Progressive Loss of Muscle Strength: The Effects of Ageing and Sarcopenia on Muscle Function in Older Females
Abstract
1. Introduction
2. Materials and Methods
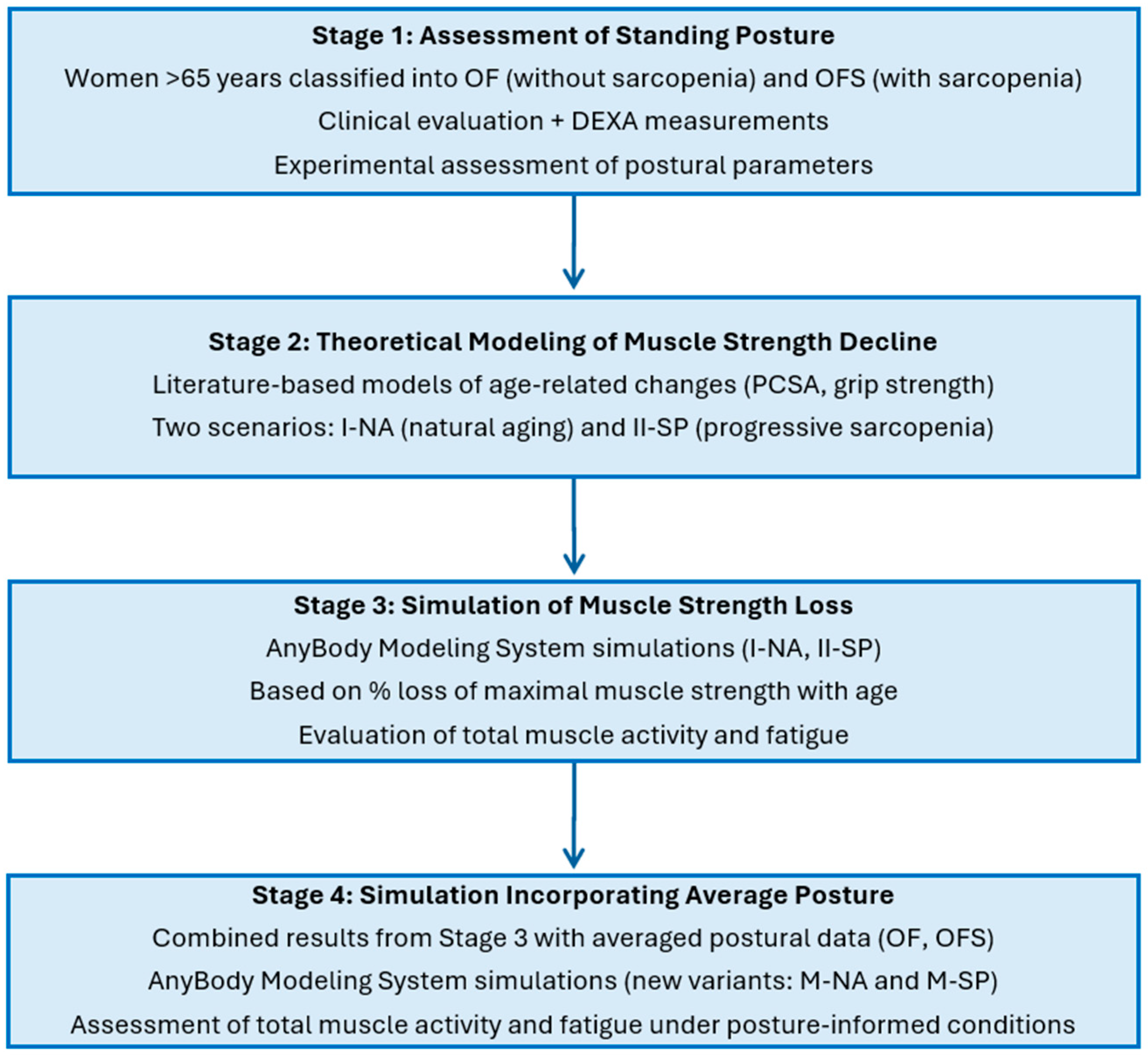
- Assessment of standing posture—Women aged over 65 were classified into groups with sarcopenia (OFS) and without sarcopenia (OF) based on clinical evaluation and DEXA measurements. Postural parameters were then assessed experimentally.
