Characterization of Patients Unsuited for Transcatheter Mitral Valve Interventions
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Population
3.2. Group Comparison of M-TEER Accepted and Refused Patients
3.3. Group Comparisons of TMVR Accepted and Refused Patients
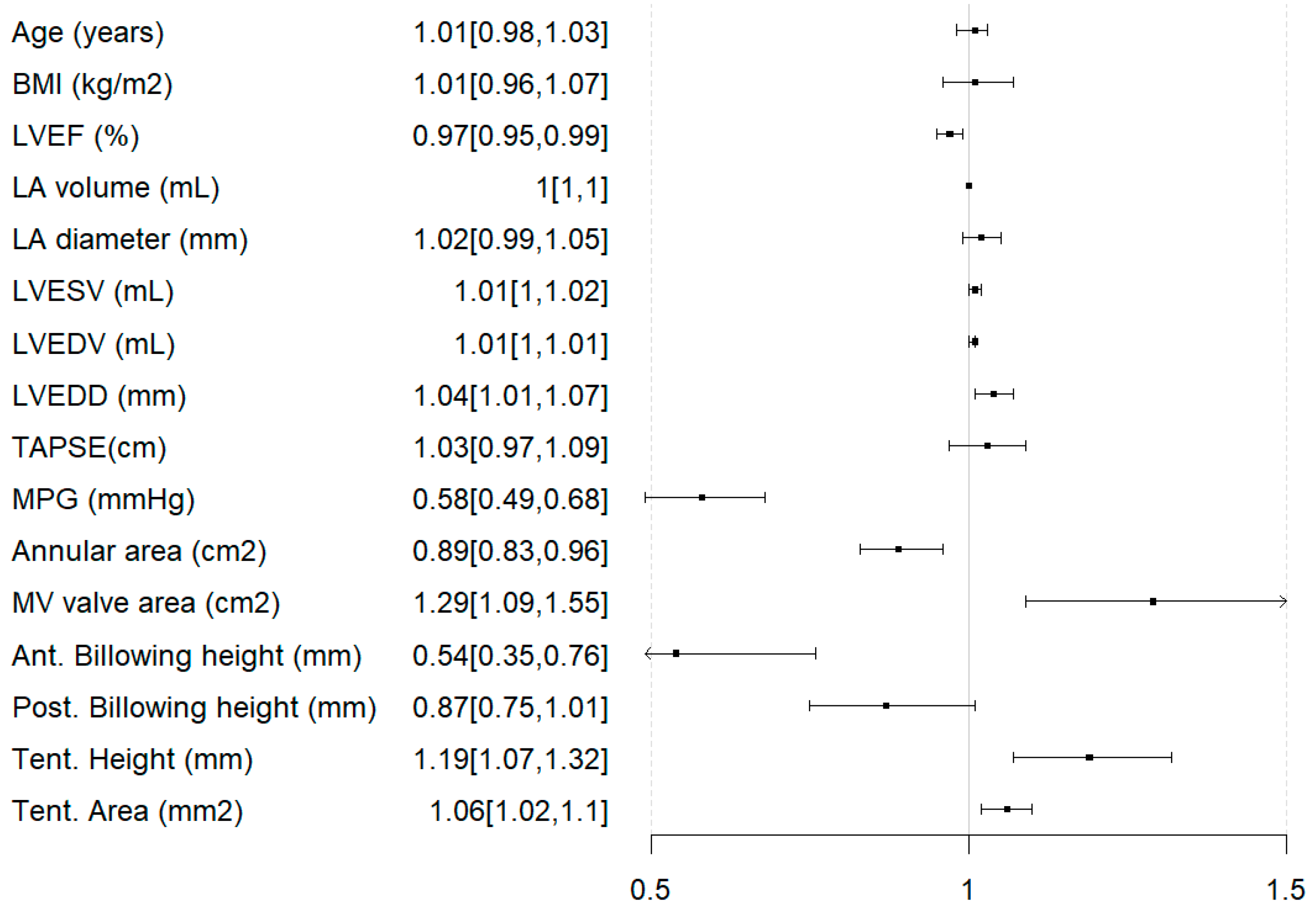
3.4. Predictors of Ineligibility for Any Intervention
4. Discussion
4.1. Identification of Suitable Transcatheter Mitral Valve Intervention Methods According to Patient Characteristics
4.2. Clinical Implications
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CE | Conformité Européenne |
| DMR | Degenerative mitral regurgitation |
| FMR | Functional mitral regurgitation |
| LA | Left atrium |
| LVEDD | Left ventricular enddiastolic diameter |
| LVEF | Left ventricular ejection fraction |
| LVESD | left ventricular endsystolic diameter |
| LVESV | left ventricular endsystolic volume |
| LVOTO | Left ventricular outflow tract obstruction |
| MPG | Mean pressure gradient |
| MR | Mitral regurgitation |
| M-TEER | Mitral transcatheter edge-to-edge repair |
| M-TEER-A | Patients accepted for Mitral transcatheter edge-to-edge repair |
| M-TEER-R | Patients rejected for Mitral transcatheter edge-to-edge repair |
| MVARC | Mitral Valve Academic Research Consortium |
| MVQ | Mitral valve quantification |
| NYHA | New York Heart Association |
| PVL | Paravalvular leakage |
| SAM | Systolic anterior motion |
| TAVR | Transcatheter aortic valve replacement |
| TMVR | Transcatheter mitral valve replacement |
| TMVR-A | Patients accepted for Transcatheter mitral valve replacement |
| TMVR-R | Patients rejected for Transcatheter mitral valve replacement |
| TEE | Transesophageal echocardiography |
| TTE | Transthoracic echocardiography |
| VC | Vena contracta |
References
- Iung, B.; Delgado, V.; Rosenhek, R.; Price, S.; Prendergast, B.; Wendler, O.; De Bonis, M.; Tribouilloy, C.; Evangelista, A.; Bogachev-Prokophiev, A.; et al. Contemporary Presentation and Management of Valvular Heart Disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation 2019, 140, 1156–1169. [Google Scholar] [CrossRef]
- Cahill, T.J.; Prothero, A.; Wilson, J.; Kennedy, A.; Brubert, J.; Masters, M.; Newton, J.D.; Dawkins, S.; Enriquez-Sarano, M.; Prendergast, B.D.; et al. Community Prevalence, Mechanisms and Outcome of Mitral or Tricuspid Regurgitation. Heart 2021, 107, 1003–1009. [Google Scholar] [CrossRef]
