AKI Subtyping and Prognostic Analysis Based on Serum Electrolyte Features in ICU
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
2.2. Patients
2.3. Variables
2.4. Methods
3. Results
3.1. Baseline Data Characteristics of AKI Patients
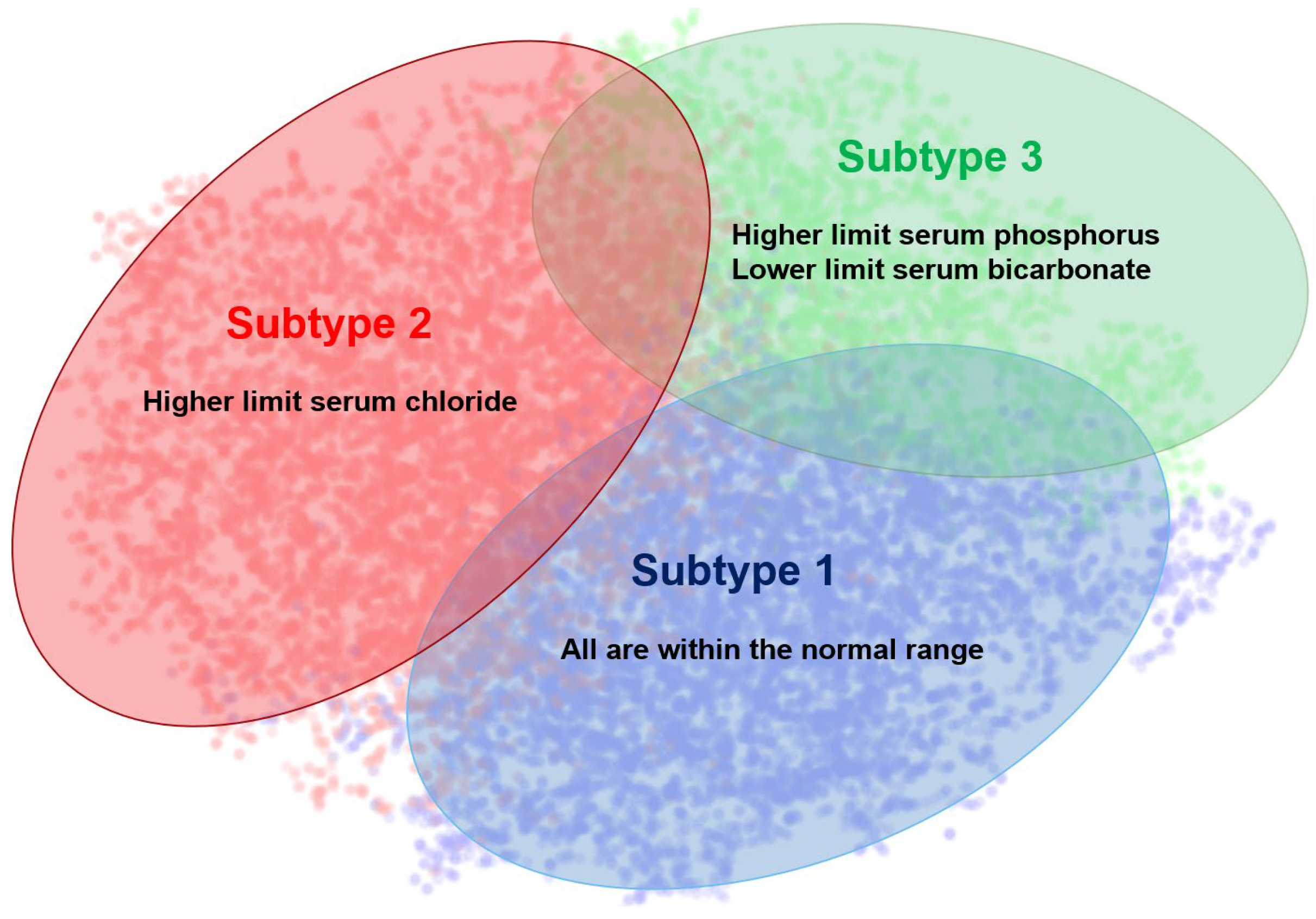
3.2. AKI Subtypes
3.3. Prognosis Analysis of AKI Subtypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | Acute Kidney Injury |
| ICU | Intensive Care Unit |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| SCr | Serum Creatinine |
| RRT | Renal Replacement Therapy |
References
- Gameiro, J.; Fonseca, J.A.; Jorge, S.; Lopes, J.A. Acute Kidney Injury Definition and Diagnosis: A Narrative Review. J. Clin. Med. 2018, 7, 307. [Google Scholar] [CrossRef]
- Shi, T.; Lin, Y.; Zhao, H.; Kong, G. Artificial intelligence models for predicting acute kidney injury in the intensive care unit: A systematic review of modeling methods, data utilization, and clinical applicability. JAMIA Open 2025, 8, ooaf065. [Google Scholar] [CrossRef]
- Maeda, A.; Inokuchi, R.; Bellomo, R.; Doi, K. Heterogeneity in the definition of major adverse kidney events: A scoping review. Intensive Care Med. 2024, 50, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Rewa, O.; Bagshaw, S.M. Acute kidney injury-epidemiology, outcomes and economics. Nat. Rev. Nephrol. 2014, 10, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; James, M.T. Acute Kidney Injury. Ann. Intern. Med. 2017, 167, Itc66–Itc80. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, T.; Kong, G. Acute Kidney Injury Prognosis Prediction Using Machine Learning Methods: A Systematic Review. Kidney Med. 2025, 7, 100936. [Google Scholar] [CrossRef]
- Yang, L.; Xing, G.; Wang, L.; Wu, Y.; Li, S.; Xu, G.; He, Q.; Chen, J.; Chen, M.; Liu, X.; et al. Acute kidney injury in China: A cross-sectional survey. Lancet 2015, 386, 1465–1471. [Google Scholar] [CrossRef]
- Bikbov, B.; Fortino, I.; Leoni, O.; Nobili, A.; Tettamanti, M. Burden of Acute Kidney Injury among Adult Hospital Patients in the Italian Lombardy Region: A 20-Year Real-World Data Analysis. Nephron 2023, 147, 599–607. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Chen, J.; Chen, Y.; Ying, Z.; Hu, Y.; Song, H.; Fu, P.; Zeng, X. The Time-Varying Impact of COVID-19 on the Acute Kidney Disorders: A Historical Matched Cohort Study and Mendelian Randomization Analysis. Health Data Sci. 2024, 4, 0159. [Google Scholar] [CrossRef]
