the approach used in the search was not revealed to him. A follow-up search was conducted within the databases to detect any retractions or corrections related to the studies included in the review.
2.4. Study Selection Process and Data Collection
Studies were organized and managed using Mendeley Reference Manager (version 2.116.1). The search process was independently undertaken by two authors (D.F.-C. and E.V.-C.) to minimize selection bias, assessing titles, abstracts, and full texts and removing duplicates. No discrepancies were detected during this review. Articles deemed eligible were further examined in detail, and reasons for excluding records that did not satisfy the inclusion criteria were documented and reported in the PRISMA flow diagram. A third reviewer (P.V.-B.) independently audited the entire selection and data extraction process.
2.5. Methodological Quality Assessment
The quality appraisal and evidence grading of the included studies followed the standards established by the Oxford Centre for Evidence-Based Medicine scale [29]. Inclusion was restricted to level 1a studies, corresponding to RCTs, with all lower evidence levels (1b–5) excluded from consideration. In addition, when methodological limitations such as bias risk, inconsistency, imprecision, lack of clarity, or suspected publication bias were identified, RCTs were reappraised and assigned a lower evidence level accordingly [29].
2.6. Data Collection Process
For each study included in the review, relevant information was extracted using a customized data collection form developed in alignment with Cochrane Collaboration standards [30]. This process was carried out using Microsoft Excel® (version 16.81). Two researchers (D.F.-C. and E.V.-C.) independently performed the extraction and compared their results to ensure accuracy. A third reviewer (P.V.-B.) supervised the entire procedure. Data extraction included bibliographic (title, authors, year, and country) and methodological details (design, evidence level, and bias assessment), participant and sampling characteristics, eligibility criteria, study context, intervention and control descriptions, and outcomes related to gross motor performance, ADL independence, and overall study conclusions.
2.7. Risk of Bias
To assess the risk of bias in the selected studies, the Cochrane tool for RCTs (RoB 2.0) was used [27]. This approach covered five key aspects: randomization, deviations in the intervention, incomplete outcome data, outcome assessment and selecting reported outcomes. The analysis was carried out collaboratively by 2 authors (D.F.-C. and E.V.-C.) and subsequently verified by an independent reviewer (P.V.-B.).
2.8. Meta-Analysis Measures
The study employed a meta-analysis approach, with the methodology registered in PROSPERO (CRD42025634706). Effect sizes were expressed as standardized mean differences (SMDs), computed for each randomized controlled trial comparison to determine mean group differences using Review Manager software (RevMan 5.4; The Cochrane Collaboration, London, UK). To account for small-sample bias, effect sizes were expressed as Hedges’ g, a corrected form of the standardized mean difference. A significance level of p < 0.05 was adopted for all analyses [31]. A random-effects model, using the Der Simonian-Laird method, was applied to estimate and combine the SMD and mean differences across outcomes, such as gross motor function and ADLs, comparing experimental and control groups before and after the intervention [32]. This model assumes variation in true intervention effects across studies due to factors like intervention type or duration, reflecting diverse effect sizes among populations. Results were pooled when at least three studies provided consistent findings [33]. Heterogeneity across studies was evaluated using the Cochrane Q test [34] and the I2 statistic, with values below 25%, between 25 and 49%, and above 50% representing low, moderate, and high heterogeneity, respectively [32]. In addition, Egger’s regression test was applied to identify potential small-study effects and publication bias [35].
2.9. Certainty of Evidence
The GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system was applied to assess the certainty and methodological robustness of the evidence across the included studies [27,36], classifying the levels of evidence as high, moderate, low, or very low. Since all studies were RCTs, a high level of certainty was used as the starting point, which was subsequently adjusted if problems such as risk of bias, lack of consistency, imprecision, lack of accuracy, opacity in results, or publication bias were identified. Two authors (D.F.-C. and E.V.-C.) carried out this assessment independently, and any differences in the results were resolved by consensus with the participation of a third author (P.V.-B.).
3. Results
3.1. Study Selection
The database search yielded 594 studies in total, identified from PubMed, ProQuest, Scopus, Web of Science, Cochrane Library, and EBSCOhost CINAHL. Following the exclusion of six duplicates, 588 studies remained for the screening process. Of these, 552 were excluded based on title and abstract review for not meeting the eligibility criteria, leaving 36 articles for full-text assessment. Following a thorough evaluation, 22 articles were excluded for the following reasons: 12 lacked a complete focus on the topic, 4 addressed unrelated subjects, and 6 did not meet the required study design criteria. Ultimately, 14 studies were retained for inclusion in the review [16,37,38,39,40,41,42,43,44,45,46,47,48,49], providing evidence on the efficacy of interventions to improve gross motor abilities and support independence in ADLs for children with CP. The selection pathway is presented in Figure 1.