- Theoretical modeling of muscle strength decline—Models were developed to represent progressive muscle strength loss across consecutive decades of life, based on literature data describing age-related changes in physiological cross-sectional area (PCSA) and grip strength. Two strength-decline scenarios were considered: natural aging (Variant I-NA) and progressive sarcopenia (Variant II-SP).
- Simulation of age-related muscle strength loss—Using the AnyBody Modeling System, simulations were conducted for both strength-decline variants (I-NA and II-SP) across consecutive decades to evaluate changes in total muscle activity and muscle fatigue, based on the percentage loss of maximal muscle strength with age.
- Simulation incorporating average body posture—Results from Stage 3 were combined with averaged experimental postural data from the OF (older females without sarcopenia) and OFS (older females with sarcopenia) groups to generate posture-specific simulation variants (M-NA and M-SP). This allowed for the assessment of muscle activity and fatigue under realistic, posture-informed conditions throughout aging.
2.1. Materials
2.2. Experimental Tests
2.3. Modeling Studies
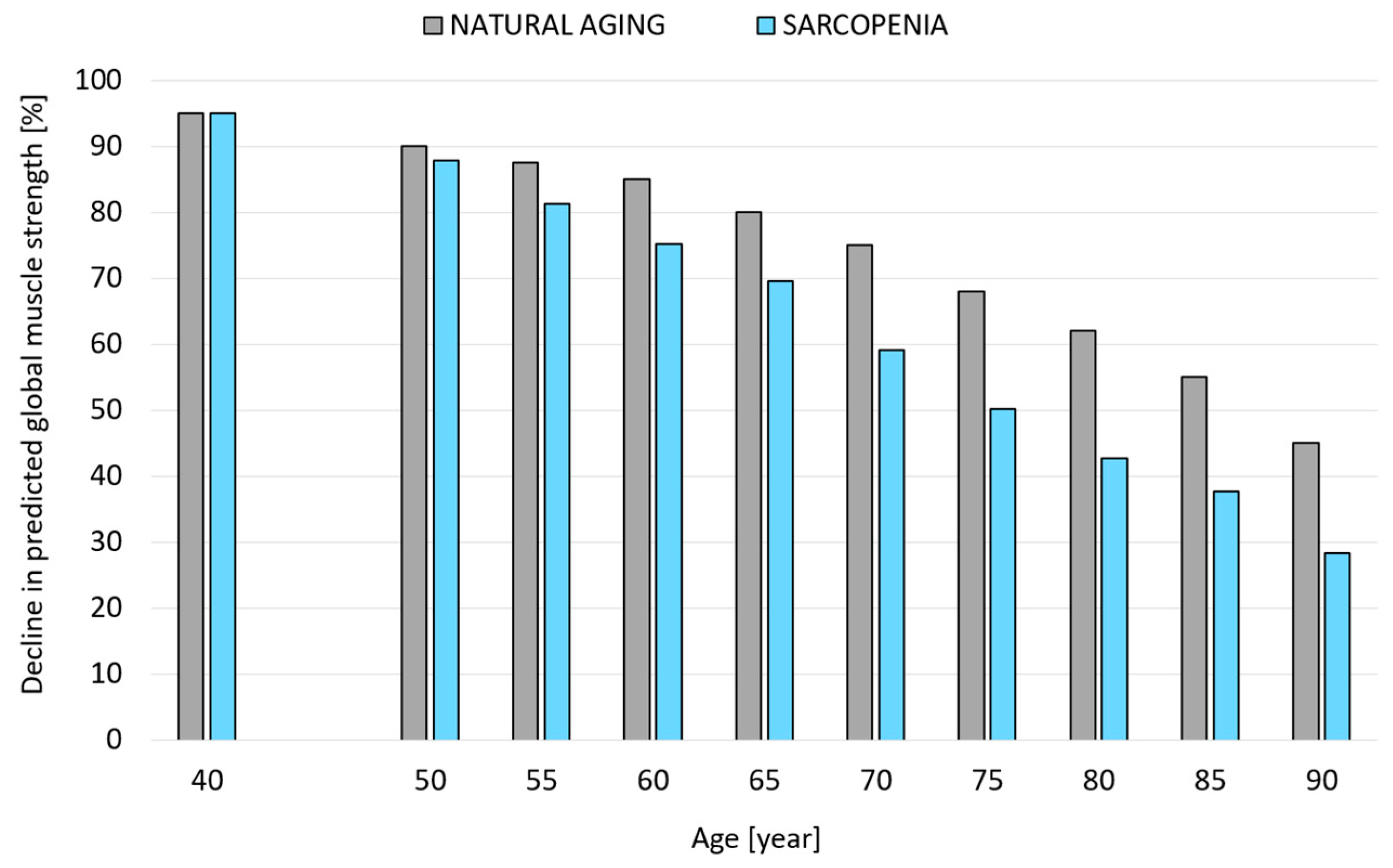
2.3.1. A Model of Changes in Muscle System Strength Considering the Percentage Loss of Maximal Muscle Strength with Age in Older Females Due to Natural Aging Processes and Progressive Sarcopenia
2.3.2. Modeling Changes in Musculoskeletal Load Associated with Reduced Muscle Strength Due to Natural Aging and Progressive Sarcopenia in Older Females
- Variant I-NA—accounting for the aging process defined by a model that assumes a percentage loss of maximum muscular strength with age due to the natural aging process.
- Variant II-SP—accounting for the aging process defined by a model assuming a percentage loss of maximal muscle strength with age in individuals with progressive sarcopenia.
2.3.3. Models of Older Females Incorporating Posture and Strength Loss Associated with Natural Aging and Progressive Sarcopenia
- The posture of OF as a result of the natural aging processes (M-NA).
- The posture of OFS as a result of progressive sarcopenia (M-SP).
3. Results
3.1. Experimental Results
3.2. Modeling Changes in the Muscle System of Older Females
3.3. Model Results
3.4. Results of Models Accounting for Posture and Loss of Strength Capacity Associated with Natural Aging Processes and Progressive Sarcopenia




4. Discussion
Limitations to This Work and Directions for Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs. World Social Report 2023: Leaving No One Behind In An Ageing World; United Nations Department of Economic and Social Affairs: New York City, NY, USA, 2023. [Google Scholar]