- Srisawat, N.; Kulvichit, W.; Mahamitra, N.; Hurst, C.; Praditpornsilpa, K.; Lumlertgul, N.; Chuasuwan, A.; Trongtrakul, K.; Tasnarong, A.; Champunot, R.; et al. The epidemiology and characteristics of acute kidney injury in the Southeast Asia intensive care unit: A prospective multicentre study. Nephrol. Dial. Transplant. 2020, 35, 1729–1738. [Google Scholar] [CrossRef]
- Melo, F.A.F.; Burdmann, E.A.; Macedo, E.; Mehta, R.; Zanetta, D.M.T. Acute kidney injury developed in the intensive care unit: A population-based prospective cohort study in the Brazilian Amazon. Sci. Rep. 2024, 14, 22954. [Google Scholar] [CrossRef] [PubMed]
- Lilly, C.M.; Mickelson, J.T. Evolution of the Intensive Care Unit Telemedicine Value Proposition. Crit. Care Clin. 2019, 35, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Amparore, D.; Campi, R.; Checcucci, E.; Piana, A.; Sica, M.; Grosso, A.A.; Presutti, M.; Barzaghi, P.; Minervini, A.; Serni, S.; et al. Patients’ perspective on the use of telemedicine for outpatient urological visits: Learning from the COVID-19 outbreak. Actas Urológicas Españolas (Engl. Ed.) 2020, 44, 637–638. [Google Scholar] [CrossRef]
- Atreya, M.R.; Sanchez-Pinto, L.N.; Kamaleswaran, R. Commentary: ‘Critical illness subclasses: All roads lead to Rome’. Crit. Care 2022, 26, 387. [Google Scholar] [CrossRef]
- Porschen, C.; Strauss, C.; Meersch, M.; Zarbock, A. Personalized acute kidney injury treatment. Curr. Opin. Crit. Care 2023, 29, 551–558. [Google Scholar] [CrossRef]
- Selby, N.M. A Comment on the Diagnosis and Definition of Acute Kidney Injury. Nephron 2019, 141, 203–206. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Chaudhari, H.; Mahendrakar, S.; Baskin, S.E.; Reddi, A.S. Contrast-Induced Acute Kidney Injury: Evidence in Support of Its Existence and a Review of Its Pathogenesis and Management. Int. J. Nephrol. Renov. Dis. 2022, 15, 253–266. [Google Scholar] [CrossRef]
- Chaudhary, K.; Vaid, A.; Duffy, Á.; Paranjpe, I.; Jaladanki, S.; Paranjpe, M.; Johnson, K.; Gokhale, A.; Pattharanitima, P.; Chauhan, K.; et al. Utilization of Deep Learning for Subphenotype Identification in Sepsis-Associated Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2020, 15, 1557–1565. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Prince, D.K.; Mansour, S.; Ikizler, T.A.; Siew, E.D.; Chinchilli, V.M.; Garg, A.X.; Go, A.S.; Kaufman, J.S.; Kimmel, P.L.; et al. Integrated Analysis of Blood and Urine Biomarkers to Identify Acute Kidney Injury Subphenotypes and Associations with Long-term Outcomes. Am. J. Kidney Dis. 2023, 82, 311–321.e1. [Google Scholar] [CrossRef]
