1. Introduction
Aberrant origins of the supra-aortic trunk (SAT) are uncommon, with a prevalence of 18% in the general population, but a higher proportion (35%) in patients with thoracic aortic disease [
1,
2,
3].
An Isolated Left Vertebral Artery (ILVA) arising directly from the aortic arch between the left common carotid artery (LCCA) and the left subclavian artery (LSA) is described to be the second most frequent variant of SAT configurations, with an incidence ranging between 0.8% and 6.3% [
1,
2,
3,
4] and a left-side predominance [
4].
Despite anatomical variations not being clinically relevant, their recognition is pivotal during treatment of cerebrovascular or thoracic aortic pathology, as their non-recognition and accidental ligation or lesion may lead to possibly devastating neurologic complications [
5,
6].
Proper understanding of anatomical variation is especially mandatory when planning endovascular and hybrid interventions involving the aortic arch, as those variants may represent significant technical challenges and deeply impact surgical strategy [
7]. Several approaches have been described for ILVA management during endovascular and hybrid aortic arch repair, including transposition to the left carotid artery or total endovascular revascularization with dedicated fenestration [
8,
9,
10].
The safety and efficacy of these approaches remain unproven due to the lack of large studies, long-term results, and follow-up data.
In this scenario, we investigated the current management strategies for aberrant left vertebral artery during total endovascular and hybrid repair of aortic arch pathologies with the aim of evaluating the effectiveness of such approaches, making the available evidence more accessible to decision makers in real-world clinical practice.
2. Methods
2.1. Study Design
This systematic review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [
11] and PICO (Patients, Intervention, Comparator, Outcomes) [
12] approach (
Supplementary Materials Tables S1 and S2; Figure S1).
A literature search was performed by two independent authors (MM and OB) based on three databases (PubMed, SCOPUS and WEB OF SCIENCE) from inception to February 2025 using the following string: (“isolated left vertebral artery” OR “ILVA” OR “aberrant left vertebral artery”) AND (“management” OR “treatment” OR “reconstruction” OR “transposition” OR “hybrid” OR “endovascular” OR “fenestration” OR “repair” OR “reparation” OR “TEVAR” OR “reimplantation” OR “replacement” OR “revascularization” OR “procedure”) AND (“safety” OR “follow-up” OR benefits OR effect OR effects OR efficacy OR outcome OR outcomes OR “process assessment” OR appropriateness OR “quality”).
All papers reporting the management (conservative or interventional) of ILVA during hybrid or endovascular treatment of aortic arch diseases (including aneurysm, chronic/acute dissection, intramural hematomas or penetrating aortic ulcer) were included as detailed below.
The titles and abstracts were screened for appropriateness and then a second round of eligibility was applied to the retrieved full text. Disagreement was resolved by discussion and consensus, and the reasons for exclusion were documented.
The reference lists of all included studies were also examined for identification of further relevant articles.
The International Prospective Register of Systematic Reviews (PROSPERO) was inquired to avoid duplication and the full protocol of the study was registered (CRD42024562104).
2.2. Endpoints
Primary and secondary outcomes were defined according to the SVS reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries [
13].
Our primary endpoint was to investigate the different interventional strategies (endovascular or open) of aberrant ILVA arising directly from the aortic arch during endovascular and hybrid treatment of aortic arch diseases and the related mortality (overall and aortic-related at 30 days and during follow-up).
As secondary outcomes, we investigated new onset (minor and major) neurological symptoms (see definition above), immediate and follow-up patency rate of the ILVA revascularization, the occurrence of endoleak and ILVA-related reintervention rate.
2.3. Definitions
New onset neurological symptoms were divided into minor and major neurological symptoms. Minor neurological symptoms were defined as transient isolated brainstem symptoms such as balance disorders, dizziness, diplopia, dysphagia, aphasia, drop attacks and bilateral homonymous hemianopia.
Major neurological symptoms were defined as permanent neurological deficit, including anterior and posterior circulation stroke, paraparesis and paraplegia.
2.4. Inclusion and Exclusion Criteria
Papers were included if:
An isolated left aberrant vertebral artery was detected on a contrasted computed tomography angiography (CTA) preoperatively;
The left aberrant ILVA arose directly from the aortic arch;
The included patients were treated for an aortic arch pathology (including aneurysm, chronic/acute dissection, intramural hematomas or penetrating aortic ulcer) with an endovascular or hybrid treatment either in an elective or emergent setting.