- Dawson, A.; Dennison, E. Measuring the musculoskeletal aging phenotype. Maturitas 2016, 93, 13–17. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, M.; Zhou, R.; Ou, W.; Yao, P. How heavy is the medical expense burden among the older adults and what are the contributing factors? A literature review and problem-based analysis. Front. Public Health 2023, 11, 1165381. [Google Scholar] [CrossRef]
- Roubenoff, R. Sarcopenia: A major modifiable cause of frailty in the elderly. J. Nutr. Health Aging 2000, 4, 140–142. [Google Scholar] [PubMed]
- Ko, F.C.; Walston, J.D. Chapter 63—What Is Frailty? In Evidence-Based Practice in Palliative Medicine; Goldstein, N.E., Morrison, R.S., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; pp. 363–370. [Google Scholar]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31, Erratum in Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Dennison, E.M.; Sayer, A.A.; Cooper, C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat. Rev. Rheumatol. 2017, 13, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Moreland, J.D.; Richardson, J.A.; Goldsmith, C.H.; Clase, C.M. Muscle weakness and falls in older adults: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2004, 52, 1121–1129. [Google Scholar] [CrossRef]
- Nikolić, M.; Bajek, S.; Bobinac, D.; Vranić, T.S.; Jerković, R. Aging of human skeletal muscles. Coll. Antropol. 2005, 29, 67–70. [Google Scholar]
- Ata, A.M.; Kara, M.; Kaymak, B.; Özçakar, L. Sarcopenia Is Not “Love”: You Have to Look Where You Lost it! Am. J. Phys. Med. Rehabil. 2020, 99, e119–e120. [Google Scholar] [CrossRef]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyère, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef]
- von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Park, S. Gender-Specific Risk Factors and Prevalence for Sarcopenia among Community-Dwelling Young-Old Adults. Int. J. Environ. Res. Public Health 2022, 19, 7232. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 2012, 24, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Kuh, D.; Cooper, C.; Sayer, A.A. Global variation in grip strength: A systematic review and meta-analysis of normative data. Age Ageing 2016, 45, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Metter, E.J.; Conwit, R.; Tobin, J.; Fozard, J.L. Age-associated loss of power and strength in the upper extremities in women and men. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, B267–B276. [Google Scholar] [CrossRef]
- Faulkner, J.A.; Larkin, L.M.; Claflin, D.R.; Brooks, S.V. Age-related changes in the structure and function of skeletal muscles. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1091–1096. [Google Scholar] [CrossRef]
- Peterson, S.J.; Mozer, M. Differentiating Sarcopenia and Cachexia Among Patients With Cancer. Nutr. Clin. Pract. 2017, 32, 30–39. [Google Scholar] [CrossRef]