- Wiersema, R.; Jukarainen, S.; Vaara, S.T.; Poukkanen, M.; Lakkisto, P.; Wong, H.; Linder, A.; van der Horst, I.C.C.; Pettilä, V. Two subphenotypes of septic acute kidney injury are associated with different 90-day mortality and renal recovery. Crit. Care 2020, 24, 150. [Google Scholar] [CrossRef]
- Lee, S.W.; Baek, S.H.; Ahn, S.Y.; Na, K.Y.; Chae, D.-W.; Chin, H.J.; Kim, S. The Effects of Pre-Existing Hyponatremia and Subsequent-Developing Acute Kidney Injury on In-Hospital Mortality: A Retrospective Cohort Study. PLoS ONE 2016, 11, e0162990. [Google Scholar] [CrossRef]
- Woitok, B.K.; Funk, G.-C.; Walter, P.; Schwarz, C.; Ravioli, S.; Lindner, G. Dysnatremias in emergency patients with acute kidney injury: A cross-sectional analysis. Am. J. Emerg. Med. 2020, 38, 2602–2606. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Gambaro, G.; Ferraro, P.M. Serum Potassium Disorders Predict Subsequent Kidney Injury: A Retrospective Observational Cohort Study of Hospitalized Patients. Kidney Blood Press. Res. 2022, 47, 270–276. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Vaitla, P.; Nissaisorakarn, V.; Mao, M.A.; Genovez, J.L.Z.; Kattah, A.G.; Pattharanitima, P.; Vallabhajosyula, S.; Keddis, M.T.; Qureshi, F.; et al. Clinically Distinct Subtypes of Acute Kidney Injury on Hospital Admission Identified by Machine Learning Consensus Clustering. Med. Sci. 2021, 9, 60. [Google Scholar] [CrossRef]
- Lai, C.-F.; Liu, J.-H.; Tseng, L.-J.; Tsao, C.-H.; Chou, N.-K.; Lin, S.-L.; Chen, Y.-M.; Wu, V.-C. Unsupervised clustering identifies sub-phenotypes and reveals novel outcome predictors in patients with dialysis-requiring sepsis-associated acute kidney injury. Ann. Med. 2023, 55, 2197290. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Huang, J.; Zhuang, J.; Huang, H.; Jiang, S.; She, M.; Tian, M.; Liu, Y.; Yu, X. Identifying acute kidney injury subphenotypes using an outcome-driven deep-learning approach. J. Biomed. Inform. 2023, 143, 104393. [Google Scholar] [CrossRef]
- Xiao, W.; Huang, L.; Guo, H.; Liu, W.; Zhang, J.; Liu, Y.; Hua, T.; Yang, M. Development and validation of potential phenotypes of serum electrolyte disturbances in critically ill patients and a Web-based application. J. Crit. Care 2024, 82, 154793. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.J.; Johnson, A.E.W.; Raffa, J.D.; Celi, L.A.; Mark, R.G.; Badawi, O. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci. Data 2018, 5, 180178. [Google Scholar] [CrossRef]
