Stress Echocardiography in Aortic Stenosis: From Diagnostic Challenges to Guideline-Endorsed Clinical Applications
Abstract
1. Introduction
2. Aim of the Review
3. Methods
4. Principles of Stress Echocardiography in Aortic Stenosis
4.1. Pathophysiological Rationale in Aortic Stenosis
4.2. Types of Stress Modalities
4.3. Protocol Standardization
4.4. Safety Considerations and Contraindications
5. Exercise Stress Echocardiography in Asymptomatic Severe Aortic Stenosis
5.1. Rationale for Exercise Testing
5.2. Evidence Base and Prognostic Markers During ESE
5.3. Clinical Decision-Making
6. Dobutamine Stress Echocardiography in Low-Flow, Low-Gradient Aortic Stenosis
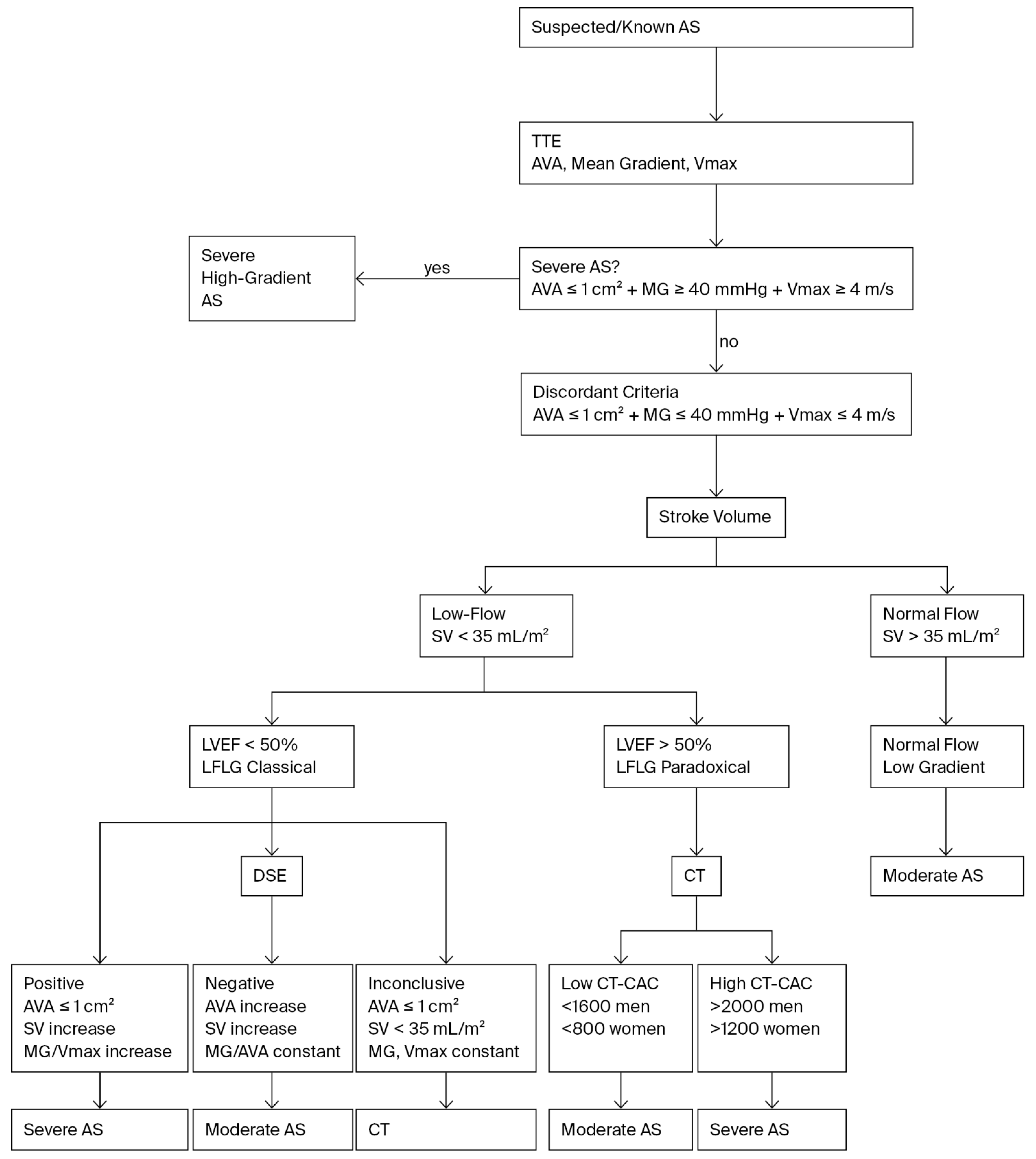
6.1. The Clinical Problem of Low-Flow, Low-Gradient Aortic Stenosis
6.2. Projected Aortic Valve Area
6.3. Prognostic Implications
6.4. Recent Refinements and Contemporary Insights
6.5. Comparative Assessment of Dobutamine Stress Echocardiography and Computed Tomography Calcium Scoring in Low-Flow, Low-Gradient Aortic Stenosis
Limitations of Stress Echocardiography and the Role of CT-CAC
7. Hemodynamic and Functional Parameters During Stress Echocardiography
7.1. Left Ventricular Systolic Reserve
7.2. Left Ventricular Diastolic Function
7.3. Transaortic Flow and Gradients
7.4. Myocardial Deformation Imaging
7.5. Valvulo-Arterial Impedance and Global Afterload
7.6. Concept of Volume–Flow (V–Q) Discordance
8. Guideline Recommendations and Consensus Statements
9. Clinical Scenario and Utility for Using SE in AS
10. Knowledge Gaps and Future Directions
10.1. Integration with Multimodality Imaging
10.2. Advanced Echocardiographic Technologies
10.3. Artificial Intelligence and Automated Analysis
10.4. Role in the Transcatheter Era
10.5. Standardization and Broader Adoption
10.6. Barriers to the Widespread Use of Stress Echocardiography in Routine Clinical Practice
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aluru, J.S.; Barsouk, A.; Saginala, K.; Rawla, P.; Barsouk, A. Valvular Heart Disease Epidemiology. Med. Sci. 2022, 10, 32. [Google Scholar] [CrossRef]
- Chen, Q.F.; Shi, S.; Wang, Y.F.; Shi, J.; Liu, C.; Xu, T.; Ni, C.; Zhou, X.; Lin, W.; Peng, Y.; et al. Global, Regional, and National Burden of Valvular Heart Disease, 1990 to 2021. J. Am. Heart Assoc. 2024, 13, e037991. [Google Scholar] [CrossRef]
- Peters, A.S.; Duggan, J.P.; Trachiotis, G.D.; Antevil, J.L. Epidemiology of Valvular Heart Disease. Surg. Clin. North. Am. 2022, 102, 517–528. [Google Scholar] [CrossRef]
- Iung, B.; Vahanian, A. Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 2011, 8, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.A.; Rasania, S.P.; Afilalo, J.; Popma, J.J.; Lipsitz, L.A.; Kim, D.H. Functional status and quality of life after transcatheter aortic valve replacement: A systematic review. Ann. Intern. Med. 2014, 160, 243–254. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Clavel, M.A.; Magne, J.; Pibarot, P. Low-gradient aortic stenosis. Eur. Heart J. 2016, 37, 2645–2657. [Google Scholar] [CrossRef]
- Alkhalaila, O.; Shehadat, M.A. Low-Gradient Aortic Stenosis; the Diagnostic Dilemma. Heart Views 2022, 23, 39–46. [Google Scholar] [CrossRef]
- Mogensen, N.S.B.; Ali, M.; Carter-Storch, R.; Annabi, M.S.; Grenier-Delaney, J.; Møller, J.E.; Øvrehus, K.A.; Pellikka, P.A.; Pibarot, P.; Clavel, M.A.; et al. Dobutamine Stress Echocardiography in Low-Gradient Aortic Stenosis. J. Am. Soc. Echocardiogr. 2024, 37, 1023–1033. [Google Scholar] [CrossRef]