- Yuan, D.; Jin, H.; Liu, Q.; Liu, Q.; Zhang, J.; Ma, B.; Xiao, W.; Li, Y. Publication Trends for Sarcopenia in the World: A 20-Year Bibliometric Analysis. Front. Med. 2022, 9, 802651. [Google Scholar] [CrossRef]
- Marini, M.; Sarchielli, E.; Brogi, L.; Lazzeri, R.; Salerno, R.; Sgambati, E.; Monaci, M. Role of adapted physical activity to prevent the adverse effects of the sarcopenia. A pilot study. Ital. J. Anat. Embryol. 2008, 113, 217–225. [Google Scholar]
- Roth, S.M.; Ferrell, R.F.; Hurley, B.F. Strength training for the prevention and treatment of sarcopenia. J. Nutr. Health Aging 2000, 4, 143–155. [Google Scholar]
- Ignasiak, D.; Valenzuela, W.; Reyes, M.; Ferguson, S.J. The effect of muscle ageing and sarcopenia on spinal segmental loads. Eur. Spine J. 2018, 27, 2650–2659. [Google Scholar] [CrossRef]
- Newman, A.B.; Haggerty, C.L.; Goodpaster, B.; Harris, T.; Kritchevsky, S.; Nevitt, M.; Miles, T.P.; Visser, M.; Health Aging And Body Composition Research Group. Strength and muscle quality in a well-functioning cohort of older adults: The Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2003, 51, 323–330. [Google Scholar] [CrossRef]
- Kutáč, P.; Bunc, V.; Sigmund, M. Whole-body dual-energy X-ray absorptiometry demonstrates better reliability than segmental body composition analysis in college-aged students. PLoS ONE 2019, 14, e0215599. [Google Scholar] [CrossRef]
- Schubert, M.M.; Seay, R.F.; Spain, K.K.; Clarke, H.E.; Taylor, J.K. Reliability and validity of various laboratory methods of body composition assessment in young adults. Clin. Physiol. Funct. Imaging 2019, 39, 150–159. [Google Scholar] [CrossRef]
- Skals, S.L.; Jung, M.; Damsgaard, M.; Andersen, M.S. Prediction of ground reaction forces and moments during sports-related movemets. Multibody Syst. Dyn. 2017, 39, 175–195. [Google Scholar] [CrossRef]
- Damsgaard, M.; Rasmussen, J.; Christensen, S.T.; Surma, E.; de Zee, M. Analysis of musculoskeletal systems in the AnyBody Modeling System. Simul. Model. Pract. Theory 2006, 14, 1100–1111. [Google Scholar] [CrossRef]
- de Zee, M.; Hansen, L.; Wong, C.; Rasmussen, J.; Simonsen, E.B. A generic detailed rigid-body lumbar spine model. J. Biomech. 2007, 40, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Han, K.S.; Zander, T.; Taylor, W.R.; Rohlmann, A. An enhanced and validated generic thoraco-lumbar spine model for prediction of muscle forces. Med. Eng. Phys. 2012, 34, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.; Zander, T.; Dreischarf, M.; Schmidt, H. Influence of lumbar spine rhythms and intra-abdominal pressure on spinal loads and trunk muscle forces during upper body inclination. Med. Eng. Phys. 2016, 38, 333–338. [Google Scholar] [CrossRef]