- Bonanni, M.; Pizzino, F.; Benedetti, G.; Capasso, R.; Manzo, R.; Iuliano, G.; Trimarchi, G.; D’Agostino, A.; Paradossi, U.; Gimelli, A.; et al. Echocardiographic Screening for Transcatheter Edge-to-Edge Mitral Valve Repair: Correlation Between Transthoracic and Transesophageal Assessment. JCDD 2025, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Samad, Z.; Shaw, L.K.; Phelan, M.; Glower, D.D.; Ersboll, M.; Toptine, J.H.; Alexander, J.H.; Kisslo, J.A.; Wang, A.; Mark, D.B.; et al. Long-Term Outcomes of Mitral Regurgitation by Type and Severity. Am. Heart J. 2018, 203, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Dziadzko, V.; Clavel, M.-A.; Dziadzko, M.; Medina-Inojosa, J.R.; Michelena, H.; Maalouf, J.; Nkomo, V.; Thapa, P.; Enriquez-Sarano, M. Outcome and Undertreatment of Mitral Regurgitation: A Community Cohort Study. Lancet 2018, 391, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.; Doenst, T.; Pfister, R.; Gummert, J.; Kessler, M.; Boekstegers, P.; Lubos, E.; Schröder, J.; Thiele, H.; Walther, T.; et al. Transcatheter Repair versus Mitral-Valve Surgery for Secondary Mitral Regurgitation. N. Engl. J. Med. 2024, 391, 1787–1798. [Google Scholar] [CrossRef]
- Anker, S.D.; Friede, T.; Von Bardeleben, R.-S.; Butler, J.; Khan, M.-S.; Diek, M.; Heinrich, J.; Geyer, M.; Placzek, M.; Ferrari, R.; et al. Transcatheter Valve Repair in Heart Failure with Moderate to Severe Mitral Regurgitation. N. Engl. J. Med. 2024, 391, 1799–1809. [Google Scholar] [CrossRef]
- Imran, H.; Ahmad, K.; Baig, M.; Elgendy, I.Y.; Iqbal, N.; Ehsan, A.; Sharaf, B.; Gordon, P.; Saad, M. Transcatheter Edge-to-Edge Repair of Mitral Valve Regurgitation: Closing the Gap to Broaden the Coverage. Rev. Cardiovasc. Med. 2023, 24, 15. [Google Scholar] [CrossRef]
- Wilde, N.; Tanaka, T.; Vij, V.; Sugiura, A.; Sudo, M.; Eicheler, E.; Silaschi, M.; Vogelhuber, J.; Bakhtiary, F.; Nickenig, G.; et al. Characteristics and Outcomes of Patients Undergoing Transcatheter Mitral Valve Replacement with the Tendyne System. Clin. Res. Cardiol. 2024, 113, 1–10. [Google Scholar] [CrossRef]
- Stone, G.W.; Adams, D.H.; Abraham, W.T.; Kappetein, A.P.; Généreux, P.; Vranckx, P.; Mehran, R.; Kuck, K.-H.; Leon, M.B.; Piazza, N.; et al. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 2: Endpoint Definitions. J. Am. Coll. Cardiol. 2015, 66, 308–321. [Google Scholar] [CrossRef]
- Fattouch, K.; Castrovinci, S.; Murana, G.; Novo, G.; Caccamo, G.; Bertolino, E.C.; Sampognaro, R.; Novo, S.; Ruvolo, G.; Lancellotti, P. Multiplane Two-Dimensional versus Real Time Three-Dimensional Transesophageal Echocardiography in Ischemic Mitral Regurgitation. Echocardiography 2011, 28, 1125–1132. [Google Scholar] [CrossRef][Green Version]