- Adamo, M.; Pagnesi, M.; Chioncel, O.; Bayes-Genis, A.; Abdelhamid, M.; Antohi, E.L.; Bucciarelli-Ducci, C.; Chieffo, A.; Cosyns, B.; Gilard, M.; et al. Management of aortic stenosis and chronic heart failure: A clinical consensus statement of the Heart Failure Association (HFA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC. Eur. J. Heart Fail. 2025. [Google Scholar] [CrossRef]
- Piérard, L.A.; Lancellotti, P. Stress testing in valve disease. Heart 2007, 93, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Blais, C.; Burwash, I.G.; Mundigler, G.; Dumesnil, J.G.; Loho, N.; Rader, F.; Baumgartner, H.; Beanlands, R.S.; Chayer, B.; Kadem, L.; et al. Projected valve area at normal flow rate improves the assessment of stenosis severity in patients with low-flow, low-gradient aortic stenosis: The multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) study. Circulation 2006, 113, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Lebois, F.; Simon, M.; Tombeux, C.; Chauvel, C.; Pierard, L.A. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005, 112, I377–I382. [Google Scholar] [CrossRef]
- Lancellotti, P.; Magne, J.; Donal, E.; O’Connor, K.; Dulgheru, R.; Rosca, M.; Pierard, L.A. Determinants and prognostic significance of exercise pulmonary hypertension in asymptomatic severe aortic stenosis. Circulation 2012, 126, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Magne, J.; Lancellotti, P.; Piérard, L.A. Exercise testing in asymptomatic severe aortic stenosis. JACC Cardiovasc. Imaging 2014, 7, 188–199. [Google Scholar] [CrossRef]
- Abergel, E.; Venner, C.; Tribouilloy, C.; Chauvel, C.; Simon, M.; Codiat, R.; Piechaud, T.; Maurin, V.; Dejour, E.; Kumble, A.; et al. Prognostic Value and Safety of Serial Exercise Echocardiography in Asymptomatic Severe Aortic Stenosis. J. Am. Heart Assoc. 2025, 14, e036599. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef]
- Picano, E.; Ciampi, Q.; Citro, R.; D’Andrea, A.; Scali, M.C.; Cortigiani, L.; Olivotto, I.; Mori, F.; Galderisi, M.; Costantino, M.F.; et al. Stress echo 2020: The international stress echo study in ischemic and non-ischemic heart disease. Cardiovasc. Ultrasound 2017, 15, 3. [Google Scholar] [CrossRef]
- Ring, L.; Shah, B.N.; Bhattacharyya, S.; Harkness, A.; Belham, M.; Oxborough, D.; Pearce, K.; Rana, B.S.; Augustine, D.X.; Robinson, S.; et al. Echocardiographic assessment of aortic stenosis: A practical guideline from the British Society of Echocardiography. Echo Res. Pract. 2021, 8, G19–G59. [Google Scholar] [CrossRef]
- Redfors, B.; Pibarot, P.; Gillam, L.D.; Burkhoff, D.; Bax, J.J.; Lindman, B.R.; Bonow, R.O.; O’Gara, P.T.; Leon, M.B.; Généreux, P. Stress Testing in Asymptomatic Aortic Stenosis. Circulation 2017, 135, 1956–1976. [Google Scholar] [CrossRef]
- Annabi, M.S.; Clavel, M.A.; Pibarot, P. Dobutamine Stress Echocardiography in Low-Flow, Low-Gradient Aortic Stenosis: Flow Reserve Does Not Matter Anymore. J. Am. Heart Assoc. 2019, 8, e012212. [Google Scholar] [CrossRef]
- Sato, K.; Sankaramangalam, K.; Kandregula, K.; Bullen, J.A.; Kapadia, S.R.; Krishnaswamy, A.; Mick, S.; Rodriguez, L.L.; Grimm, R.A.; Menon, V.; et al. Contemporary Outcomes in Low-Gradient Aortic Stenosis Patients Who Underwent Dobutamine Stress Echocardiography. J. Am. Heart Assoc. 2019, 8, e011168. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, Q.; Zagatina, A.; Cortigiani, L.; Gaibazzi, N.; Borguezan Daros, C.; Zhuravskaya, N.; Wierzbowska-Drabik, K.; Kasprzak, J.D.; de Castro, E.S.P.J.L.; D’Andrea, A.; et al. Functional, Anatomical, and Prognostic Correlates of Coronary Flow Velocity Reserve During Stress Echocardiography. J. Am. Coll. Cardiol. 2019, 74, 2278–2291. [Google Scholar] [CrossRef]
