Relationship Between Bone Metabolic Markers and Presence of Sarcopenia in Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Study
Abstract
1. Introduction
2. Methods
2.1. Research Subjects
2.2. Data Collection and Definition of Osteoporosis and Sarcopenia
2.3. Statistical Analysis
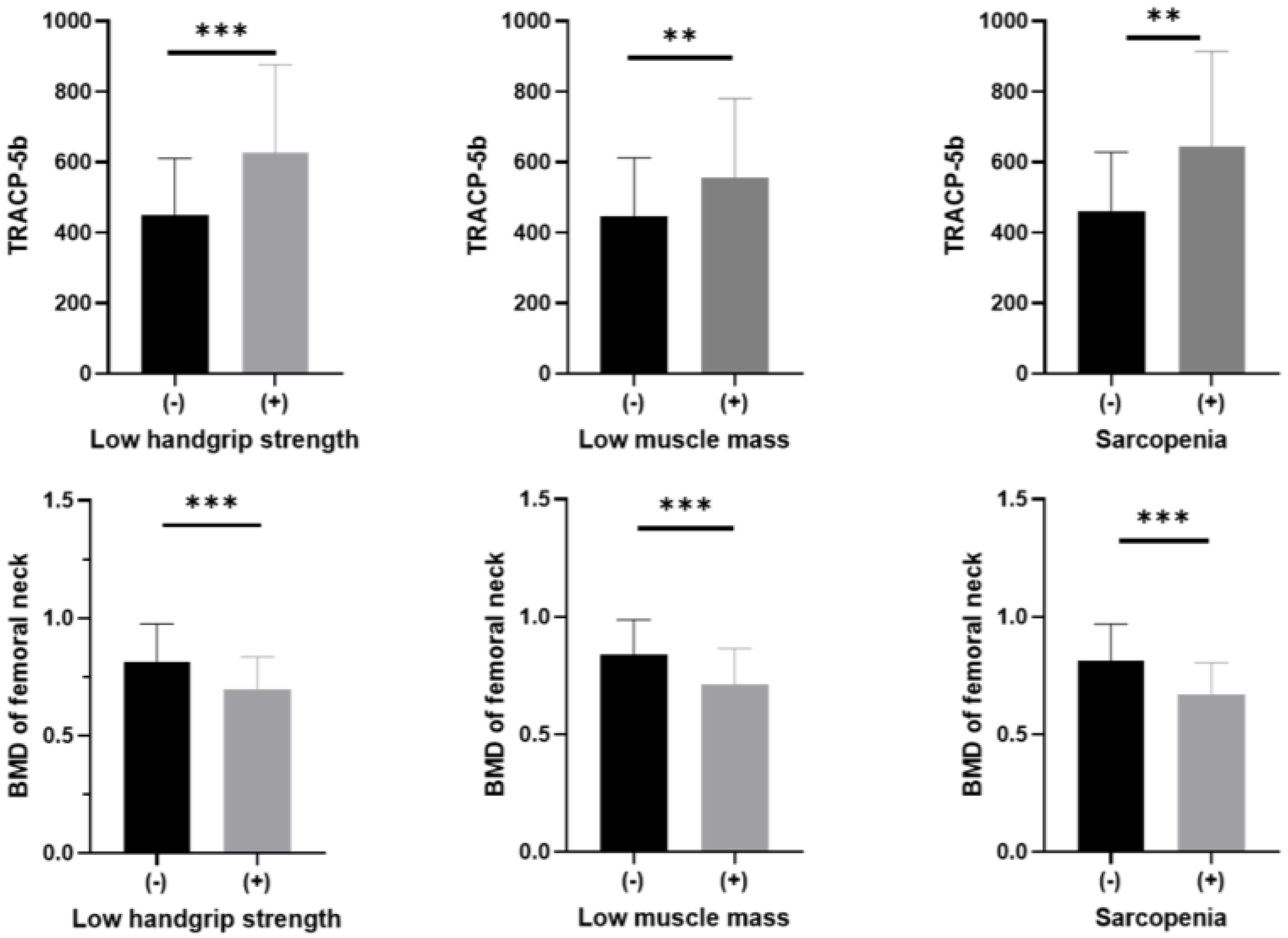
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, M.I.; Hinton, P.S. Physical Activity and Bone Health. Mo. Med. 2014, 111, 59–64. [Google Scholar]
- Fujimoto, K.; Inage, K.; Eguchi, Y.; Orita, S.; Suzuki, M.; Kubota, G.; Sainoh, T.; Sato, J.; Shiga, Y.; Abe, K.; et al. Use of Bioelectrical Impedance Analysis for the Measurement of Appendicular Skeletal Muscle Mass/Whole Fat Mass and Its Relevance in Assessing Osteoporosis among Patients with Low Back Pain: A Comparative Analysis Using Dual X-Ray Absorptiometry. Asian Spine J. 2018, 12, 839–845. [Google Scholar] [CrossRef]
- Miyakoshi, N.; Hongo, M.; Mizutani, Y.; Shimada, Y. Prevalence of Sarcopenia in Japanese Women with Osteopenia and Osteoporosis. J. Bone Miner. Metab. 2013, 31, 556–561. [Google Scholar] [CrossRef]
- Sjöblom, S.; Suuronen, J.; Rikkonen, T.; Honkanen, R.; Kröger, H.; Sirola, J. Relationship between Postmenopausal Osteoporosis and the Components of Clinical Sarcopenia. Maturitas 2013, 75, 175–180. [Google Scholar] [CrossRef]
- Licata, A.A. Bone Density, Bone Quality, and FRAX: Changing Concepts in Osteoporosis Management. Am. J. Obstet. Gynecol. 2013, 208, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Ewing, S.K.; Taylor, B.C.; Fink, H.A.; Stone, K.L.; Cauley, J.A.; Tracy, J.K.; Hochberg, M.C.; Rodondi, N.; Cawthon, P.M. Frailty and Risk of Falls, Fracture, and Mortality in Older Women: The Study of Osteoporotic Fractures. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Looker, A.C. Dysmobility Syndrome and Mortality Risk in US Men and Women Age 50 Years and Older. Osteoporos. Int. 2015, 26, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Lyles, K.W.; Colón-Emeric, C.S.; Magaziner, J.S.; Adachi, J.D.; Pieper, C.F.; Mautalen, C.; Hyldstrup, L.; Recknor, C.; Nordsletten, L.; Moore, K.A.; et al. Zoledronic Acid and Clinical Fractures and Mortality after Hip Fracture. N. Eng. J. Med. 2007, 357, 1799–1809. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Brueck, C.C.; Singh, S.K.; Dobnig, H. Osteoporosis in Patients With Diabetes Mellitus. J. Bone Miner. Res. 2007, 22, 1317–1328. [Google Scholar] [CrossRef]
- Yamauchi, M.; Sugimoto, T. Lifestyle-Related Diseases and Osteoporosis. Nihon Naika Gakkai Zasshi 2015, 104, 2414–2420. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a Risk Factor for Falls in Elderly Individuals: Results from the IlSIRENTE Study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xu, J. Association between Muscle Mass, Bone Mineral Density and Osteoporosis in Type 2 Diabetes. J. Diabetes Investig. 2022, 13, 351–358. [Google Scholar] [CrossRef]