- Lim, D.S.; Herrmann, H.C.; Grayburn, P.; Koulogiannis, K.; Ailawadi, G.; Williams, M.; Ng, V.G.; Chau, K.H.; Sorajja, P.; Smith, R.L.; et al. Consensus Document on Non-Suitability for Transcatheter Mitral Valve Repair by Edge-to-Edge Therapy. Struct. Heart 2021, 5, 227–233. [Google Scholar] [CrossRef]
- Rudolph, F.; Kirchner, J.; Ivannikova, M.; Fortmeier, V.; Rudolph, T.K.; Friedrichs, K.P.; Rudolph, V.; Gerçek, M. A Comparative Study of 1-Year Postprocedural Outcomes in Transcatheter Mitral Valve Repair in Advanced Primary Mitral Regurgitation: PASCAL vs. MitraClip. JCM 2024, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Koell, B.; Weimann, J.; Donal, E.; Patel, D.; Stolz, L.; Tanaka, T.; Scotti, A.; Trenkwalder, T.; Rudolph, F.; et al. Impact of Intraprocedural Mitral Regurgitation and Gradient Following Transcatheter Edge-to-Edge Repair for Primary Mitral Regurgitation. JACC Cardiovasc. Interv. 2024, 17, 1559–1573. [Google Scholar] [CrossRef]
- Zafar, H.; Soleimani, S.; Ijaz, M.; Zafar, J.; Sharif, F. Complex Mitral Valve Anatomy and Open Issues in Transcatheter Mitral Valve Replacement. Surg. Pract. Sci. 2023, 13, 100182. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Imbrie-Moore, A.M.; Zhu, Y.; Wilkerson, R.J.; Wang, H.; Park, G.H.; Wu, C.A.; Pandya, P.K.; Mullis, D.M.; Marin-Cuartas, M.; et al. The Critical Biomechanics of Aortomitral Angle and Systolic Anterior Motion: Engineering Native Ex Vivo Simulation. Ann. Biomed. Eng. 2023, 51, 794–805. [Google Scholar] [CrossRef]
- Denti, P.; Saccocci, M.; Buzzatti, N.; Ascione, G.; Margonato, D.; Gatto, P.; Palloshi, A.; Sarais, C.; Longoni, M.; Maisano, F. Transseptal BATMAN for High-Risk Valve-in-Ring Procedures. JACC Case Rep. 2024, 29, 102200. [Google Scholar] [CrossRef]
- Case, B.C.; Lisko, J.C.; Babaliaros, V.C.; Greenbaum, A.B.; Satler, L.; Ben-Dor, I.; Forrestal, B.J.; Yerasi, C.; Kamioka, N.; Rogers, T.; et al. LAMPOON Techniques to Prevent or Manage Left Ventricular Outflow Tract Obstruction in Transcatheter Mitral Valve Replacement. Ann. Cardiothorac. Surg. 2021, 10, 172–179. [Google Scholar] [CrossRef]
- Thaden, J.J.; Malouf, J.F.; Nkomo, V.T.; Pislaru, S.V.; Holmes, D.R.; Reeder, G.S.; Rihal, C.S.; Eleid, M.F. Mitral Valve Anatomic Predictors of Hemodynamic Success with Transcatheter Mitral Valve Repair. JAHA 2018, 7, e007315. [Google Scholar] [CrossRef]