Only retrospective or prospective series, in English language and reporting sufficient data about management strategies and related outcomes, were included.
Literature review studies, conference abstracts, theses, case reports, case series, dissertations and book chapters were not included in our analysis.
2.5. Data Collection
The following data were retrieved from the included papers in a predefined database: authors, year of publication, country, study design, overall number of patients, number of patients presenting with ILVA, patients’ demographics, type of aortic arch disease, type of treatment, type of endograft used, setting for repair, 30-day and long-term mortality (for all causes and aortic-related), new neurological symptoms, aortic-related reinterventions patency of the revascularization, and the follow-up length.
In patients undergoing surgical or endovascular revascularization of the ILVA, the immediate and long-term patency rate and overall endoleak rate were also reported. No further search was conducted to retrieve any unpublished data.
2.6. Assessment of Study Quality and Risk of Bias
The studies were analyzed in terms of design, heterogeneity, and possible bias. As there were no randomized studies, papers reporting insufficient data or at high risk of bias according to the Newcastle Ottawa-Score (NOS) [
14] were ruled out from the analysis (
Supplementary Materials Table S3).
2.7. Statistical Analysis
Categorical data are presented as counts and percentages. Continuous variables are reported as mean and range. Taking into consideration the small number of patients, no subgroups were analyzed, and no further statistical analyses were performed. Because of the heterogeneity of reported data, no meta-analysis of pooled data was performed. The extracted data are reported in percentages and absolute values or narratively synthesized. SPSS statistical software (v. 25, SPSS Inc., Chicago, IL, USA) was used for all analyses.
3. Results
3.1. Study Selection and Characteristics
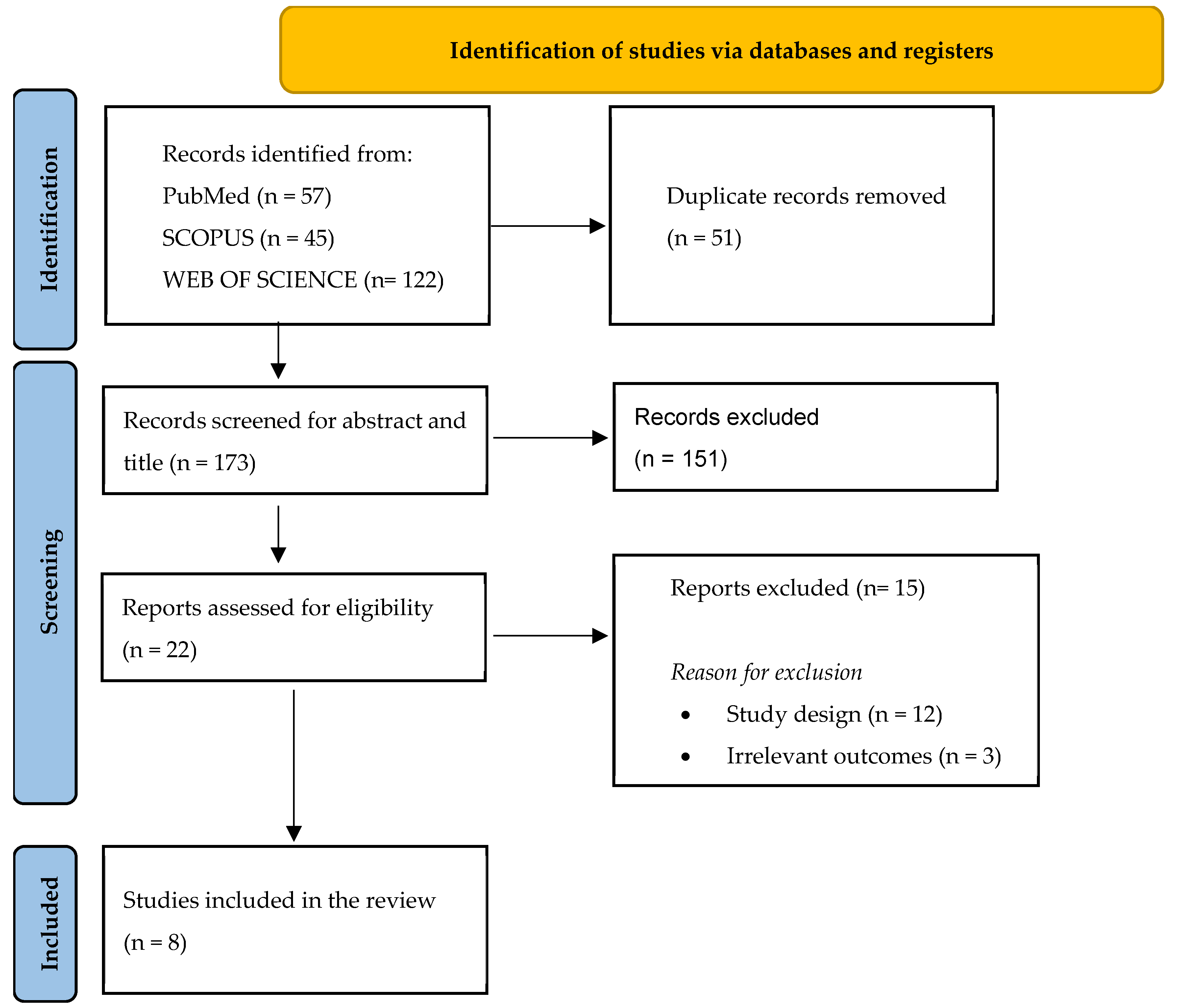
The search yielded 224 articles, and after duplication removal, a total of 173 papers were screened for title and abstract (
Figure 1). Twenty-two papers were retrieved and assessed for text eligibility, among which eight (two retrospective multicenter cohort studies and six retrospective single-center cohort studies) were included according to the above-mentioned inclusion and exclusion criteria, totaling 199 patients.
The main characteristics of the included papers are reported in
Table 1.
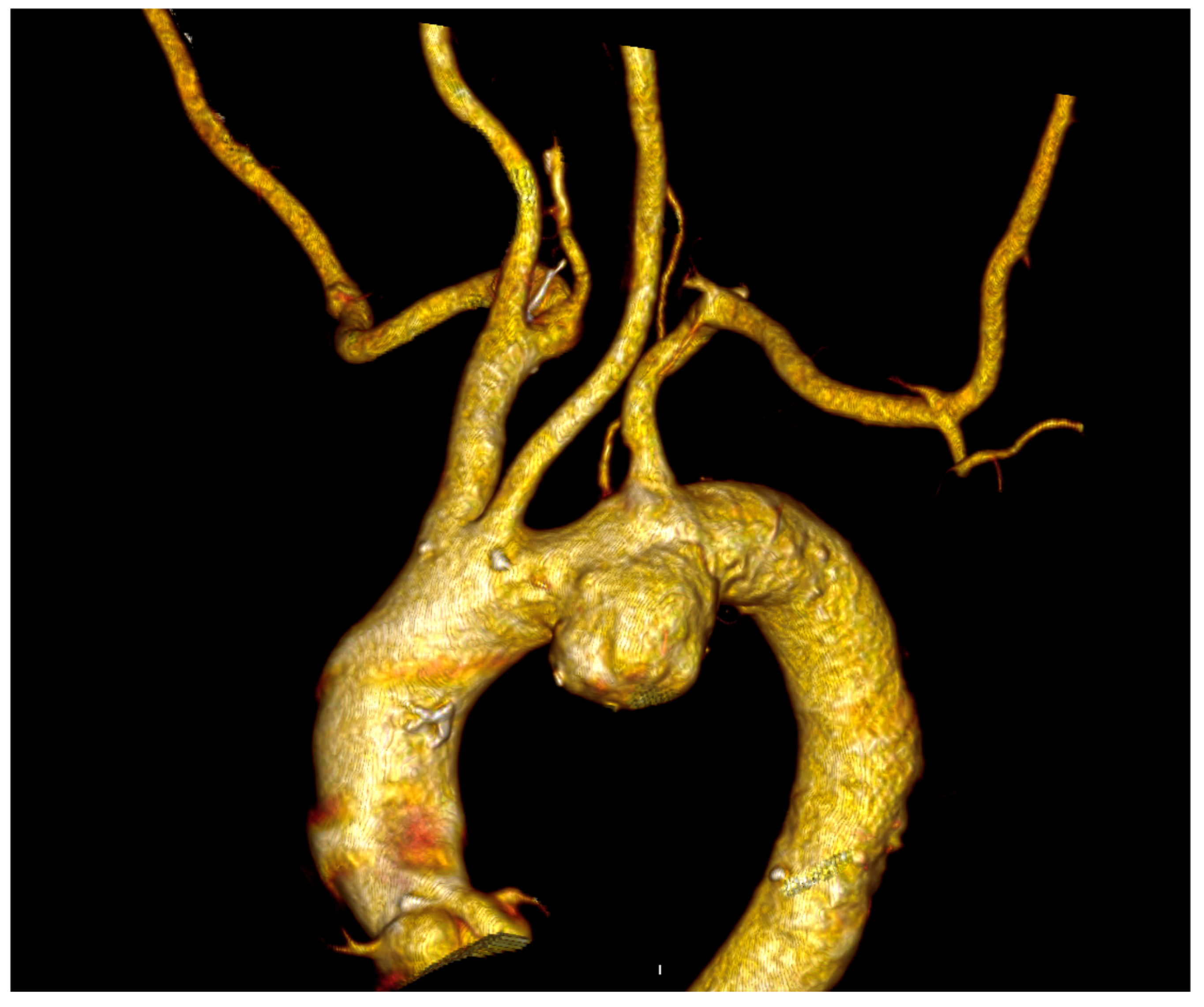
In all cases, the ILVA was detected on the preoperative computed tomography angiography (CTA) (
Figure 2).