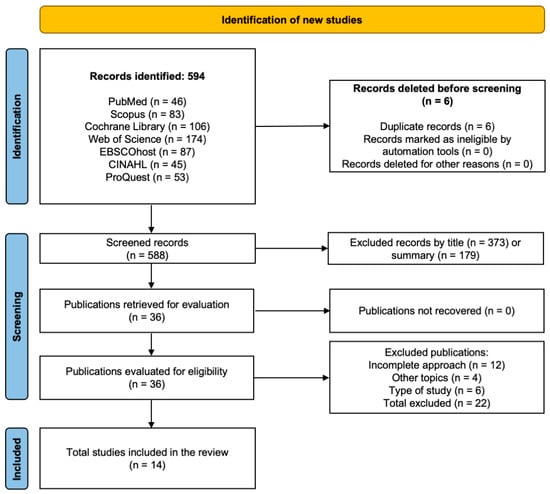
Figure 1.
PRISMA flow diagram illustrating the study selection process for the systematic review.
3.2. Methodological Quality
This systematic review and meta-analysis comprised studies of high methodological quality, all of which were RCTs [16,37,38,39,40,41,42,43,44,45,46,47,48,49], corresponding to level 1a evidence according to the Oxford classification.
3.3. Risk of Bias
The risk of bias assessment identified one study with low risk [42], twelve with some concerns [16,37,38,39,41,43,44,45,46,47,48,49], and one with high risk [40]. These results collectively suggest a moderate concern for bias across the reviewed evidence. Figure 2 and Figure 3 present a visual overview of these findings.
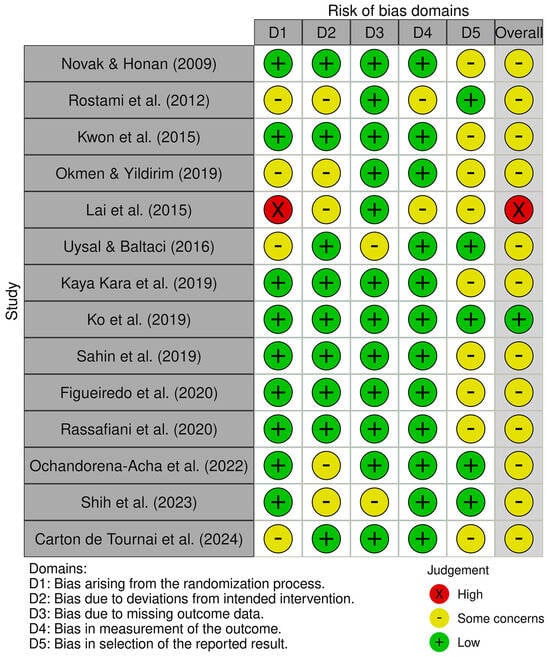
Figure 2.
Risk of bias tool: traffic light chart [26,37,38,39,40,41,42,43,44,45,46,47,48,49].
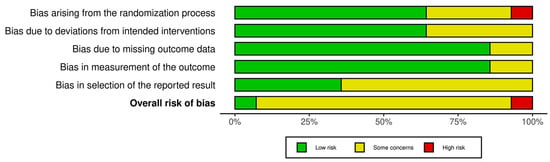
Figure 3.
Risk of bias tool: summary table by domain.
3.4. Characteristics of the Studies
Of the 14 studies reviewed, various interventions demonstrated positive effects on gross motor function and ADLs in children with CP. Virtual reality interventions, like Kinect, were particularly effective for motor function and independence [39,48]. Game-based interventions, such as the Nintendo Wii™, improved balance and occupational activities [41]. Combined approaches, like aquatic therapy with functional exercises and intensive programs like Hand-Arm Bimanual Intensive Therapy, also yielded positive results [40,45]. Additionally, therapies such as hippotherapy, mirror therapy with muscle strength exercises, and early interventions like HABIT-ILE significantly enhanced motor skills, balance, and bimanual performance [38,43,49]. These findings highlight the value of diverse, individualized interventions in improving both functional abilities and overall well-being in children with CP. The summary of the characteristics of each study and their main results is described in Table 2.

Table 2.
Summary table of results characteristics.
3.5. Sample Characteristics
A total of 510 pediatric participants with CP were included in the studies analyzed within this systematic review and meta-analysis. The mean age was 7.6 years, with a range varying between 1.1 years (13 months) and 12 years. Sample sizes varied from a minimum of 14 participants [48] to a maximum of 91 participants [38], considering the diverse interventions implemented and the outcomes reported in each case.