- Oguz, D.; Padang, R.; Rashedi, N.; Pislaru, S.V.; Nkomo, V.T.; Mankad, S.V.; Malouf, J.F.; Guerrero, M.; Reeder, G.S.; Eleid, M.F.; et al. Risk for Increased Mean Diastolic Gradient after Transcatheter Edge-to-Edge Mitral Valve Repair: A Quantitative Three-Dimensional Transesophageal Echocardiographic Analysis. J. Am. Soc. Echocardiogr. 2021, 34, 595–603.e2. [Google Scholar] [CrossRef] [PubMed]
- Hohneck, A.; Ansari, U.; Natale, M.; Wittig, K.; Overhoff, D.; Riffel, P.; Boettcher, M.; Akin, I.; Duerschmied, D.; Papavassiliu, T. Description of a New Clinical Syndrome: Thoracic Constriction without Evidence of the Typical Funnel-Shaped Depression-the “Invisible” Pectus Excavatum. Sci. Rep. 2023, 13, 12036. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M. Impact of Chest Wall Conformation on the Outcome of Primary Mitral Regurgitation Due to Mitral Valve Prolapse. J. Cardiovasc. Echogr. 2022, 32, 29–37. [Google Scholar] [CrossRef] [PubMed]
- d’Arcy, J.L.; Coffey, S.; Loudon, M.A.; Kennedy, A.; Pearson-Stuttard, J.; Birks, J.; Frangou, E.; Farmer, A.J.; Mant, D.; Wilson, J.; et al. Large-Scale Community Echocardiographic Screening Reveals a Major Burden of Undiagnosed Valvular Heart Disease in Older People: The OxVALVE Population Cohort Study. Eur. Heart J. 2016, 37, 3515–3522. [Google Scholar] [CrossRef]
- Ahmed, A.; Aziz, T.A.A.; AlAsaad, M.M.R.; Majthoob, M.; Toema, A. Transcatheter Mitral Valve Implantation with Tendyne System Ten Years since the First In-Human Implant A Systematic Review. J. Cardiothorac. Surg. 2023, 18, 315. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Terre, J.A.; George, I. Over 15 Years: The Advancement of Transcatheter Aortic Valve Replacement. Ann. Cardiothorac. Surg. 2020, 9, 442–451. [Google Scholar] [CrossRef]
- Gheorghe, L.; Brouwer, J.; Wang, D.D.; Wunderlich, N.; Rana, B.; Rensing, B.; Eefting, F.; Timmers, L.; Swaans, M. Current Devices in Mitral Valve Replacement and Their Potential Complications. Front. Cardiovasc. Med. 2020, 7, 531843. [Google Scholar] [CrossRef]
- Tzeis, S.; Andrikopoulos, G.; Deisenhofer, I.; Ho, S.Y.; Theodorakis, G. Transseptal Catheterization: Considerations and Caveats. Pacing Clin. Electrophysiol. 2010, 33, 231–242. [Google Scholar] [CrossRef]
- Alkhouli, M.; Sarraf, M.; Zack, C.J.; Holmes, D.R.; Rihal, C.S. Iatrogenic Atrial Septal Defect Following Transseptal Cardiac Interventions. Int. J. Cardiol. 2016, 209, 142–148. [Google Scholar] [CrossRef]





| Characteristic | M-TEER Refused | M-TEER Accepted | p-Value 1 |
|---|---|---|---|
| n = 21 | n = 168 | ||
| Age, years [median (IQR)] | 75 (70–79) | 80 (74–83) | 0.02 |
| Female [n (%)] | 6 (29) | 74 (44) | 0.20 |
| BMI, kg/m2 [mean ± SD] | 27.4 ± 8.0 | 26.4 ± 5.7 | 0.50 |