- Rassi, A.N.; Aljaroudi, W.; Naderi, S.; Alraies, M.C.; Menon, V.; Rodriguez, L.; Grimm, R.; Griffin, B.; Jaber, W.A. Exercise stress echocardiography in patients with aortic stenosis: Impact of baseline diastolic dysfunction and functional capacity on mortality and aortic valve replacement. Cardiovasc. Diagn. Ther. 2013, 3, 205–215. [Google Scholar] [CrossRef]
- Maréchaux, S.; Hachicha, Z.; Bellouin, A.; Dumesnil, J.G.; Meimoun, P.; Pasquet, A.; Bergeron, S.; Arsenault, M.; Le Tourneau, T.; Ennezat, P.V.; et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur. Heart J. 2010, 31, 1390–1397. [Google Scholar] [CrossRef]
- Gahl, B.; Çelik, M.; Head, S.J.; Vanoverschelde, J.L.; Pibarot, P.; Reardon, M.J.; van Mieghem, N.M.; Kappetein, A.P.; Jüni, P.; Da Costa, B.R. Natural History of Asymptomatic Severe Aortic Stenosis and the Association of Early Intervention With Outcomes: A Systematic Review and Meta-analysis. JAMA Cardiol. 2020, 5, 1102–1112. [Google Scholar] [CrossRef]
- Sharma, N.; Sachedina, A.K.; Kumar, S. Low-flow, Low-gradient Severe Aortic Stenosis: A Review. Heart Int. 2023, 17, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Snir, A.D.; Ng, M.K.; Strange, G.; Playford, D.; Stewart, S.; Celermajer, D.S. Prevalence and Outcomes of Low-Gradient Severe Aortic Stenosis-From the National Echo Database of Australia. J. Am. Heart Assoc. 2021, 10, e021126. [Google Scholar] [CrossRef] [PubMed]
- Maes, F.; Lerakis, S.; Barbosa Ribeiro, H.; Gilard, M.; Cavalcante, J.L.; Makkar, R.; Herrmann, H.C.; Windecker, S.; Enriquez-Sarano, M.; Cheema, A.N.; et al. Outcomes From Transcatheter Aortic Valve Replacement in Patients With Low-Flow, Low-Gradient Aortic Stenosis and Left Ventricular Ejection Fraction Less Than 30%: A Substudy From the TOPAS-TAVI Registry. JAMA Cardiol. 2019, 4, 64–70. [Google Scholar] [CrossRef]
- Clavel, M.A.; Ennezat, P.V.; Maréchaux, S.; Dumesnil, J.G.; Capoulade, R.; Hachicha, Z.; Mathieu, P.; Bellouin, A.; Bergeron, S.; Meimoun, P.; et al. Stress echocardiography to assess stenosis severity and predict outcome in patients with paradoxical low-flow, low-gradient aortic stenosis and preserved LVEF. JACC Cardiovasc. Imaging 2013, 6, 175–183. [Google Scholar] [CrossRef]
- Clavel, M.A.; Burwash, I.G.; Mundigler, G.; Dumesnil, J.G.; Baumgartner, H.; Bergler-Klein, J.; Sénéchal, M.; Mathieu, P.; Couture, C.; Beanlands, R.; et al. Validation of conventional and simplified methods to calculate projected valve area at normal flow rate in patients with low flow, low gradient aortic stenosis: The multicenter TOPAS (True or Pseudo Severe Aortic Stenosis) study. J. Am. Soc. Echocardiogr. 2010, 23, 380–386. [Google Scholar] [CrossRef]
- Fougères, E.; Tribouilloy, C.; Monchi, M.; Petit-Eisenmann, H.; Baleynaud, S.; Pasquet, A.; Chauvel, C.; Metz, D.; Adams, C.; Rusinaru, D.; et al. Outcomes of pseudo-severe aortic stenosis under conservative treatment. Eur. Heart J. 2012, 33, 2426–2433. [Google Scholar] [CrossRef]