Additionally, in 145 (73%) cases, a preoperative CTA of the brain was performed to rule out other concomitant diseases or anatomical variants and check the integrity of the Willis circle.
The classification of the vertebral artery variable origin defined by Lazaridis and colleagues [
4] was used in only two studies [
15,
17] that reported LA2.2 configuration in all cases (19 patients).
Only six included studies reported the aortic stent-graft (SG) used during TEVAR for a total of 33 Castor single-branched stent grafts (MicroPort Medical, Shanghai, China), 21 TAG (W.L. Gore & Associates, Flagstaff, AZ, USA), 23 Valiant (Medtronic, Minneapolis, MN, USA), 14 Ankura (Lifetech Scientific, Shenzhen, China), 6 Zenith (Cook, Bloomington, IN, USA). 8 Aegis (MicroPort, Shanghai, China) and 2 RelayPro (Terumo Aortic, Tokyo, Japan).
3.2. Indication for Revascularization
Among the 199 patients with ILVA who underwent total endovascular or hybrid treatment of aortic arch disease, 149 patients (75% male; mean age 63 years, range 55–76 years) required additional procedures for ILVA revascularization (
Table 2).
The underlying aortic arch disease was as follows: acute or chronic thoracic aortic dissection (80 patients, 53.7%), thoracic aortic aneurysm (28 patients, 18.9%), intramural hematoma (10 patients, 6.7%), aortic pseudoaneurysm (5 patients, 3.3%), penetrating aortic ulcer (5 patients, 3.3%) or not disclosed (21 patients, 14%).
The indication for revascularization was given for TEVAR requiring coverage of ILVA origin and one of the following reasons:
dominant ILVA (54 cases, 36.2%) [
9,
10,
17];
symmetric vertebral arteries with an incomplete circle of Willis (45 cases, 30.2%) [
9,
10,
17];
increased risk of spinal cord ischemia for extensive coverage of the aorta (>200 mm or previous aortic surgery) (2 cases, 1.3%) [
17].
A systematic transposition of the ILVA was performed in the remaining cases (48 cases, 32.2%) [
8,
15,
18].
Conversely, in 50 cases ILVA was not revascularized due to:
hypoplastic LVA (vertebral artery diameter < 2.0 mm) or right vertebral artery dominance (17 patients, 34%) [
9];
ILVA not involved in the aortic pathology or not covered by the stent-graft when Ishimaru zone 3-TEVAR was performed (32 patients, 64%) [
15,
16] or for chronic occlusion at the origin (1 patient, 2%) [
15].
These patients were excluded from the analysis.
3.3. Strategies for Revascularization
Several strategies for ILVA revascularization have been described in the included studies: the studies by Piffaretti et al., Yang et al., and Shergill et al. described the results achieved with ILVA open surgical revascularization during TEVAR (40 patients) [
8,
15]; the studies by Shen et al., Luo et al., and Wang et al. focused on total endovascular reconstruction through physician-modified fenestration (PMF) (39 patients) and in situ fenestration (ISF) (3 patients) [
10,
17,
18]. Finally, Zhang and colleagues compared the outcomes of fenestration (24 patients), transposition (15 patients) and chimney technique (28 patients) for ILVA revascularization (
Figure 3) [
9].