| STS Score, % [median (IQR)] | 1.3 (0.8–2.7) | 2.6 (1.5–4.6) | 0.016 |
| EuroSCORE II, % [median (IQR)] | 2.3 (1.5–3.5) | 4.5 (2.7–7.4) | 0.004 |
| Atrial fibrillation [n (%)] | 15 (71) | 109 (65) | 0.60 |
| Prior MI [n (%)] | 4 (19) | 27 (16) | 0.80 |
| Prior cardiac surgery [n (%)] | 4 (19) | 38 (23) | >0.90 |
| NYHA class baseline ≥3 [n (%)] | 17 (81) | 144 (86) | 0.50 |
| Prior MV intervention | 0.003 | ||
| None [n (%)] | 17 (81) | 165 (98) | |
| Prior MV replacement [n (%)] | 2 (9.5) | 0 (0) | |
| Prior MV repair [n (%)] | 2 (9.5) | 3 (2) | |
| MV disease | 0.20 | ||
| MV regurgitation [n (%)] | 20 (90) | 161 (96) | |
| Combined MV disease [n (%)] | 1 (0.1) | 7 (0.04) | |
| MV Stenosis [n (%)] | 0 (0) | 0 (0) | |
| MV regurgitation etiology | 0.002 | ||
| Degenerative [n (%)] | 12 (57) | 56 (33) | |
| Functional [n (%)] | 4 (19) | 96 (57) | |
| Mixed [n (%)] | 5 (24) | 16 (10) | |
| Residual [n (%)] | 0 (0) | 0 (0) | |
| MR severity | 0.20 | ||
| Mild [n (%)] | 1 (5) | 0 (0) | |
| Mild to moderate [n (%)] | 0 (0) | 5 (3) | |
| Moderate to severe [n (%)] | 9 (43) | 81 (48) | |
| Severe [n (%)] | 11 (52) | 82 (49) |
| Characteristic | M-TEER Refused | M-TEER Accepted | p-Value 1 |
|---|---|---|---|
| n = 21 | n = 168 | ||
| LVEF, % [mean ± SD] | 50 ± 15 | 57 ± 16 | 0.04 |
| LVEDD, mm [median (IQR)] | 56 (51–61) | 57 (51–63) | 0.70 |
| LVEDV, ml [median (IQR)] | 113 (84–143) | 100 (71–158) | 0.80 |
| LVESD, mm [median (IQR)] | 36 (32–44) | 39 (33–50) | 0.30 |
| LVESV, ml [median (IQR)] | 48 (23–69) | 47 (31–87) | 0.40 |
| LA Diameter, mm [median (IQR)] | 48 (39–55) | 52 (46–59) | 0.048 |
| LA Volume, mL [mean ± SD] | 131 ± 99 | 127 ± 60 | 0.30 |
| RA Area, cm2 [mean ± SD] | 21 ± 10 | 24 ± 10 | 0.14 |
| RV Basal Diameter, mm [mean ± SD] | 33 ± 10 | 38 ± 10 | 0.088 |
| TAPSE, mm [mean ± SD] | 19 ± 4 | 19 ± 5 | 0.90 |
| MR EROA, cm2 [mean ± SD] | 0.4 ± 0.3 | 0.4 ± 0.3 | 0.60 |
| MR Volume, ml [mean ± SD] | 58 ± 28 | 58 ± 34 | 0.70 |
| MR Vmax, m/s [mean ± SD] | 6 ± 1 | 5 ± 1 | 0.006 |
| MR EROA (TEE), mm [mean ± SD] | 0.4 ± 0.2 | 0.4 ± 0.3 | 0.082 |
| MR Volume (TEE), ml [mean ± SD] | 70± 33 | 50 ± 31 | 0.030 |
| MR Vmax (TEE), m/s [mean ± SD] | 5 ± 1 | 5 ± 1 | 0.085 |
| Mitral Valve Orifice Area, cm2 [mean ± SD] | 4.9 ± 2.1 | 4.8 ± 1.6 | 0.80 |
| MV MPG, mmHg [mean ± SD] | 4.0 ± 1.5 | 2.2 ± 1.1 | <0.001 |
| Annular Area (measured in 3D), cm2 [mean ± SD] | 12.2 ± 3.5 | 11.5 ± 3.2 | 0.40 |
| Annular Circumference, cm [mean ± SD] | 12.4 ± 1.8 | 12.1 ± 1.7 | 0.40 |
| Aortomitral Angle, ° [mean ± SD] | 135 ± 12 | 139 ± 14 | 0.30 |