- Dalmaijer, E.S.; Nord, C.L.; Astle, D.E. Statistical power for cluster analysis. BMC Bioinform. 2022, 23, 205. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Kim, H.; Park, S.; Jhee, J.H.; Yun, H.-R.; Kim, H.; Kee, Y.K.; Yoon, C.-Y.; Oh, H.J.; Chang, T.I.; et al. Electrolyte and mineral disturbances in septic acute kidney injury patients undergoing continuous renal replacement therapy. Medicine 2016, 95, e4542. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.G.; Yun, D.; Kang, M.W.; Kim, Y.C.; Kim, D.K.; Oh, K.-H.; Joo, K.W.; Koo, H.S.; Han, S.S. Longitudinal trajectory of acidosis and mortality in acute kidney injury requiring continuous renal replacement therapy. BMC Nephrol. 2022, 23, 411. [Google Scholar] [CrossRef] [PubMed]
- Bullivant, E.M.; Wilcox, C.S.; Welch, W.J. Intrarenal vasoconstriction during hyperchloremia: Role of thromboxane. Am. J. Physiol. Ren. Physiol. 1989, 256 Pt 2, F152–F157. [Google Scholar] [CrossRef]
- Boniatti, M.M.; Cardoso, P.R.; Castilho, R.K.; Vieira, S.R. Is hyperchloremia associated with mortality in critically ill patients? A prospective cohort study. J. Crit. Care 2011, 26, 175–179. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcellos KSkinner, D.L. Hyperchloraemia is associated with acute kidney injury and mortality in the critically ill: A retrospective observational study in a multidisciplinary intensive care unit. J. Crit. Care 2018, 45, 45–51. [Google Scholar] [CrossRef]
- Itoh, J.; Aoki, Y.; Omoto, M.; Katsuragawa, T.; Mimuro, S.; Nakajima, Y. Association Between Early Hyponatremia and Clinical Outcomes in Critically Ill Patients: A Retrospective Cohort Study. Cureus 2024, 16, 56138. [Google Scholar] [CrossRef]
- Vaara, S.T.; Bhatraju, P.K.; Stanski, N.L.; McMahon, B.A.; Liu, K.; Joannidis, M.; Bagshaw, S.M. Subphenotypes in acute kidney injury: A narrative review. Crit. Care 2022, 26, 251. [Google Scholar] [CrossRef]
- Zhao, G.-J.; Xu, C.; Ying, J.-C.; Lü, W.-B.; Hong, G.-L.; Li, M.-F.; Wu, B.; Yao, Y.-M.; Lu, Z.-Q. Association between furosemide administration and outcomes in critically ill patients with acute kidney injury. Crit. Care 2020, 24, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ren, Q.; Li, X.; Han, H.; Xie, K.; Wang, G. Association between furosemide administration and clinical outcomes in patients with sepsis-associated acute kidney injury receiving renal replacement therapy: A retrospective observational cohort study based on MIMIC-IV database. BMJ Open 2023, 13, e074046. [Google Scholar] [CrossRef]