3.3.1. Open Surgical Revascularization
Overall, a total of 55/149 (37%) patients underwent open surgical revascularization of the ILVA as depicted following:
In case of zone 2 TEVAR:
A single-stage intervention with revascularization of the left subclavian artery through a carotid-subclavian bypass and transposition of the ILVA onto the bypass graft or directly onto the left common carotid artery was performed in 27% of cases (40/149 patients).
ILVA revascularization through a saphenous vein bypass from the left carotid was performed in 5.3% of cases (8/149 patients) through a supraclavicular approach.
In 3 cases (2%), the method of vertebral revascularization was unclear.
In patients who underwent open arch graft replacement (4/149, 2.7%),
3.3.2. Endovascular Revascularization
Endovascular revascularization was performed in a total of 94/149 (63%) patients:
Among patients who underwent PMF, 33 had zone 2 TEVAR and ILVA revascularization via on-table fenestration of a Castor (MicroPort Medical, Shanghai, China) single-branch stent graft [
10,
18]; 30 of them underwent TEVAR with single or double on-table fenestrations for ILVA and subclavian artery revascularization (with the use of a bridging bare metal stent in only three cases) [
9,
17].
The 28 patients treated with chimney technique underwent a novel approach based on the delivery of a chimney stent in the LSA, ensuring that it crosses the LVA origin to guarantee its perfusion through the mesh of the stent and the gutter between the LSA chimney and the aortic stent-graft [
9].
Finally, three cases of ISF were performed using a liver biopsy needle through a surgical supraclavicular approach for exposure of ILVA with a bare metal bridging stent [
17].
3.4. Primary Endpoints
The overall 30-day mortality was 1.3% (one patient in the surgical revascularization group died during hospital admission for a ruptured thoracic aneurysm, and one patient in the endovascular group died 29 days after the procedure from a hemodialysis-related brain hemorrhage).
Among the remaining 147 patients, the late mortality rate was 5.4% (mean follow-up of 46 months, range 6–120 months), as two additional deaths occurred in the endovascular group (2/93, 2.1%) and six in the surgical group, respectively (6/54, 11.1%). Causes of death were respiratory failure in oxygen-dependent chronic respiratory insufficiency (one), advanced liver cancer (one), or not disclosed (six). No late aortic-related deaths were reported (
Table 3).
3.5. Secondary Endpoints
3.5.1. New Onset Neurological Symptoms
New onset neurological symptoms occurred in 11.4% of patients (17/149) as detailed following.
Minor neurological symptoms occurred in 7.4% of patients (16.4% in the open revascularization group and 2.1% in the endovascular group).
Major neurological symptoms were reported in 3.3% of patients (7.4% in the open revascularization group and 1% in the endovascular group). Specifically, one patient experienced an anterior stroke and another a posterior stroke (both patients had undergone transposition); one stroke localization was not disclosed (and occurred in a patient who had undergone PMF); one patient suffered from paraparesis and another from paraplegia (both patients had undergone transposition).
Furthermore, Horner syndrome was observed in one patient who had undergone surgical ILVA transposition; in this case, the central or peripheral etiology of the symptom was not disclosed [
1].
3.5.2. Secondary ILVA-Related Reintervention
Secondary ILVA-related interventions were needed in 5 patients symptomatic for dizziness who presented with ILVA stenosis during the follow-up period [
9] (the reintervention rate was 11.7% in the surgical group and 3.1% in the endovascular group, respectively).
Among them, a stenting procedure was performed in two patients initially treated with IFS, while angioplasty was performed in three patients who had previously undergone transposition. These data were calculated excluding the paper from Shergill et al. Due to the inability to disambiguate reintervention data from this publication.
No further reinterventions were conversely performed in the subgroup of patients experiencing ILVA revascularization occlusion during follow-up (11/149 cases, 7.3%) (16.3% in the surgical group and 2.1% in the endovascular group respectively), of which nine were asymptomatic cases and two presented with dizziness.
3.5.3. Endoleak
Eleven cases of endoleaks (EL) were observed (11.7%) in the endovascular group. Notably, 5/28 (17.8%) patients treated with chimney technique experienced a type IA EL that was closely followed up without need for any additional intervention, and 6/63 (9.5%) having undergone PMF presented a type IA EL (four) or IIIC EL (two), resulting in one reintervention for bridging stent positioning.
Reported data in the included studies were insufficient to disambiguate the relationship between the underlying aortic pathology, or the anamnestic characteristics, and the outcomes achieved with the different vascular interventions performed.