- Sakai, R.; Hashimoto, Y.; Ushigome, E.; Miki, A.; Okamura, T.; Matsugasumi, M.; Fukuda, T.; Majima, S.; Matsumoto, S.; Senmaru, T.; et al. Late-Night-Dinner Is Associated with Poor Glycemic Control in People with Type 2 Diabetes: The KAMOGAWA-DM Cohort Study. Endocr. J. 2018, 65, 395–402. [Google Scholar] [CrossRef]
- Yamada, S.; Inaba, M.; Kurajoh, M.; Shidara, K.; Imanishi, Y.; Ishimura, E.; Nishizawa, Y. Utility of Serum Tartrate-resistant Acid Phosphatase (TRACP5b) as a Bone Resorption Marker in Patients with Chronic Kidney Disease: Independence from Renal Dysfunction. Clin. Endocrinol. 2008, 69, 189–196. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Genant, H.K.; Cooper, C.; Poor, G.; Reid, I.; Ehrlich, G.; Kanis, J.; Nordin, B.E.C.; Barrett-Connor, E.; Black, D.; Bonjour, J.-P.; et al. Interim Report and Recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos. Int. 1999, 10, 259–264. [Google Scholar] [CrossRef]
- Alexeeva, L.; Burkhardt, P.; Christiansen, C.; Cooper, C.; Delmas, P.; Johnell, O.; Johnston, C.; Kanis, J.A.; Lips, P.; Melton, L.J.; et al. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis; World Health Organization (WHO) Technical Report Series; World Health Organization (WHO): Geneva, Switzerland, 1994; pp. 1–129. [Google Scholar]
- Winzenrieth, R.; Michelet, F.; Hans, D. Three-Dimensional (3D) Microarchitecture Correlations with 2D Projection Image Gray-Level Variations Assessed by Trabecular Bone Score Using High-Resolution Computed Tomographic Acquisitions: Effects of Resolution and Noise. J. Clin. Densitom. 2013, 16, 287–296. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Odén, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Barkmann, R.; Boutroy, S.; Brown, J.; Chapurlat, R.; et al. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. J. Bone Miner. Res. 2016, 31, 940–948. [Google Scholar] [CrossRef]
- Janssen, I. Skeletal Muscle Cutpoints Associated with Elevated Physical Disability Risk in Older Men and Women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Hida, T.; Shimokata, H.; Sakai, Y.; Ito, S.; Matsui, Y.; Takemura, M.; Kasai, T.; Ishiguro, N.; Harada, A. Sarcopenia and Sarcopenic Leg as Potential Risk Factors for Acute Osteoporotic Vertebral Fracture among Older Women. Eur. Spine J. 2016, 25, 3424–3431. [Google Scholar] [CrossRef]
- Yoo, J.-I.; Ha, Y.-C.; Kwon, H.-B.; Lee, Y.-K.; Koo, K.-H.; Yoo, M.-J. High Prevalence of Sarcopenia in Korean Patients after Hip Fracture: A Case-Control Study. J. Korean Med. Sci. 2016, 31, 1479. [Google Scholar] [CrossRef]
- Sølling, A.S.; Harsløf, T.; Jørgensen, N.R.; Langdahl, B. Changes in RANKL and TRAcP 5b after Discontinuation of Denosumab Suggest RANKL Mediated Formation of Osteoclasts Results in the Increased Bone Resorption. Osteoporos. Int. 2023, 34, 599–605. [Google Scholar] [CrossRef]