3.6. Administered Dosages and Executed Interventions
The reviewed programs included activities aimed at improving gross motor function and independence in ADLs, varying in duration, frequency, and intensity. Several studies implemented 8-week interventions with 2 weekly sessions, such as Shih et al. [48] with Kinect-based and therapist-based CIMT (135 min/session), Ko et al. [42] with task-oriented training (30 min/session), and Sahin et al. [44] with virtual reality therapy via Kinect (45 min/session), all at moderate intensity. More intensive interventions included Figueiredo et al. [45] with HABIT (three weeks, five sessions of 360 min/week) and Carton de Tournai et al. [49] with HABIT-ILE (2 weeks, 5 sessions of 300 min/week). Different strategies were used, such as mirror therapy and strength exercises [43], pediatric aquatic therapy [40], treadmill training combined with virtual reality [47], and hippotherapy [38], with intensities ranging from moderate to high.
3.7. Gross Motor Function
Overall, the meta-analytic results favored OT interventions for improving gross motor function (Table 3). As illustrated in Figure 4, small to large effect sizes (ES = 0.01–0.63) indicated significant gains in GMFM-66 outcomes. Funnel plot of standard error by Hedge’s g can be found in Figure S1.

Table 3.
Effectiveness of occupational therapy-based intervention on gross motor functions in children with cerebral palsy.
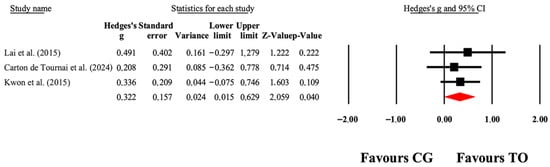
Figure 4.
The forest plot illustrates the changes in GMFM-66 scores among patients with cerebral palsy who participated in occupational therapy compared to those assigned as controls. The values presented represent effect sizes (Hedges’ g) along with 95% CI. The size of the squares in the plot indicates the statistical weight of each study [38,40,49].
3.8. Activities of Daily Living
The overall effects of OT interventions on the independence of ADLs (as assessed by functional capacity, occupational performance, and satisfaction) are shown in Table 4. Forest plots are shown in Figure 5, Figure 6, Figure 7 and Figure 8. Small to large effect sizes (ES = 0.19 to 2.63) showed significant differences related to the OT intervention evidenced in PEDI-Mobility, COPM-Performance and COPM-Satisfaction, with no significant differences found in PEDI-Self Care. Funnel plot of standard error by Hedge’s g for Figure 5, Figure 6, Figure 7 and Figure 8 can be found in Figures S2–S5, respectively.

Table 4.
Summary of results of the meta-analysis.
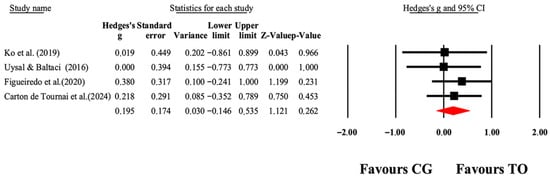
Figure 5.
The forest plot illustrates the changes in PEDI-Self-Care scores among patients with cerebral palsy participating in occupational therapy compared to those assigned as controls. The values presented represent effect sizes (Hedges’ g) along with 95% CI. The size of the squares in the plot indicates the statistical weight of each study [41,42,45,49].
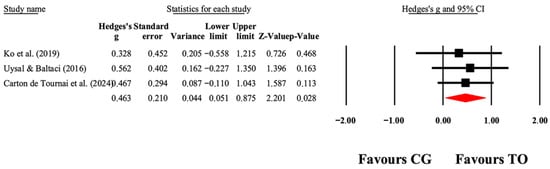
Figure 6.
The forest plot illustrates the changes in PEDI-Mobility in patients with cerebral palsy participating in occupational therapy compared with patients with cerebral palsy assigned as controls. Values shown are effect sizes (Hedges’ g) with 95% CI. The size of the squares plotted reflects the statistical weight of each study [41,42,49].
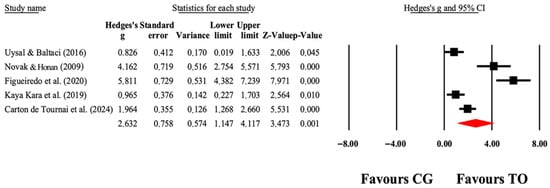
Figure 7.