- Kadem, L.; Rieu, R.; Dumesnil, J.G.; Durand, L.G.; Pibarot, P. Flow-dependent changes in Doppler-derived aortic valve effective orifice area are real and not due to artifact. J. Am. Coll. Cardiol. 2006, 47, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Pawade, T.; Clavel, M.A.; Tribouilloy, C.; Dreyfus, J.; Mathieu, T.; Tastet, L.; Renard, C.; Gun, M.; Jenkins, W.S.A.; Macron, L.; et al. Computed Tomography Aortic Valve Calcium Scoring in Patients With Aortic Stenosis. Circ. Cardiovasc. Imaging 2018, 11, e007146. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.R.; Clavel, M.A.; Messika-Zeitoun, D.; Cueff, C.; Malouf, J.; Araoz, P.A.; Mankad, R.; Michelena, H.; Vahanian, A.; Enriquez-Sarano, M. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ. Cardiovasc. Imaging 2013, 6, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.C.; Hung, J.C.-C.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 254–275. [Google Scholar] [CrossRef] [PubMed]
- Besir, B.; Ramu, S.K.; Majeed-Saidan, M.M.A.; Rajendran, J.; Iskandar, O.; Reed, G.; Puri, R.; Yun, J.; Harb, S.; Miyasaka, R.; et al. Outcomes and Predictors of Different Flow-Gradient Patterns of Aortic Stenosis After Transcatheter Aortic Valve Replacement. Am. J. Cardiol. 2025, 242, 42–52. [Google Scholar] [CrossRef]
- Zhu, D.; Ito, S.; Miranda, W.R.; Nkomo, V.T.; Pislaru, S.V.; Villarraga, H.R.; Pellikka, P.A.; Crusan, D.J.; Oh, J.K. Left Ventricular Global Longitudinal Strain Is Associated With Long-Term Outcomes in Moderate Aortic Stenosis. Circ. Cardiovasc. Imaging 2020, 13, e009958. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Chen, H.; Li, H. Prognostic Value of Global Longitudinal Strain in Asymptomatic Aortic Stenosis: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 778027. [Google Scholar] [CrossRef]
- Picano, E.; Ciampi, Q.; Cortigiani, L.; Arruda-Olson, A.M.; Borguezan-Daros, C.; de Castro, E.S.P.J.L.; Cocchia, R.; Bossone, E.; Merli, E.; Kane, G.C.; et al. Stress Echo 2030: The Novel ABCDE-(FGLPR) Protocol to Define the Future of Imaging. J. Clin. Med. 2021, 10, 3641. [Google Scholar] [CrossRef] [PubMed]
- Vollema, E.M.; Sugimoto, T.; Shen, M.; Tastet, L.; Ng, A.C.T.; Abou, R.; Marsan, N.A.; Mertens, B.; Dulgheru, R.; Lancellotti, P.; et al. Association of Left Ventricular Global Longitudinal Strain With Asymptomatic Severe Aortic Stenosis: Natural Course and Prognostic Value. JAMA Cardiol. 2018, 3, 839–847. [Google Scholar] [CrossRef]
- Dahl, J.S.; Videbæk, L.; Poulsen, M.K.; Rudbæk, T.R.; Pellikka, P.A.; Møller, J.E. Global Strain in Severe Aortic Valve Stenosis. Circ. Cardiovasc. Imaging 2012, 5, 613–620. [Google Scholar] [CrossRef]
- Stens, N.A.; van Iersel, O.; Rooijakkers, M.J.P.; van Wely, M.H.; Nijveldt, R.; Bakker, E.A.; Rodwell, L.; Pedersen, A.L.D.; Poulsen, S.H.; Kjønås, D.; et al. Prognostic Value of Preprocedural LV Global Longitudinal Strain for Post-TAVR-Related Morbidity and Mortality: A Meta-Analysis. JACC Cardiovasc. Imaging 2023, 16, 332–341. [Google Scholar] [CrossRef]
- Levy-Neuman, S.; Meledin, V.; Gandelman, G.; Goland, S.; Zilberman, L.; Edri, O.; Shneider, N.; Abaeh, N.; Bdolah-Abram, T.; George, J.; et al. The Association Between Longitudinal Strain at Rest and Stress and Outcome in Asymptomatic Patients with Moderate and Severe Aortic Stenosis. J. Am. Soc. Echocardiogr. 2019, 32, 722–729. [Google Scholar] [CrossRef]
- Briand, M.; Dumesnil, J.G.; Kadem, L.; Tongue, A.G.; Rieu, R.; Garcia, D.; Pibarot, P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: Implications for diagnosis and treatment. J. Am. Coll. Cardiol. 2005, 46, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, Z.; Dumesnil, J.G.; Pibarot, P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J. Am. Coll. Cardiol. 2009, 54, 1003–1011. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.W.; Kane, G.C.; et al. The Clinical Use of Stress Echocardiography in Non-Ischaemic Heart Disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 101–138. [Google Scholar] [CrossRef] [PubMed]