- Kawao, N.; Moritake, A.; Tatsumi, K.; Kaji, H. Roles of Irisin in the Linkage from Muscle to Bone During Mechanical Unloading in Mice. Calcif. Tissue Int. 2018, 103, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, T.; Ruan, Y.; Yao, J.; Shen, H.; Xu, Y.; Zheng, B.; Zhang, Z.; Wang, J.; Tan, Q. The Association of Serum Irisin with Bone Mineral Density and Turnover Markers in New-Onset Type 2 Diabetic Patients. Int. J. Endocrinol. 2022, 2022, 7808393. [Google Scholar] [CrossRef]
- Guo, M.; Yao, J.; Li, J.; Zhang, J.; Wang, D.; Zuo, H.; Zhang, Y.; Xu, B.; Zhong, Y.; Shen, F.; et al. Irisin Ameliorates Age-associated Sarcopenia and Metabolic Dysfunction. J. Cachexia Sarcopenia Muscle 2023, 14, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical Stimulation of Bone in Vivo Reduces Osteocyte Expression of Sost/Sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, E.; Shibata, M.; Yoshino, Y.; Sekiguchi, S.; Kakita, A.; Takayanagi, T.; Makino, M.; Hayakawa, N.; Suzuki, A. Serum Sclerostin Concentration Reflects Bone Turnover and Glycation in Men with Type 2 Diabetes Mellitus. Fujita Med. J. 2018, 4, 1–5. [Google Scholar]
- Kim, J.A.; Roh, E.; Hong, S.; Lee, Y.-B.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, N.H.; Kim, S.G.; Baik, S.H.; et al. Association of Serum Sclerostin Levels with Low Skeletal Muscle Mass: The Korean Sarcopenic Obesity Study (KSOS). Bone 2019, 128, 115053. [Google Scholar] [CrossRef]
- Oei, L.; Zillikens, M.C.; Dehghan, A.; Buitendijk, G.H.S.; Castaño-Betancourt, M.C.; Estrada, K.; Stolk, L.; Oei, E.H.G.; van Meurs, J.B.J.; Janssen, J.A.M.J.L.; et al. High Bone Mineral Density and Fracture Risk in Type 2 Diabetes as Skeletal Complications of Inadequate Glucose Control. Diabetes Care 2013, 36, 1619–1628. [Google Scholar] [CrossRef]
- Bjørnerem, Å. The Clinical Contribution of Cortical Porosity to Fragility Fractures. BoneKEy Rep. 2016, 5, 846. [Google Scholar] [CrossRef]
- Burghardt, A.J.; Issever, A.S.; Schwartz, A.V.; Davis, K.A.; Masharani, U.; Majumdar, S.; Link, T.M. High-Resolution Peripheral Quantitative Computed Tomographic Imaging of Cortical and Trabecular Bone Microarchitecture in Patients with Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2010, 95, 5045–5055. [Google Scholar] [CrossRef] [PubMed]
- Samelson, E.J.; Demissie, S.; Cupples, L.A.; Zhang, X.; Xu, H.; Liu, C.-T.; Boyd, S.K.; McLean, R.R.; Broe, K.E.; Kiel, D.P.; et al. Diabetes and Deficits in Cortical Bone Density, Microarchitecture, and Bone Size: Framingham HR-PQCT Study. J. Bone Miner. Res. 2018, 33, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Johansson, J.; McMillan, L.B.; Ebeling, P.R.; Nordstrom, P.; Nordstrom, A. Associations of Sarcopenia and Its Components with Bone Structure and Incident Falls in Swedish Older Adults. Calcif. Tissue Int. 2019, 105, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Yamaguchi, T.; Yamauchi, M.; Kaji, H.; Sugimoto, T. Diabetic Patients Have an Increased Risk of Vertebral Fractures Independent of BMD or Diabetic Complications. J. Bone Miner. Res. 2009, 24, 702–709. [Google Scholar] [CrossRef]