The forest plot illustrates the changes in COPM-Performance in patients with cerebral palsy participating in occupational therapy compared with patients with cerebral palsy assigned as controls. Values shown are effect sizes (Hedges’ g) with 95% CI. The size of the squares plotted reflects the statistical weight of each study [26,41,43,45,49].
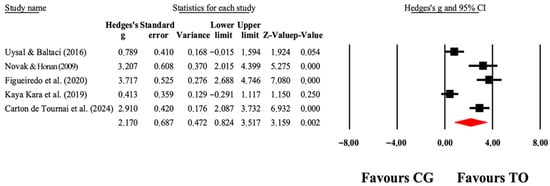
Figure 8.
The forest plot illustrates the changes in COPM-Satisfaction in patients with cerebral palsy participating in occupational therapy compared with patients with cerebral palsy assigned as controls. Values shown are effect sizes (Hedges’ g) with 95% CI. The size of the squares plotted reflects the statistical weight of each study [26,41,43,45,49].
3.9. Certainty of Evidence
Evidence concerning OT interventions in children with CP provides moderate certainty for benefits in gross motor function and ADLs. However, certain studies revealed methodological weaknesses that suggest some degree of bias, they do not raise any issues regarding inconsistency or precision, which supports their clinical relevance (Table 5).

Table 5.
Evaluation of methodological quality using the GRADEpro tool.
3.10. Effects Adverse and Adherence
The pooled data from the included studies revealed a 76.4% adherence rate and no reported adverse effects. These results indicate that the interventions were both feasible and well tolerated in children with CP, reinforcing their suitability for broader clinical application.
4. Discussion
4.1. Gross Motor Function
The meta-analysis identified significant gains in gross motor function assessed via the GMFM-66 (p = 0.04). Supporting these findings, Szturm et al. [50] reported that a 12-week game-based intervention significantly improved balance (p = 0.03) and GMFM-88 subdomains of standing (p = 0.05) and walking/running/jumping (p = 0.03). Likewise, Rastgar Koutenaei et al. [51] observed greater post-intervention and 9-week GMFM-88 improvements following Swiss ball training compared with stable surface exercises (p < 0.001). Matusiak-Wieczorek et al. [52] observed that hippotherapy enhanced trunk control, with significant gains in the group receiving two sessions per week (p = 0.005), whereas improvements with one session weekly were not significant (p = 0.028). Conversely, Jha et al. [53] found that virtual reality combined with physical therapy improved GMFM-88 scores within groups but not between groups (p = 0.519). Overall, these findings highlight the importance of individualized interventions to optimize motor function in children with CP.
4.2. Activities of Daily Living
Significant improvements were identified in ADLs following occupational therapy-based intervention including occupational performance and satisfaction (COPM-Performance, p = 0.001; COPM-Satisfaction, p = 0.002) and mobility (PEDI-Mobility, p = 0.02). However, no significant change was observed in self-care outcomes (PEDI Self-Care, p = 0.26). Consistent with the meta-analytic results, Choi et al. [54] observed significant ADL gains following four weeks of virtual reality rehabilitation compared with conventional occupational therapy (PEDI-CAT, p < 0.01), with greater improvements among children with severe motor limitations. Consistently, Yang et al. [55] found that a rehabilitation program grounded in the ICF framework significantly improved ADLs after three months (p < 0.001), showing superior outcomes compared with traditional therapy. Özen et al. [56] observed significant ADL improvements following functional electrical stimulation in children with spastic diplegic CP (p = 0.004). Conversely, Jha et al. [53] reported that both virtual reality and control groups improved on the WeeFIM (p < 0.001 and p < 0.05, respectively), but without between-group differences (p = 0.33). Overall, these findings suggest that OT-based rehabilitation can effectively enhance ADLs in children with CP, though outcomes vary depending on intervention type and motor severity.
4.3. Limitations and Strengths
This systematic review and meta-analysis acknowledge several limitations: (i) the restricted number of included studies may have reduced the analytical breadth, (ii) although methodological rigor was generally high, twelve studies raised some concerns regarding potential bias; and (iii) small sample sizes and the heterogeneity of intervention protocols limit the ability to draw definitive conclusions.
Nevertheless, this review also has notable strengths. It exclusively included RCTs. Furthermore, it incorporated innovative interventions, such as virtual reality and functional electrical stimulation, which reflect emerging therapeutic trends. The included studies reported significant improvements in motor function and ADLs. Additionally, the combined sample size of 510 participants contributes to the external validity of the findings.