- Eleid, M.F.; Sorajja, P.; Michelena, H.I.; Malouf, J.F.; Scott, C.G.; Pellikka, P.A. Flow-gradient patterns in severe aortic stenosis with preserved ejection fraction: Clinical characteristics and predictors of survival. Circulation 2013, 128, 1781–1789. [Google Scholar] [CrossRef]
- Minners, J.; Allgeier, M.; Gohlke-Baerwolf, C.; Kienzle, R.P.; Neumann, F.J.; Jander, N. Inconsistent grading of aortic valve stenosis by current guidelines: Haemodynamic studies in patients with apparently normal left ventricular function. Heart 2010, 96, 1463–1468. [Google Scholar] [CrossRef]
- Hungerford, S.L.; Song, N.; Loo, B.; Rye, E.; Sritharan, H.; Everett, K.D.; Hayward, C.S.; Kapur, N.K.; Muller, D.W.M.; Adji, A.I. The Effect of Volume-Flow Discordance on Survival in Severe Aortic Stenosis. JACC Asia 2025, 34, S455–S456. [Google Scholar] [CrossRef]
- Kusunose, K.; Yamada, H.; Nishio, S.; Torii, Y.; Hirata, Y.; Seno, H.; Saijo, Y.; Ise, T.; Yamaguchi, K.; Yagi, S.; et al. Preload Stress Echocardiography Predicts Outcomes in Patients with Preserved Ejection Fraction and Low-Gradient Aortic Stenosis. Circ. Cardiovasc. Imaging 2017, 10, e006690. [Google Scholar] [CrossRef]
- Hachicha, Z.; Dumesnil, J.G.; Bogaty, P.; Pibarot, P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007, 115, 2856–2864. [Google Scholar] [CrossRef]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Ajmone Marsan, N.; Barili, F.; Bonaros, N.; Cosyns, B.; et al. 2025 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2025, 67, ezaf276. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; La Canna, G.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; Vannan, M.A.; et al. Multi-modality imaging assessment of native valvular regurgitation: An EACVI and ESC council of valvular heart disease position paper. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e171–e232. [Google Scholar] [CrossRef] [PubMed]
- Garbi, M.; Habib, G.; Plein, S.; Neglia, D.; Kitsiou, A.; Donal, E.; Pinto, F.; Bax, J.; Achenbach, S.; Popescu, B.A.; et al. Appropriateness criteria for cardiovascular imaging use in clinical practice: A position statement of the ESC/EACVI taskforce. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Loganath, K.; Bing, R.; Treibel, T.A.; McCann, G.P.; Newby, D.E.; Leipsic, J.; Fraccaro, C.; Paolisso, P.; Cosyns, B.; et al. Multi-modality imaging in aortic stenosis: An EACVI clinical consensus document. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1430–1443. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; Seiffert, M.; Vonthein, R.; Baumgartner, H.; Bleiziffer, S.; Borger, M.A.; Choi, Y.H.; Clemmensen, P.; Cremer, J.; Czerny, M.; et al. Transcatheter or Surgical Treatment of Aortic-Valve Stenosis. N. Engl. J. Med. 2024, 390, 1572–1583. [Google Scholar] [CrossRef]
- Gasparovic, H.; Tokic, T. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N. Engl. J. Med. 2024, 390, 865–866. [Google Scholar] [CrossRef] [PubMed]
| Feature | Exercise Stress Echocardiography | Dobutamine Stress Echocardiography |
|---|---|---|
| Primary patient population | Asymptomatic or equivocal-symptom severe AS with preserved LVEF | LFLG AS with reduced LVEF (<50%) |
| Main purpose | Risk stratification and unmasking of latent symptoms Functional hemodynamic assessment under physiological load | Differentiates true severe vs. pseudo-severe AS in LFLG states Evaluates CR |
| Stress modality | Semi-supine bicycle ergometry (preferred) Treadmill with post-exercise imaging (less ideal) | Low dose dobutamine infusion (≤20 μg/kg/min) |