4. Discussion
In recent years, endovascular strategies in association or not with rerouting of the supra-aortic vessels have been increasingly applied to treat arch and descending thoracic aorta pathologies. Indeed, these approaches mitigate the peri- and postoperative morbidity and mortality rates that have lately been reported to be about 5.7% for open surgery versus 1.9% for endovascular treatment, respectively [
7].
Hence, the understanding of the anatomic variant of aortic arch vessels has become even more important to allow proper preoperative planning for the endovascular procedure.
ILVA has been described to be the second most frequent variant of SAT configurations [
1,
2,
3,
4]. With the steady increase in the number of endovascular procedures being performed worldwide, it is expected that vascular surgeons will face more and more such anatomical variants. However, whether ILVA should be revascularized or not in all cases is unclear. Several authors suggest a systematic revascularization of the ILVA in all cases [
8,
15,
16]. When neurological risk for the brain or spinal cord is increased the revascularization of an aberrant ILVA is always indicated [
8,
15,
16,
20,
21]. Indeed, other authors advocate for revascularization of the ILVA only under specific circumstances. This is the case of dominant ILVA during zone 2 TEVAR,9,10,15 incomplete circle of Willis or when an extensive coverage of the descending thoracic aorta is planned with occlusion of the intercostal vessels [
17].
The current management strategies for revascularization of ILVA during endovascular/hybrid treatment of aortic arch diseases include both open and endovascular options, but no sufficient data from comparative studies are currently available to indicate the definitive superiority of one revascularization technique over another. According to the data presented above, an anatomical revascularization of the ILVA (both via open reimplantation or fenestrated technology) was the technique more frequently applied, as it guarantees a straightforward flow to the target vessels. Chimney technique and extra-anatomical surgical bypasses seem indeed to be burdened with potentially higher risk of patency loss during follow-up [
22].
Endovascular approaches represented the preferred option in most of the included centers (63% of treated cases), probably because patients selected for these strategies since the beginning are poor candidates for any kind of open surgery or because a total endovascular intervention allows for ILVA revascularization during the index procedure avoiding the risk associated with surgical dissection (i.e., vagus/recurrent laryngeal nerve palsy, Horner’s syndrome and lymphocele) [
23,
24,
25,
26].
However, the frequency of reporting should not be conflated with superiority over open strategies. Indeed, despite reported data being too heterogeneous to draw any definitive conclusion, no aortic-related death was reported in any cases regardless of the intervention performed.
A recent retrospective study by Shergill and colleagues compared the results achieved in 143 patients who underwent vertebral artery revascularization during TEVAR, dividing patients into a direct vertebral revascularization cohort and an indirect (carotid-subclavian bypass or through subclavian-carotid transposition) vertebral revascularization cohort. The authors reported a significantly higher incidence of complications in the direct group than in the indirect one (bypass thrombosis 33.3% vs. 0.8%,
p < 0.0001; and hoarseness 57.1% vs. 18.0%,
p < 0.001, respectively), but there was no significant difference in mortality rates at 30 days, 1, 3, 5, and 10 years of follow-up [
19].
Long-term data are yet to be defined for both techniques, but it seems that even in the case of occlusion of the reconstruction during follow-up, patients mostly remain asymptomatic, and no further intervention is required. Considering the frequent asymptomatic nature of vertebral revascularization occlusion and the rate of neurological complications, albeit predominantly minor, associated with both surgical and endovascular procedures, a more selective strategy for ILVA revascularization may be warranted.
Data currently available do not allow us to analyze nor compare the results achieved with these two techniques in the long term nor to completely comment on the comparative neurological outcomes.
As a result of what emerged from the analysis of the literature, we believe that until further and more robust data are available, when it is indicated to perform the revascularization of an ILVA, the procedure should be tailored to the anatomic characteristics of the patient, their comorbidity, and the setting of the procedure but also based on the center experience.
5. Conclusions
Overall, our findings underscore the need for prospective studies with standardized protocols. However, despite data currently available being extremely heterogeneous to draw any definitive conclusion, it seems that both surgical and endovascular techniques for ILVA revascularization during total endovascular and hybrid treatment of aortic arch pathologies allow for an acceptable rate of mortality and neurological complications.
Until more robust data are available to indicate the superiority of one strategy over another, the revascularization technique for ILVA should be tailored based on the anatomic characteristics of the patient and the center experience.