4.4. Practical Applications
This systematic review and meta-analysis highlight the efficacy of occupational therapy-based intervention in improving gross motor function and ADLs among children with CP. Evidence supports the effectiveness of modalities such as virtual reality, game-based programs, intensive bimanual hand and arm therapy, hippotherapy, mirror therapy, and aquatic therapy [39,41,44,45,47,48]. Collectively, these findings advocate for individualized, evidence-based rehabilitation strategies that consider each child’s functional profile and promote both motor improvement and overall well-being. From a translational standpoint, these results emphasize the need to integrate multimodal, client-centered interventions into clinical practice. Future research should prioritize high-quality comparative trials to determine the relative effectiveness of these approaches, explore long-term functional outcomes, and evaluate their feasibility and scalability across diverse rehabilitation contexts.
5. Conclusions
OT interventions in children with CP showed significant improvements in gross motor function, measured with GMFM-66, and in independence in ADL, assessed with COPM-Performance, COPM-Satisfaction, and PEDI-Mobility. However, no significant differences were observed in the results measured with PEDI-Self Care.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm14217624/s1, Table S1. PRISMA checklist; Figure S1. Forest plot of the Gross Motor Function Measure (GMFM-66) outcomes. The meta-analysis indicates low heterogeneity among included studies (I2 = 0.00%, p = 0.84), demonstrating consistent results across trials evaluating motor function after Occupational therapy-based intervention; Figure S2. Forest plot of the Pediatric Evaluation of Disability Inventory—Self-Care (PEDI—Self Care) outcomes. Results show low heterogeneity (I2 = 0.00%, p = 0.86), indicating stable effects among studies assessing self-care performance improvements; Figure S3. Forest plot of the Pediatric Evaluation of Disability Inventory—Mobility (PEDI—Mobility) outcomes. The analysis shows low heterogeneity (I2 = 0.00%, p = 0.92), suggesting consistent findings regarding mobility improvements following OT-based rehabilitation; Figure S4. Forest plot of the Canadian Occupational Performance Measure—Performance (COPM—Performance) outcomes. This analysis reveals high heterogeneity (I2 = 92.2%, p = 0.00), reflecting variability among studies in performance-related outcomes; Figure S5. Forest plot of the Canadian Occupational Performance Measure—Satisfaction (COPM—Satisfaction) outcomes. Results show high heterogeneity (I2 = 91.4%, p = 0.00), suggesting considerable differences among studies in satisfaction-related measures. Ref. [57] is listed in the supplementary.
Author Contributions
Conceptualization, J.P.-C., J.H.-M., E.V.-C. and P.V.-B.; methodology, J.P.-C., J.H.-M. and E.V.-C.; software, J.P.-C., J.H.-M. and E.V.-C.; validation, J.P.-C., J.H.-M., E.V.-C., D.F.-C., C.S.-G., C.S., E.C.-P., C.L. and E.F.-R.; formal analysis, J.P.-C., J.H.-M. and P.V.-B.; investigation, J.P.-C., J.H.-M., E.V.-C., D.F.-C., C.S.-G., C.S., E.C.-P., C.L., P.V.-B. and E.F.-R.; resources, C.S. and P.V.-B.; data curation, J.P.-C., J.H.-M. and E.V.-C.; writing—original draft preparation, J.P.-C., J.H.-M., E.V.-C., D.F.-C., C.S.-G., C.S., E.C.-P., C.L., P.V.-B. and E.F.-R.; writing—review and editing, J.P.-C., J.H.-M., E.V.-C., D.F.-C., C.S.-G., C.S., E.C.-P., C.L., P.V.-B. and E.F.-R.; visualization, J.P.-C. and J.H.-M.; supervision, P.V.-B. and E.F.-R.; project administration, P.V.-B.; funding acquisition, C.L. and E.F.-R. All authors have read and agreed to the published version of the manuscript.