| Key parameters assessed | Symptom provocation (angina, dyspnea, and syncope) Abnormal blood pressure response Change in mean transvalvular gradient with exercise Exercise-induced pulmonary hypertension | Change in stroke volume (CR: ≥20% rise) Flow-induced changes in mean gradient AVA stability (true severe: ≤1.0 cm2 despite ↑ flow; pseudo-severe: AVA enlarges) |
| Parameter | Definition/Threshold | Clinical Significance | Impact |
|---|---|---|---|
| Rise in mean transvalvular gradient [14,27] | Exercise-induced increase ≥18–20 mmHg | Associated with accelerated symptom development and adverse prognosis | First demonstration of incremental prognostic value of ESE beyond resting indices; validated ESE as a stratification tool in asymptomatic patients |
| Exercise-induced pulmonary hypertension [16] | Pulmonary artery systolic pressure >60 mmHg | Reflects impaired LV–pulmonary vascular coupling; strong predictor of mortality and AVR | Introduced pulmonary hypertension as a novel prognostic marker |
| Blood pressure response [17] | Abnormal or blunted rise in systolic pressure during exercise | Indicates limited LV contractile reserve; associated with worse outcomes | Validated prognostic relevance; established ESE as valuable adjunct in severe AS with preserved EF |
| Symptom provocation during ESE [18,22] | Development of exertional dyspnea, angina, or presyncope | Provides unequivocal evidence of decompensation despite stable resting echocardiography | Strong clinical indicator; supported broader application in guidelines |
| Safety/feasibility of ESE [18,26] | — | Demonstrates that ESE is a practical and generally safe tool in severe AS | Confirmed feasibility and safety in clinical practice |
| Serial/longitudinal ESE value [18] | — | Useful for disease monitoring and timing of AVR intervention | Highlighted role of repeated ESE in longitudinal surveillance |
| Approach | Definition/Method | Strengths | Limitations |
|---|---|---|---|
| Standard DSE criteria [13,14,23,24,32] | Low dose dobutamine infusion up to 20 μg/kg/min; interpretation based on the following:
|
|
|
| Projected AVA (AVAproj) [5,6,12,14,19,32,33] | Mathematical extrapolation of AVA to a standardized flow of 250 mL/s, derived from slope of AVA–flow relationship during DSE |
|
|
| Marker | Definition/Finding | Prognostic Significance | Therapeutic Implications |
|---|---|---|---|
| Contractile reserve (CR) [13,23,24] | ≥20% increase in stroke volume during low dose dobutamine | Historically, absence of CR predicted high operative mortality (30–50%) in surgical AVR | Presence of CR = better surgical/TAVR outcomes; absence no longer absolute contraindication with contemporary AVR |
| True severe AS [13,14,23] | Mean gradient ≥40 mmHg with AVA ≤1.0 cm2 during flow augmentation | Identifies patients with fixed obstruction at high risk of adverse outcomes if untreated | AVR (SAVR or TAVR) confers substantial survival benefit |
| Pseudo-severe AS | AVA increases >1.0 cm2 with minimal gradient rise during DSE | Reflects flow limitation rather than fixed obstruction; not associated with improved survival after AVR | Conservative management preferred; avoids unnecessary intervention |
| Paradoxical LFLG AS [31,32] | Preserved EF, small LV cavity, impaired filling; gradient remains low despite flow | DSE refines risk stratification, identifying those with severe obstruction despite preserved EF | Guides selection for AVR vs. watchful waiting |
| Refinement/Insight | Definition/Key Proposal | Clinical Impact |
|---|---|---|