- Mehta, R.L.; Pascual, M.T.; Soroko, S.; Chertow, G.M. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. Jama 2002, 288, 2547–2553. [Google Scholar] [CrossRef]
- Uchino, S.; Doig, G.S.; Bellomo, R.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N.; et al. Diuretics and mortality in acute renal failure. Crit. Care Med. 2004, 32, 1669–1677. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Gibney, R.N.; Kruger, P.; Hassan, I.; McAlister, F.A.; Bellomo, R. The effect of low-dose furosemide in critically ill patients with early acute kidney injury: A pilot randomized blinded controlled trial (the SPARK study). J. Crit. Care 2017, 42, 138–146. [Google Scholar] [CrossRef]
- Clec’h, C.; Darmon, M.; Lautrette, A.; Chemouni, F.; Azoulay, E.; Schwebel, C.; Dumenil, A.-S.; Garrouste-Orgeas, M.; Goldgran-Toledano, D.; Cohen, Y.; et al. Efficacy of renal replacement therapy in critically ill patients: A propensity analysis. Crit. Care 2012, 16, R236. [Google Scholar] [CrossRef] [PubMed]
- Metnitz, P.G.H.; Krenn, C.G.; Steltzer, H.; Lang, T.; Ploder, J.; Lenz, K.; Le Gall, J.-R.; Druml, W. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit. Care Med. 2002, 30, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, M. Loop diuretic use in patients with AKI: Different severity, different response. Crit. Care 2018, 22, 202. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F.P.; Yang, W.; Machado, C.A.; Mariani, L.H.; Borovskiy, Y.; Berns, J.S.; Feldman, H.I. Dialysis versus nondialysis in patients with AKI: A propensity-matched cohort study. Clin. J. Am. Soc. Nephrol. 2014, 9, 673–681. [Google Scholar] [CrossRef]
- Libório, A.B.; Leite, T.T.; Neves, F.M.d.O.; Teles, F.; Bezerra, C.T.d.M. AKI complications in critically ill patients: Association with mortality rates and RRT. Clin. J. Am. Soc. Nephrol. 2015, 10, 21–28. [Google Scholar] [CrossRef]
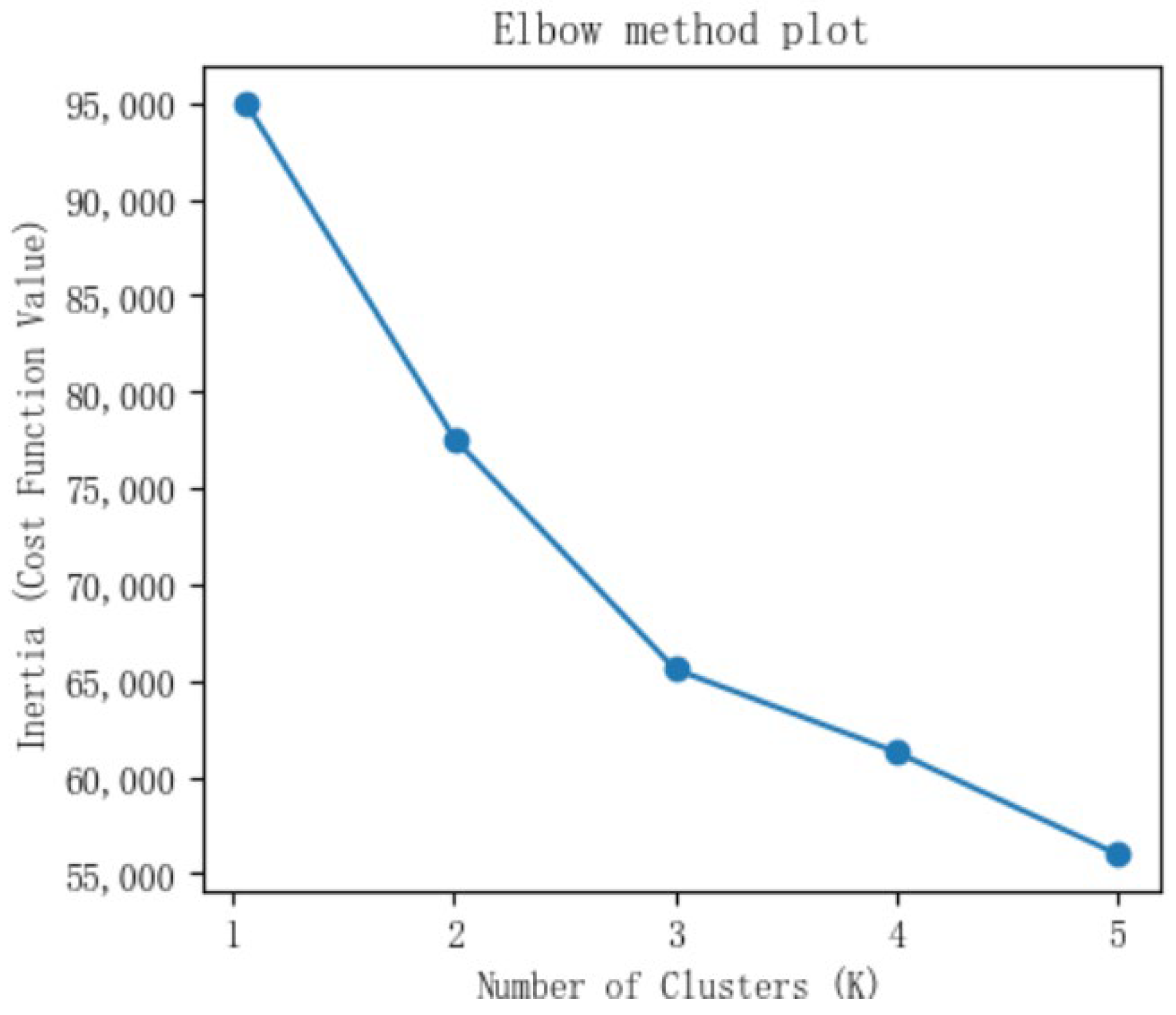

| Variables | Mean Value (±Standard Deviation)/ Frequency (Percentage) | |
|---|---|---|
| eICU-CRD (n= 15,838) | Chinese Local Critical Care Database (n = 431) | |
| Age | 62.22 ± 16.29 | 68.80 ± 8.11 |
| Male, n (%) | 8654 (54.64) | 239 (55.45) |
| Heart rate, BPM | 119.88 ± 24.73 | 103.89 ± 33.63 |
| Respiratory rate, breaths/min | 33.51 ± 9.70 | 35.33 ± 16.90 |