6. Limitation
This study is inherently limited by the non-standardized nature of the cases selected for analysis, which affects the uniformity of variables of interest. To start with, the findings are primarily based on single-center experiences, which may introduce procedural bias related to specific expertise or preferred techniques at those centers. The rarity of the condition also limits the available sample size, reducing the overall strength of the study. Additionally, a direct comparison between the two groups is not feasible, further weakening the robustness of the conclusions.
Moreover, most of the included studies were of fair methodological quality, with common limitations such as incomplete data, lack of control groups, and underreporting of complications. Indeed, some articles imprecisely reported or did not clearly specify neurological outcomes, making interpretation difficult.
Altogether, these factors limit the generalizability of our findings and highlight the need for prospective studies employing standardized protocols. Therefore, the reported results should be interpreted with caution.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/jcm14217626/s1, Table S1: PRISMA checklist (Moher et al. 2009). Table S2: PICO framework. Table S3: Risk of Bias Assessment. Newcastle-Ottawa Scale Items. Table S4: Risk Of Bias in Non-randomized Studies – of Exposures (ROBINS-E) assessment.
Author Contributions
Conception and design: M.M., A.L., O.B. and Y.T.; analysis and interpretation: M.M., A.L., D.P., O.B. and L.R.; data collection: M.M., A.L., D.P. and L.R.; writing the article: M.M., A.L. and O.B.; critical revision of the article: O.B., T.D. and Y.T.; final approval of the article: M.M., A.L., D.P., L.R., O.B., G.T., T.D. and Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript.
| CTA | computed tomography angiography |
| ILVA | isolated left vertebral artery |
| IMH | intramural haematoma |
| ISF | in-situ fenestration |
| LCCA | left common carotid artery |
| LSA | left subclavian artery |
| PAU | penetrating aortic ulcer |
| PMF | Physician-modified fenestration |
| SAT | supra-aortic trunk |
| TEVAR | thoracic endovascular aortic aneurysm repair |
References
- Celikyay, Z.R.; Koner, A.E.; Celikyay, F.; Denız, C.; Acu, B.; Firat, M.M. Frequency and imaging findings of variations in human aortic arch anatomy based on multidetector computed tomography data. Clin. Imaging 2013, 37, 1011–1119. [Google Scholar] [CrossRef]
- Popieluszko, P.; Henry, B.M.; Sanna, B.; Hsieh, W.C.; Saganiak, K.; Pekala, P.A.; Walocha, J.A.; Tomaszewzki, K.A. A systematic review and meta-analysis of variations in branching patterns of the adult aortic arch. J. Vasc. Surg. 2018, 68, 298–306. [Google Scholar] [CrossRef]
- Dumfarth, J.; Chou, A.S.; Ziganshin, B.A.; Bhandari, R.; Peterss, S.; Tranquilli, M.; Mojibian, H.; Fang, H.; Rizzo, J.A.; Elefteriades, J.A. Atypical aortic arch branching variants: A novel marker for thoracic aortic disease. J. Thorac. Cardiovasc. Surg. 2015, 149, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, N.; Piagkou, M.; Loukas, M.; Piperaki, E.T.; Totlis, T.; Noussios, G.; Natsis, K. A systematic classification of the vertebral artery variable origin: Clinical and surgical implications. Surg. Radiol. Anat. 2018, 40, 779–797. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, K.; Shiiya, N.; Washiyama, N.; Yamashita, K.; Takahashi, D.; Tsuda, K.; Kando, Y. Vertebral artery variations in thoracic aortic patients. Eur. J. Cardiothorac. Surg. 2014, 46, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Wooster, M. Hybrid and Endovascular Management of Aortic Arch Pathology. J. Clin. Med. 2024, 13, 6248. [Google Scholar] [CrossRef]
- Matsumura, J.S.; Cambria, R.P.; Dake, M.D.; Moore, R.D.; Svensson, L.G.; Snyder, S.; TX2 Clinical Investigators. International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results. J. Vasc. Surg. 2008, 47, 247–257. [Google Scholar] [CrossRef]