| Flow rate vs. stroke volume index [36] | Use of stress transaortic flow rate instead of stroke volume index as the preferred marker of flow augmentation | Improves diagnostic accuracy by directly reflecting transvalvular hemodynamics; reduces risk of misclassification |
| Contemporary multicenter evidence [37] | Large-scale registry analysis of DSE across a spectrum of LVEF; refined diagnostic cut-offs for contractile reserve and AVAproj | Confirms safety of DSE; standardizes interpretation criteria; supports use in both classical and paradoxical LFLG AS |
| Integration with multimodality imaging [5,6] | Incorporation of CT-CAC when DSE is inconclusive, particularly in paradoxical LFLG AS | Enhances diagnostic certainty; complements DSE by confirming anatomical severity of stenosis |
| Feature | Dobutamine Stress Echocardiography | Computed Tomography Calcium Scoring |
|---|---|---|
| Principle [13,36,37] | Flow-dependent, functional assessment of AS severity under pharmacologic augmentation of stroke volume | Flow-independent, anatomic quantification of valve calcification |
| Key diagnostic criteria [13,37] | True severe AS: AVA ≤ 1.0 cm2 and/or mean gradient ≥ 40 mmHg with ≥20% ↑ stroke volume | Severe AS: Agatston score ≥2000 AU (men), ≥1200 AU (women) |
| Additional information [24,36] | Evaluates contractile reserve (predicts outcomes) | Quantifies calcification burden, predicts rapid progression and adverse events |
| Best use [5,7] | First-line test in LFLG AS to differentiate true vs. pseudo-severe | When DSE is inconclusive, not feasible, or contractile reserve absent |
| Limitations [28,40] | Requires good acoustic windows and sinus rhythm; unreliable if no contractile reserve | No functional data; radiation exposure and possible contrast use |
| Guideline status [5,6] | Recommended as initial evaluation in LFLG AS (Class I) | Recommended when DSE is inconclusive or discordant (Class I) |
| Outcome prediction [24,36] | Contractile reserve→better surgical/TAVR outcomes | High calcium score→worse prognosis and faster progression |
| Parameter | Preferred SE Modality | Key Measurement/Definition | Clinical Use and Prognostic Value |
|---|---|---|---|
| Left ventricular systolic reserve (CR) [13,24,25,29] | DSE (low dose dobutamine) | ≥20% increase in stroke volume during stress | Distinguishes true vs. pseudo-severe LFLG AS; preserved CR historically associated with lower surgical mortality; absence no longer absolute contraindication to AVR/TAVR but still signals higher operative risk |
| Left ventricular diastolic reserve [17,20] | ESE (exercise) | Rise in E/e′ ratio or other surrogates of filling pressure under exercise | Detects occult diastolic dysfunction; correlates with exertional dyspnea and earlier symptom development in severe AS |
| Transaortic flow and gradient dynamics [35] | ESE and DSE | Flow rate = stroke volume ÷ LV ejection time; gradient response to stress | Improves accuracy in inconclusive or LFLG AS; dynamic gradients reflect functional severity under physiological load |
| Myocardial deformation (GLS) [40,43,44,45,46,53] | ESE or DSE (with speckle tracking) | Failure of GLS to augment during stress indicates limited myocardial reserve | Early marker of LV dysfunction even with preserved LVEF; impaired or non-augmenting GLS predicts symptom onset, remodeling, adverse outcomes; prognostic in surgical and TAVR populations |
| Valvulo-arterial impedance (Zva) [9,44,47,48,49,50,54] | ESE or DSE | Zva = (SAP + mean gradient) ÷ stroke volume index | Integrates valvular + vascular afterload; stress-induced rise indicates poor arterial compliance, accelerated symptom onset, and worse survival; adds risk stratification beyond valve area/gradient |