- Iki, M.; Tamaki, J.; Kadowaki, E.; Sato, Y.; Dongmei, N.; Winzenrieth, R.; Kagamimori, S.; Kagawa, Y.; Yoneshima, H. Trabecular Bone Score (TBS) Predicts Vertebral Fractures in Japanese Women Over 10 Years Independently of Bone Density and Prevalent Vertebral Deformity: The Japanese Population-Based Osteoporosis (JPOS) Cohort Study. J. Bone Miner. Res. 2014, 29, 399–407. [Google Scholar] [CrossRef]


| n | 119 |
|---|---|
| Age (years) | 72.3 ± 8.1 |
| Sex (men/women) | 43 (36.1)/76 (63.9) |
| Duration of diabetes (years) | 13.8 ± 10.2 |
| Height (cm) | 157.1 ± 8.9 |
| Body weight (kg) | 59.9 ± 11.8 |
| Body mass index (kg/m2) | 24.2 ± 4.2 |
| Biguanide (yes) | 39 (32.8) |
| Thiazolidine (yes) | 2 (1.7) |
| Sulfonylurea (yes) | 21 (17.6) |
| Glinide (yes) | 8 (6.7) |
| DPP4 inhibitor (yes) | 46 (38.7) |
| SGLT2 inhibitor (yes) | 16 (13.4) |
| α glucosidase inhibitor (yes) | 17 (14.3) |
| GLP-1 receptor agonist (yes) | 40 (33.6) |
| Insulin (yes) | 38 (31.9) |
| Exercise (yes) | 51 (42.9) |
| Current smoker (yes) | 13 (10.9) |
| HbA1c (%) | 7.4 ± 1.1 |
| Creatinine (umol/L) | 70.9 ± 25.5 |
| Estimated glomerular filtration rate (mL/min/1.73m2) | 65.5 ± 17.7 |
| Trabecular bone score (n = 118) | 1.33 ± 0.10 |
| Abnormal TBS | 19 (16.1) |
| Overlap of abnormal TBS with low BMD | 18 (15.3) |
| Overlap of abnormal TBS with sarcopenia | 3 (2.5) |
| Bone mineral density of the lumbar spine (g/cm2) | 0.95 ± 0.22 |
| Normal | 74 (62.2) |
| Osteopenia | 29 (24.4) |
| Osteoporosis | 16 (13.4) |
| Bone mineral density of the whole neck (g/cm2) | 0.79 ± 0.16 |
| Normal | 60 (50.4) |
| Osteopenia | 40 (33.6) |
| Osteoporosis | 19 (16.0) |
| Bone mineral density of the femoral neck (g/cm2) | 0.64 ± 0.16 |
| Normal | 40 (33.6) |
| Osteopenia | 34 (28.6) |
| Osteoporosis | 45 (37.8) |
| Bone-specific alkaline phosphatase (U/L) | 15.4 ± 7.0 |
| Tartrate-resistant acid phosphatase 5b (mU/dL) | 488.1 ± 196.4 |
| Handgrip strength (kg) | 25.5 ± 8.0 |
| Low handgrip strength (yes) | 26 (21.8) |
| Skeletal muscle mass index (kg/m2) | 6.5 ± 1.0 |
| Low muscle mass (yes) | 45 (37.8) |
| Sarcopenia (yes) | 18 (15.1) |
| Prior fragility fractures (yes) | 91 (76.5) |
| Men | Handgrip Strength | SMI | ||
| r | p | r | p | |
| Bone-specific alkaline phosphatase | −0.31 | 0.040 | −0.38 | 0.012 |
| Tartrate-resistant acid phosphatase 5b | −0.50 | <0.001 | −0.41 | 0.007 |
| BMD of the lumbar spine | 0.16 | 0.311 | 0.15 | 0.348 |
| BMD of whole neck | 0.36 | 0.018 | 0.44 | 0.003 |
| BMD of femoral neck | 0.39 | 0.011 | 0.35 | 0.020 |
| Women | Handgrip strength | SMI | ||
| r | p | r | p | |
| Bone-specific alkaline phosphatase | −0.02 | 0.848 | −0.08 | 0.519 |