Funding
Author P.V.-B. acknowledges support from the National Research and Development Agency of Chile (Agencia Nacional de Investigación y Desarrollo, ANID) through the FONDECYT project No. 11220035.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Krigger, K.W. Cerebral palsy: An overview. Am. Fam. Physician 2006, 73, 91–100. [Google Scholar] [PubMed]
- Vitrikas, K.; Dalton, H.; Breish, D. Cerebral palsy: An overview. Am. Fam. Physician 2020, 101, 213–220. [Google Scholar] [PubMed]
- Upadhyay, J.; Tiwari, N.; Ansari, M.N. Cerebral palsy: Aetiology, pathophysiology and therapeutic interventions. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1891–1901. [Google Scholar] [CrossRef]
- Wimalasundera, N.; Stevenson, V.L. Cerebral palsy. Pract. Neurol. 2016, 16, 184–194. [Google Scholar] [CrossRef]
- Panda, S.; Singh, A.; Kato, H.; Kokhanov, A. Cerebral palsy: A contemporary perspective. NeoReviews 2024, 25, e350–e360. [Google Scholar] [CrossRef]
- Gulati, S.; Sondhi, V. Cerebral palsy: An overview. Indian J. Pediatr. 2018, 85, 1006–1016. [Google Scholar] [CrossRef]
- Wood, E.; Rosenbaum, P. The Gross Motor Function Classification System for cerebral palsy: A study of reliability and stability over time. Dev. Med. Child Neurol. 2000, 42, 292–296. [Google Scholar] [CrossRef]
- Mahasup, N.; Sritipsukho, P.; Lekskulchai, R.; Keawutan, P. Inter-rater and intra-rater reliability of the Gross Motor Function Measure (GMFM-66) by Thai pediatric physical therapists. J. Med. Assoc. Thail. 2011, 94 (Suppl. S7), S139–S144. [Google Scholar]
- Eliasson, A.C.; Krumlinde-Sundholm, L.; Rösblad, B.; Beckung, E.; Arner, M.; Öhrvall, A.M.; Rosenbaum, P. The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Dev. Med. Child Neurol. 2006, 48, 549–554. [Google Scholar] [CrossRef]
- Morris, C.; Kurinczuk, J.J.; Fitzpatrick, R.; Rosenbaum, P.L. Reliability of the Manual Ability Classification System for children with cerebral palsy. Dev. Med. Child Neurol. 2006, 48, 950–953. [Google Scholar] [CrossRef]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Hidecker, M.J.C.; Paneth, N.; Rosenbaum, P.L.; Kent, R.D.; Lillie, J.; Eulenberg, J.B.; Chester, K., Jr.; Johnson, B.; Michalsen, L.; Evatt, M.; et al. Developing and validating the Communication Function Classification System for individuals with cerebral palsy. Dev. Med. Child Neurol. 2011, 53, 704–710. [Google Scholar] [CrossRef]
- Piscitelli, D.; Ferrarello, F.; Ugolini, A.; Verola, S.; Pellicciari, L. Measurement properties of GMFCS, GMFCS-ER, MACS, and CFCS in cerebral palsy: A systematic review with meta-analysis. Dev. Med. Child Neurol. 2021, 63, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Rauf, W.; Sarmad, S.; Khan, I.; Jawad, M. Effect of positioning on gross motor function and spasticity in children with spastic cerebral palsy. J. Pak. Med. Assoc. 2021, 71, 801–805. [Google Scholar] [CrossRef]
- Elbasan, B.; Kayıhan, H.; Duzgun, I. Sensory integration and activities of daily living in children with developmental coordination disorder. Ital. J. Pediatr. 2012, 38, 14. [Google Scholar] [CrossRef]
- Mendoza-Sengco, P.; Lee Chicoine, C.; Vargus-Adams, J. Early cerebral palsy detection and intervention. Pediatr. Clin. N. Am. 2023, 70, 385–398. [Google Scholar] [CrossRef]
- Hoare, B.J.; Wallen, M.A.; Thorley, M.N.; Jackman, M.L.; Carey, L.M.; Imms, C. Constraint-induced movement therapy in children with unilateral cerebral palsy. Cochrane Database Syst. Rev. 2019, 2019, CD004149. [Google Scholar] [CrossRef] [PubMed]
- Damiano, D.L.; Longo, E. Evidence for early intervention in infants with or at high risk of cerebral palsy: An overview of systematic reviews. Dev. Med. Child Neurol. 2021, 63, 771–784. [Google Scholar] [CrossRef]
- Park, E.S.; Rha, D.W.; Shin, J.S.; Kim, S.; Jung, S. Effects of hippotherapy on gross motor function and functional performance in children with cerebral palsy. Yonsei Med. J. 2014, 55, 1736–1742. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Yu, S.; Guo, X.; Xun, B.; Zhang, Y. Effect of ultra-early NDT therapy intervention on neural and motor development in infants at high risk of cerebral palsy. Folia Neuropathol. 2023, 61, 419–425. [Google Scholar] [CrossRef]