| Volume–flow (V–Q) discordance [52] | ESE or DSE (flow-based analysis) | Mismatch between stroke volume index (SVi) and transaortic flow rate (TFR) (e.g., SVi < 35 mL/m2 with TFR > 210 mL/s) | Novel marker of adverse outcome; low V–Q discordance linked to better survival after TAVR; offers superior prognostic discrimination vs. SVi or TFR alone; may refine low-flow AS risk stratification |
| Document/Guideline | Year | Main Contributions | Specific Role of SE in AS | Class of Recommendation/LOE |
|---|---|---|---|---|
| EACVI/ASE Expert Consensus [19] | 2016 | First unified framework for SE beyond ischemic heart disease | Recommended ESE to unmask symptoms in asymptomatic severe AS; DSE for LFLG AS with reduced EF | Consensus document (no formal class/LOE) |
| Stress Echo 2020 (ABCDE protocol) [20] | 2020 | Introduced multiparametric protocol (A–E: wall motion, B-lines, contractile reserve, diastolic reserve, arrhythmias) | Extended SE into a holistic hemodynamic tool; highlighted potential role in valvular disease | Protocol paper (no formal class/LOE) |
| ACC/AHA Valve Guidelines [6] | 2020 | American guideline update for valvular heart disease |
| ESE: Class IIa, LOE B-NR DSE: Class I, LOE B-NR |
| ESC/EACTS Valve Guidelines [5] | 2021 | European guideline update for valvular disease |
| ESE: Class I, LOE C DSE: Class I, LOE C |
| EACVI Position Papers (Imaging Toolbox) [56,57] | 2022 | Consolidated SE as part of multimodality imaging strategy | Emphasized multiparametric SE in discordant AS and complex cases; integration with CT-CAC and strain | Position paper (no formal class/LOE) |
| ABCDEG refinement for AS [20,42] | 2023 | Extension of Stress Echo 2020 | Added systematic evaluation of transvalvular gradients (G) during stress as a dedicated AS parameter | Consensus refinement (no formal class/LOE) |
| ESC/EACTS Guidelines for VHD [55] | 2025 | Major update of 2021 guidelines; refined integrative imaging algorithm for AS and intervention thresholds | SE has an explicit role in the algorithm: DSE LFLG AS to assess flow reserve and distinguish true from pseudo-severe disease—with integration of CT-CAC (thresholds for severe AS: ≥2000 AU in men and ≥1200 in women); ESE to confirm the asymptomatic status and to identify risk markers (e.g., a sustained fall in SBP > 20 mmHg) | Evidence refers to the post-test decision, not to the test itself:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodas, R.; Pop, C.; Petris, A.O. Stress Echocardiography in Aortic Stenosis: From Diagnostic Challenges to Guideline-Endorsed Clinical Applications. J. Clin. Med. 2025, 14, 7424. https://doi.org/10.3390/jcm14207424
Hodas R, Pop C, Petris AO. Stress Echocardiography in Aortic Stenosis: From Diagnostic Challenges to Guideline-Endorsed Clinical Applications. Journal of Clinical Medicine. 2025; 14(20):7424. https://doi.org/10.3390/jcm14207424
Chicago/Turabian StyleHodas, Roxana, Călin Pop, and Antoniu Octavian Petris. 2025. "Stress Echocardiography in Aortic Stenosis: From Diagnostic Challenges to Guideline-Endorsed Clinical Applications" Journal of Clinical Medicine 14, no. 20: 7424. https://doi.org/10.3390/jcm14207424
APA StyleHodas, R., Pop, C., & Petris, A. O. (2025). Stress Echocardiography in Aortic Stenosis: From Diagnostic Challenges to Guideline-Endorsed Clinical Applications. Journal of Clinical Medicine, 14(20), 7424. https://doi.org/10.3390/jcm14207424