| Tartrate-resistant acid phosphatase 5b | −0.25 | 0.032 | −0.21 | 0.073 |
| BMD of the lumbar spine | 0.09 | 0.463 | 0.39 | <0.001 |
| BMD of whole neck | 0.37 | 0.001 | 0.59 | <0.001 |
| BMD of femoral neck | 0.34 | 0.003 | 0.50 | <0.001 |
| Men | Handgrip Strength | SMI | ||
| r | p | r | p | |
| Trabecular bone score | 0.24 | 0.113 | −0.001 | 0.995 |
| Women | Handgrip strength | SMI | ||
| r | p | r | p | |
| Trabecular bone score | 0.11 | 0.340 | 0.09 | 0.459 |
| Low Handgrip Strength | Low SMI | Sarcopenia | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age (year) | 1.26 (1.12–1.41) | <0.001 | 1.12 (1.05–1.20) | <0.001 | 1.34 (1.13–1.58) | <0.001 |
| Men | 1.85 (0.55–6.20) | 0.321 | 1.46 (0.58–3.63) | 0.421 | 6.03 (1.30–28.1) | 0.021 |
| Exercise | 0.66 (0.20–2.19) | 0.495 | 1.39 (0.57–3.37) | 0.463 | 0.49 (0.11–2.13) | 0.339 |
| Smoking | 0.46 (0.04–4.50) | 0.508 | 1.54 (0.42–5.70) | 0.519 | 0.95 (0.09–10.3) | 0.964 |
| TRACP-5b (per 10 mU/dL incremental) | 1.04 (1.01–1.08) | 0.008 | 1.03 (1.00–1.05) | 0.008 | 1.05 (1.01–1.10) | 0.008 |
| Low Handgrip strength | Low SMI | Sarcopenia | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age (year) | 1.29 (1.14–1.47) | <0.001 | 1.13 (1.05–1.22) | <0.001 | 1.47 (1.18–1.84) | <0.001 |
| Men | 3.70 (0.90–15.2) | 0.069 | 3.55 (1.15–11.0) | 0.028 | 26.8 (3.22–223) | 0.002 |
| Exercise | 1.07 (0.31–3.64) | 0.913 | 1.97 (0.77–5.04) | 0.159 | 1.59 (0.29–8.88) | 0.595 |
| Smoking | 0.58 (0.06–5.51) | 0.633 | 1.56 (0.42–5.79) | 0.505 | 1.45 (0.12–17.3) | 0.771 |
| BMD of femoral neck (per 10 g/cm2 incremental) | 0.48 (0.28–0.84) | 0.010 | 0.50 (0.34–0.75) | <0.001 | 0.30 (0.14–0.68) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuyama, T.; Hashimoto, Y.; Kitagawa, N.; Osaka, T.; Hamaguchi, M.; Fukui, M. Relationship Between Bone Metabolic Markers and Presence of Sarcopenia in Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Study. J. Clin. Med. 2025, 14, 5973. https://doi.org/10.3390/jcm14175973
Matsuyama T, Hashimoto Y, Kitagawa N, Osaka T, Hamaguchi M, Fukui M. Relationship Between Bone Metabolic Markers and Presence of Sarcopenia in Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(17):5973. https://doi.org/10.3390/jcm14175973
Chicago/Turabian StyleMatsuyama, Tomoyuki, Yoshitaka Hashimoto, Noriyuki Kitagawa, Takafumi Osaka, Masahide Hamaguchi, and Michiaki Fukui. 2025. "Relationship Between Bone Metabolic Markers and Presence of Sarcopenia in Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Study" Journal of Clinical Medicine 14, no. 17: 5973. https://doi.org/10.3390/jcm14175973
APA StyleMatsuyama, T., Hashimoto, Y., Kitagawa, N., Osaka, T., Hamaguchi, M., & Fukui, M. (2025). Relationship Between Bone Metabolic Markers and Presence of Sarcopenia in Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Study. Journal of Clinical Medicine, 14(17), 5973. https://doi.org/10.3390/jcm14175973