- Amirthalingam, J.; Paidi, G.; Alshowaikh, K.; Jayarathna, A.I.; Salibindla, D.B.A.M.R.; Karpinska-Leydier, K.; Ergin, H.E. Virtual reality intervention to improve motor function in patients undergoing rehabilitation for cerebral palsy, Parkinson’s disease, or stroke: A systematic review of randomized controlled trials. Cureus 2021, 13, e16763. [Google Scholar] [CrossRef]
- Zai, W.; Xu, N.; Wu, W.; Wang, Y.; Wang, R. Effect of task-oriented training on gross motor function, balance and activities of daily living in children with cerebral palsy: A systematic review and meta-analysis. Medicine 2002, 101, e31565. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.L. Portrait of occupational therapy. J. Interprof. Care 2005, 19, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Steultjens, E.M.; Dekker, J.; Bouter, L.M.; van de Nes, J.C.; Lambregts, B.L.; van den Ende, C.H. Occupational therapy for children with cerebral palsy: A systematic review. Clin. Rehabil. 2004, 18, 1–14. [Google Scholar] [CrossRef]
- American Occupational Therapy Association. Occupational therapy practice framework: Domain and process (4th ed.). Am. J. Occup. Ther. 2020, 74 (Suppl. S2), 7412410010. [Google Scholar] [CrossRef]
- Novak, I.; Honan, I. Effectiveness of pediatric occupational therapy for children with disabilities: A systematic review. Aust. Occup. Ther. J. 2019, 66, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions, Version 6.4; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. PRISMA 2020 statement: An updated guideline for the publication of systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Manterola, C.; Zavando, D. Cómo interpretar los “niveles de evidencia” en los diferentes escenarios clínicos. Rev. Chil. Cir. 2009, 61, 582–595. [Google Scholar] [CrossRef]
- Pereira, M.F.; Prahm, C.; Kolbenschlag, J.; Oliveira, E.; Rodrigues, N.F. Application of AR and VR in hand rehabilitation: A systematic review. J. Biomed. Inform. 2020, 111, 103584. [Google Scholar] [CrossRef]
- Verhagen, A.P.; de Vet, H.C.W.; de Bie, R.A.; Kessels, A.G.H.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 5.0.0; The Cochrane Collaboration: London, UK; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Davey, J.; Turner, R.M.; Clarke, M.J.; Higgins, J.P.T. Characteristics of meta-analyses and their component studies in the Cochrane Database of Systematic Reviews: A cross-sectional, descriptive analysis. BMC Med. Res. Methodol. 2011, 11, 160. [Google Scholar] [CrossRef]
- Morris, S.B.; DeShon, R.P. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol. Methods 2002, 7, 105–125. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Xie, C.X.; Machado, G.C. Clinimetrics: Grading of Recommendations, Assessment, Development and Evaluation (GRADE). J. Physiother. 2021, 67, 66. [Google Scholar] [CrossRef] [PubMed]
- Rostami, H.R.; Arastoo, A.A.; Nejad, S.J.; Mahany, M.K.; Malamiri, R.A.; Goharpey, S. Effect of treatment environment on modified constraint-induced movement therapy results in children with spastic hemiplegic cerebral palsy: A randomized controlled trial. NeuroRehabilitation 2012, 31, 357–365. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Chang, H.J.; Yi, S.H.; Lee, J.Y.; Shin, H.Y.; Kim, Y.H. Effect of hippotherapy on gross motor function in children with cerebral palsy: A randomized controlled trial. J. Altern. Complement. Med. 2015, 21, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ökmen, M.; Yildirim, S.A. Effect of virtual reality therapy on functional development in children with cerebral palsy: A single-blind, prospective, randomized-controlled study. Turk. J. Phys. Med. Rehabil. 2019, 65, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-J.; Liu, W.-Y.; Yang, T.-F.; Chen, C.-L.; Wu, C.-Y.; Chan, R.-C. Pediatric aquatic therapy on motor function and enjoyment in children diagnosed with cerebral palsy of various motor severities. J. Child Neurol. 2015, 30, 200–208. [Google Scholar] [CrossRef]
- Uysal, S.A.; Baltaci, G. Effects of Nintendo Wii™ training on occupational performance, balance, and daily living activities in children with spastic hemiplegic cerebral palsy: A single-blind and randomized trial. Games Health J. 2016, 5, 311–317. [Google Scholar] [CrossRef]
- Ko, E.J.; Sung, I.Y.; Moon, H.J.; Yuk, J.S.; Kim, H.S.; Lee, N.H. Effect of group-task-oriented training on gross and fine motor function, and activities of daily living in children with spastic cerebral palsy. Phys. Occup. Ther. Pediatr. 2019, 40, 18–30. [Google Scholar] [CrossRef]