| Blood glucose, mg/dL | 153.71 ± 88.78 | 132.40 ± 25.23 |
| Platelets, 109/L | 194.52 ± 106.02 | 150.22 ± 62.65 |
| White blood cells, 109/L | 12.88 ± 9.50 | 14.51 ± 8.32 |
| Blood urea nitrogen, μmol/L | 24.21 ± 11.14 | 27.68 ± 12.92 |
| Hemoglobin, g/dL | 10.38 ± 2.01 | 10.41 ± 3.88 |
| Diuretics, n (%) | 1381 (8.72) | 109 (25.29) |
| RRT, n (%) | 1128 (7.12) | 74 (17.17) |
| In-hospital mortality, n (%) | 2748 (17.35) | 158 (36.66) |
| Variables (mmol/L) | Mean Value (±Standard Deviation) | ||
|---|---|---|---|
| Subtype 1 (n = 6364) | Subtype 2 (n = 6624) | Subtype 3 (n = 2850) | |
| Serum Sodium | 136.26 ± 4.63 | 142.95 ± 4.75 | 136.24 ± 5.42 |
| Serum Potassium | 3.96 ± 0.52 | 3.89 ± 0.52 | 5.01 ± 0.81 |
| Serum Chloride | 100.48 ± 4.95 | 110.89 ± 4.73 | 102.51 ± 6.72 |
| Serum Phosphate | 1.04 ± 0.33 | 0.98 ± 0.35 | 1.88 ± 0.67 |
| Serum Magnesium | 0.79 ± 0.14 | 0.79 ± 0.16 | 0.90 ± 0.22 |
| Serum Bicarbonate | 26.68 ± 4.91 | 22.03 ± 4.20 | 19.30 ± 5.12 |
| Variables | In-Hospital Mortality | In-Hospital Mortality | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Subtype 1 | ||||||
| Subtype 2 | 1.13 | 1.02–1.25 | 0.025 | |||
| Subtype 3 | 1.52 | 1.33–1.73 | <0.001 | 1.43 | 1.25–1.63 | <0.001 |
| Treatments | Subtype 1 | Subtype 2 | Subtype 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Diuretics | 1.25 | 0.99–1.58 | 0.066 | 1.30 | 1.01–1.66 | 0.044 | 0.71 | 0.50–0.99 | 0.010 |
| RRT | 1.30 | 0.95–1.77 | 0.097 | 1.56 | 1.17–2.09 | 0.003 | 0.78 | 0.61–0.99 | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Shi, T.; Xu, H.; Zhao, H.; Kong, G. AKI Subtyping and Prognostic Analysis Based on Serum Electrolyte Features in ICU. J. Clin. Med. 2025, 14, 7623. https://doi.org/10.3390/jcm14217623
Liu W, Shi T, Xu H, Zhao H, Kong G. AKI Subtyping and Prognostic Analysis Based on Serum Electrolyte Features in ICU. Journal of Clinical Medicine. 2025; 14(21):7623. https://doi.org/10.3390/jcm14217623
Chicago/Turabian StyleLiu, Wentie, Tongyue Shi, Haowei Xu, Huiying Zhao, and Guilan Kong. 2025. "AKI Subtyping and Prognostic Analysis Based on Serum Electrolyte Features in ICU" Journal of Clinical Medicine 14, no. 21: 7623. https://doi.org/10.3390/jcm14217623
APA StyleLiu, W., Shi, T., Xu, H., Zhao, H., & Kong, G. (2025). AKI Subtyping and Prognostic Analysis Based on Serum Electrolyte Features in ICU. Journal of Clinical Medicine, 14(21), 7623. https://doi.org/10.3390/jcm14217623