- Liu, T.; Khalaf, K.; Adeeb, S.; El-Rich, M. Numerical Investigation of Intra-abdominal Pressure Effects on Spinal Loads and Load-Sharing in Forward Flexion. Front. Bioeng. Biotechnol. 2019, 7, 428. [Google Scholar] [CrossRef]
- Nowakowska-Lipiec, K.; Michnik, R.; Linek, P.; Myśliwiec, A.; Zadoń, H.; Gorwa, J. Effect of strengthening and weakening of abdominal and dorsal muscles on lumbar spine loads in parents of disabled children. J. Biomech. 2023, 161, 111864. [Google Scholar] [CrossRef]
- Bassani, T.; Stucovitz, E.; Qian, Z.; Briguglio, M.; Galbusera, F. Validation of the AnyBody full body musculo-skeletal model in computing lumbar spine loads at L4L5 level. J. Biomech. 2017, 58, 89–96. [Google Scholar] [CrossRef]
- Koblauch, H. Low back load in airport baggage handlers. Dan. Med. J. 2016, 63, B5233. [Google Scholar]
- Ignasiak, D.; Dendorfer, S.; Ferguson, S.J. Thoracolumbar Spine Model with Articulated Ribcage for the Prediction of Dynamic Spinal Loading. J. Biomech. 2016, 49, 959–966. [Google Scholar] [CrossRef]
- Rajaee, M.A.; Arjmand, N.; Shirazi-Adl, A.; Plamondon, A.; Schmidt, H. Comparative evaluation of six quantitative lifting tools to estimate spine loads during static activities. Appl. Ergon. 2015, 48, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.; de Zee, M.; Carbes, S. Validation of a Biomechanical Model of the Lumbar Spine. In Proceedings of the XXIInd Congress of the International Society of Biomechanics 2009, Cape Town, South Africa, 5–9 July 2009. [Google Scholar]
- Szaflik, P.; Zadoń, H.; Michnik, R.; Nowakowska-Lipiec, K. Handgrip Strength as an Indicator of Overall Strength and Functional Performance—Systematic Review. Appl. Sci. 2025, 15, 1847. [Google Scholar] [CrossRef]
- Zadoń, H.; Michnik, R.; Nowakowska-Lipiec, K. Exploring the impact of body mass change on fatigue and activity of the muscular system during daily routine. Technol. Health Care 2023, 31, 2487–2498. [Google Scholar] [CrossRef] [PubMed]
- Distefano, G.; Goodpaster, B.H. Effects of Exercise and Aging on Skeletal Muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska-Lipiec, K.; Michnik, R.; Linek, P.; Myśliwiec, A.; Jochymczyk-Woźniak, K.; Gzik, M. A numerical study to determine the effect of strengthening and weakening of the transversus abdominis muscle on lumbar spine loads. Comput. Methods Biomech. Biomed. Engin 2020, 23, 1287–1296. [Google Scholar] [CrossRef]
- Pišot, R.; Marusic, U.; Biolo, G.; Mazzucco, S.; Lazzer, S.; Grassi, B.; Reggiani, C.; Toniolo, L.; Di Prampero, P.E.; Passaro, A.; et al. Greater loss in muscle mass and function but smaller metabolic alterations in older compared with younger men following 2 wk of bed rest and recovery. J. Appl. Physiol. 2016, 120, 922–929. [Google Scholar] [CrossRef]