- Yang, G.; Chen, H.; Sun, G.; Lou, W.; Chen, X.; Zhang, L. Transposition of Isolated Left Vertebral Artery in Hybrid Thoracic Endovascular Aortic Repair. Front. Cardiovasc. Med. 2021, 8, 783656. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, J.; Guo, M.; Liu, J.; Xu, D.; Lu, Y.; Zhu, H.; Liu, M.; Feng, R. Management of an Isolated Left Vertebral Artery on the Arch During Zone 2 Landing Thoracic Endovascular Aortic Repair: A Multicentre Retrospective Study. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 330–337. [Google Scholar] [CrossRef]
- Luo, Z.R.; Li, S.L.; Chen, L.W.; Huang, R.D. Utilizing physician modified fenestration on the castor branched stent technique for reconstruction of an isolated left vertebral artery on the aortic arch. Sci. Rep. 2024, 14, 4051. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Oderich, G.S.; Forbes, T.L.; Chaer, R.; Davies, M.G.; Lindsay, T.F.; Mastracci, T.; Singh, M.J.; Timaran, C.; Woo, E.Y.; Writing Committee Group. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J. Vasc. Surg. 2021, 73 (Suppl. 1), S4–S52. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’ Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2014. [Google Scholar]
- Piffaretti, G.; Gelpi, G.; Tadiello, M.; Ferrarese, S.; Socrate, A.M.; Tozzi, M.; Bellosta, R. Transposition of the left vertebral artery during endovascular stent-graft repair of the aortic arch. J. Thorac. Cardiovasc. Surg. 2020, 159, 2189–2198.e1. [Google Scholar] [CrossRef]
- Ding, H.; Zhu, Y.; Wang, H.; Luo, S.; Liu, Y.; Huang, W.; Dong, H.; Xue, L.; Fan, R.; Luo, J. Management of type B aortic dissection with an isolated left vertebral artery. J. Vasc. Surg. 2019, 70, 1065–1071. [Google Scholar] [CrossRef]
- Shen, P.; Li, D.; Wu, Z.; He, Y.; Wang, X.; Shang, T.; Zhu, Q.; Tian, L.; Li, Z.; Zhang, H. Physician-modified fenestration or in situ fenestration for preservation of isolated left vertebral artery in thoracic endovascular aortic repair. Front. Cardiovasc. Med. 2023, 10, 1055549. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, C.; Song, H.; Wei, D.; Meng, X.; Bai, X.; Liu, C.; Zhao, X. Endovascular repair of thoracic aortic disease with isolated left vertebral artery and unfavorable proximal landing zone using fenestrated castor stent-graft. Front. Cardiovasc. Med. 2023, 10, 1168180. [Google Scholar] [CrossRef]
- Shergill, E.S.; Udwadia, F.R.; Grubisic, M.; Salata, K.; Misskey, J.; Faulds, J. Comparative study of left vertebral artery revascularization in patients with and without aberrant left vertebral anatomy. J. Vasc. Surg. 2024, 79, 991–996. [Google Scholar] [CrossRef]
- Potter, H.A.; Ziegler, K.R.; Weaver, F.A.; Han, S.M.; Magee, G.A. Transposition of anomalous left vertebral to carotid artery during the management of thoracic aortic dissections and aneurysms. J. Vasc. Surg. 2022, 76, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Borghese, O.; Brisard, L.; Le Corvec, T.; Hauguel, A.; Guimbretière, G.; Maurel, B. Spinal Cord Protection During Thoracic and Thoracoabdominal Endovascular Aortic Repair: 5-Year Results of a Preventive Protocol Including Prophylactic Cerebrospinal Fluid Drainage in High-Risk Patients. J. Endovasc. Ther. 2023, 32, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, X.; Wu, S.; Li, X.; Xin, S.; Zhang, J. Comparison of Chimney and Fenestrated Techniques for Supra-Aortic Branch Revascularization During Thoracic Endovascular Aortic Repair: A Systematic Review and Meta-Analysis. Cardiovasc. Interv. Radiol. 2023, 46, 1315–1328. [Google Scholar] [CrossRef]
- Melissano, G.; Tshomba, Y.; Bertoglio, L.; Rinaldi, E.; Chiesa, R. Analysis of stroke after TEVAR involving the aortic arch. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 269–275, Erratum in: Eur. J. Vasc. Endovasc. Surg. 2012, 43, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Spanos, K.; Tsilimparis, N.; Rohlffs, F.; Wipper, S.; Detter, C.; Behrendt, C.A.; Debus, S.E.; Kölbel, T. Total endovascular arch repair is the procedure of the future. J. Cardiovasc. Surg. 2018, 59, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Riambau, V.; Böckler, D.; Brunkwall, J.; Cao, P.; Chiesa, R.; Coppi, G.; Czerny, M.; Fraedrich, G.; Haulon, S.; Jacobs, M.J.; et al. Editor’s Choice—Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.H.; Sillesen, H.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).