- Kara, O.K.; Yardimci, B.N.; Sahin, S.; Orhan, C.; Livanelioglu, A.; Soylu, A.R. Combined effects of mirror therapy and exercises on the upper extremities in children with unilateral cerebral palsy: A randomized controlled trial. Dev. Neurorehabil. 2019, 22, 423–429. [Google Scholar] [CrossRef]
- Şahin, S.; Köse, B.; Aran, O.T.; Ağce, Z.B.; Kayıhan, H. The effects of virtual reality on motor functions and daily life activities in unilateral spastic cerebral palsy: A single-blind randomized controlled trial. Games Health J. 2019, 8, 449–456. [Google Scholar] [CrossRef]
- Figueiredo, P.R.P.; Mancini, M.C.; Feitosa, A.M.; Teixeira, C.M.M.F.; Guerzoni, V.P.D.; Elvrum, A.G.; Ferre, C.L.; Gordon, A.M.; Brandão, M.B. Hand–arm bimanual intensive therapy and daily functioning of children with bilateral cerebral palsy: A randomized controlled trial. Dev. Med. Child Neurol. 2020, 62, 1274–1282. [Google Scholar] [CrossRef]
- Rassafiani, M.; Akbarfaimi, N.; Hosseini, S.A.; Shahshahani, S.; Karimlou, M.; Tabatabai Ghomsheh, F. The effect of the combination of active vestibular interventions and occupational therapy on balance in children with bilateral spastic cerebral palsy: A pilot randomized controlled trial. Iran. J. Child Neurol. 2020, 14, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Ochandorena-Acha, M.; Terradas-Monllor, M.; Nunes Cabrera, T.F.; Torrabias Rodas, M.; Grau, S. Effectiveness of virtual reality on functional mobility during treadmill training in children with cerebral palsy: A single-blind, two-arm parallel group randomised clinical trial (VirtWalkCP Project). BMJ Open 2022, 12, e061988. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.-Y.; Chang, Y.-J.; Wu, C.-Y. Comparative effects of kinect-based versus therapist-based constraint-induced movement therapy on motor control and daily motor function in children with unilateral cerebral palsy: A randomized controlled trial. J. Neuroeng. Rehabil. 2023, 20, 13. [Google Scholar] [CrossRef]
- Carton de Tournai, A.; Herman, E.; Ebner-Karestinos, D.; Bleyenheuft, C.; Simmat-Durand, L.; Richard, I.; Chabrol, B.; Bertoncelli, C.M.; Lefevre, F.X.; Bleyenheuft, Y. Hand-arm bimanual intensive therapy including lower extremities in infants with unilateral cerebral palsy: A randomized clinical trial. JAMA Netw. Open 2024, 7, e2445133. [Google Scholar] [CrossRef]
- Szturm, T.; Parmar, S.T.; Mehta, K.; Shetty, D.R.; Kanitkar, A.; Eskicioglu, R.; Gaonkar, N. Game-based dual-task exercise program for children with cerebral palsy: Combined balance, visuomotor, and cognitive training—A feasibility randomized controlled trial. Sensors 2022, 22, 761. [Google Scholar] [CrossRef]
- Rastgar Koutenaei, F.; Noorizadeh Dehkordi, S.; Amini, M.; ShahAli, S. Effect of Swiss ball stabilization training on trunk control, abdominal muscle thickness, balance, and motor skills in children with spastic cerebral palsy: A superiority randomized trial. Arch. Phys. Med. Rehabil. 2023, 104, 1755–1766. [Google Scholar] [CrossRef]
- Matusiak-Wieczorek, E.; Dziankowska-Zaborszczyk, E.; Synder, M.; Borowski, A. The influence of hippotherapy on sitting posture in children with cerebral palsy. Int. J. Environ. Res. Public Health 2020, 17, 6846. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.K.; Karunanithi, G.B.; Sahana, A.; Karthikbabu, S. Randomized trial of virtual reality games and physiotherapy on balance, gross motor performance, and daily functions in children with bilateral spastic cerebral palsy. Somatosens. Mot. Res. 2021, 38, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Yi, S.H.; Ao, L.; Tang, X.; Xu, X.; Shim, D.; Yoo, B.; Park, E.S.; Rha, D.W. Virtual reality rehabilitation in children with brain injury: A randomized controlled trial. Dev. Med. Child Neurol. 2021, 63, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, S.S.; Zhang, G.Y.; Wang, M.M.; Chen, G.X.; Zhu, D.N. Effect of rehabilitation training based on ICF-CY core sets on daily living activities in children with cerebral palsy: A prospective randomized controlled study. Zhongguo Dang Dai Er Ke Za Zhi 2021, 23, 608–612. [Google Scholar] [CrossRef]
- Özen, N.; Unlu, E.; Karaahmet, O.Z.; Gurcay, E.; Gundogdu, I.; Umay, E. Effectiveness of functional electrical stimulation—Cyclic treatment in children with cerebral palsy. Malawi Med. J. 2021, 33, 144–152. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).