- McPhee, J.S.; French, D.P.; Jackson, D.; Nazroo, J.; Pendleton, N.; Degens, H. Physical activity in older age: Perspectives for healthy ageing and frailty. Biogerontology 2016, 17, 567–580. [Google Scholar] [CrossRef]
- Lang, T.; Streeper, T.; Cawthon, P.; Baldwin, K.; Taaffe, D.R.; Harris, T.B. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos. Int. 2010, 21, 543–559. [Google Scholar] [CrossRef] [PubMed]
- McGregor, R.A.; Cameron-Smith, D.; Poppitt, S.D. It is not just muscle mass: A review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Healthspan 2014, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Bailey, M.; Lee, R.Y. Ageing modifies the fibre angle and biomechanical function of the lumbar extensor muscles. Clin. Biomech. 2011, 26, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.V.; Maffulli, N. Sarcopenia: Characteristics, mechanisms and functional significance. Br. Med. Bull. 2010, 95, 139–159. [Google Scholar] [CrossRef]

| Parameters | OF (n = 10) | OFS (n = 10) |
|---|---|---|
| Age (years) | 76 ± 4 | 80 ± 7 |
| Height (m) | 1.57 ± 0.06 | 1.57 ± 0.05 |
| Weight (kg) | 65 ± 12 | 70 ± 7 |
| BMI (kg/m2) | 26.5 ± 5.3 | 28.2 ± 3.4 |
| ALM (kg/m2) | 6.25 ± 0.42 | 5.07 ± 0.42 |
| Muscle mass (%) | 41.1 ± 3.2 | 35.1 ± 4.1 |
| Body fat (%) | 40.3 ± 7.2 | 41.8± 3.6 |
| Parameters | OF | OFS | p-Value (Cohen’s d) |
|---|---|---|---|
| Trunk tilted in the sagittal plane (α) [°] | 5.1° ± 3.8° | 12.2° ± 6.2° | 0.008 * (−1.38) |
| Pelvis aligned in the sagittal plane (β) [°] | 9.7° ± 5.3° | 14.3° ± 7.3° | 0.126 (−0.72) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowakowska-Lipiec, K.; Zadoń, H.; Michnik, R.; Nawrat-Szołtysik, A. Progressive Loss of Muscle Strength: The Effects of Ageing and Sarcopenia on Muscle Function in Older Females. J. Clin. Med. 2025, 14, 7276. https://doi.org/10.3390/jcm14207276
Nowakowska-Lipiec K, Zadoń H, Michnik R, Nawrat-Szołtysik A. Progressive Loss of Muscle Strength: The Effects of Ageing and Sarcopenia on Muscle Function in Older Females. Journal of Clinical Medicine. 2025; 14(20):7276. https://doi.org/10.3390/jcm14207276
Chicago/Turabian StyleNowakowska-Lipiec, Katarzyna, Hanna Zadoń, Robert Michnik, and Agnieszka Nawrat-Szołtysik. 2025. "Progressive Loss of Muscle Strength: The Effects of Ageing and Sarcopenia on Muscle Function in Older Females" Journal of Clinical Medicine 14, no. 20: 7276. https://doi.org/10.3390/jcm14207276
APA StyleNowakowska-Lipiec, K., Zadoń, H., Michnik, R., & Nawrat-Szołtysik, A. (2025). Progressive Loss of Muscle Strength: The Effects of Ageing and Sarcopenia on Muscle Function in Older Females. Journal of Clinical Medicine, 14(20), 7276. https://doi.org/10.3390/jcm14207276