| Tenting Height, cm [mean ± SD] | 0.4 ± 0.2 | 0.6 ± 0.4 | 0.020 |
| Tenting Area, cm2 [mean ± SD] | 1.0 ± 0.7 | 1.9 ± 1.5 | 0.004 |
| Tenting Volume, mL [mean ± SD] | 1.7 ± 1.2 | 2.6 ± 1.9 | 0.059 |
| Anterior Billowing Height, mm [mean ± SD] | 0.6± 1.0 | 0.1 ± 0.4 | <0.001 |
| Posterior Billowing Height, mm [mean ± SD] | 2.2 ± 2.7 | 1.0 ± 1.8 | 0.023 |
| TMVR Refused | TMVR Accepted | p-Value 1 | |
|---|---|---|---|
| Characteristic | n = 77 | n = 27 | |
| Age, years [median (IQR)] | 80 (76.5–83) | 79 (72–83) | 0.70 |
| Female [n (%)] | 40 (53) | 11 (41) | 0.30 |
| BMI, kg/m2 [mean ± SD] | 25.8 ± 4.3 | 25.6 ± 3.2 | 0.70 |
| STS Score, % [median (IQR)] | 2.7 (1.5–3.4) | 3.7 (2.8–6.5) | 0.017 |
| EuroSCORE II, % [median (IQR)] | 4.5 (2.7–9.0) | 6.6 (4.7–12.1) | 0.041 |
| Atrial fibrillation [n (%)] | 56 (74) | 22 (81) | 0.40 |
| Prior MI [n (%)] | 10 (13) | 5 (19) | 0.05 |
| Prior cardiac surgery [n (%)] | 57 (75) | 25 (93) | 0.05 |
| NYHA class baseline ≥ 3 [n (%)] | 65 (84) | 21 (78) | 0.60 |
| Prior MV intervention | 0.80 | ||
| None [n (%)] | 68 (88) | 26 (96) | |
| Prior MV replacement [n (%)] | 2 (3) | 0 | |
| Prior MV repair [n (%)] | 7 (9) | 1 (4) | |
| MV disease | 0.70 | ||
| MV regurgitation [n (%)] | 49 (64) | 16 (59) | |
| Combined MV disease [n (%)] | 27 (35) | 9 (41) | |
| MV Stenosis [n (%)] | 1 (1) | 0 (0) | |
| MV regurgitation etiology | 0.20 | ||
| Degenerative [n (%)] | 37 (48) | 11 (41) | |
| Functional [n (%)] | 21 (27) | 13 (48) | |
| Mixed [n (%)] | 14 (18) | 2 (7) | |
| Residual [n (%)] | 5 (7) | 1 (4) | |
| MR severity | 0.70 | ||
| Mild [n (%)] | 1 (1.5) | 0 (0) | |
| Mild to moderate [n (%)] | 1 (1.5) | 0 (0) | |
| Moderate to severe [n (%)] | 21 (27) | 5 (19) | |
| Severe [n (%)] | 54 (70) | 22 (81) |
| TMVR Refused | TMVR Accepted | p-Value 1 | |
|---|---|---|---|
| Characteristic | n = 77 | n = 27 | |
| LVEF, % [mean ± SD] | 54 ± 10 | 49 ± 9 | 0.014 |
| LVEDD, mm [median (IQR)] | 53 (46–59) | 57 (53–62) | 0.027 |
| LVEDV, ml [median (IQR)] | 95 (74–141) | 111 (85–134) | 0.40 |
| LVESD, mm [median (IQR)] | 37 (30–46) | 41 (36–50) | 0.014 |
| LVESV, ml [median (IQR)] | 42 (29–70) | 53 (42–76) | 0.046 |
| LA Diameter, mm [median (IQR)] | 49 (43–58) | 55 (50–59) | 0.07 |
| LA Volume, ml [mean ± SD] | 142 ± 76 | 141 ± 57 | 0.50 |
| RA Area, cm2 [mean ± SD] | 24 ± 11 | 26 ± 10 | 0.30 |
| RV Basal Diameter, mm [mean ± SD] | 35 ± 10 | 38 ± 12 | 0.10 |
| TAPSE, mm [mean ± SD] | 18 ± 5 | 17 ± 4 | 0.50 |
| MR EROA, cm2 [mean ± SD] | 0.5 ± 0.4 | 0.5 ± 0.2 | 0.20 |
| MR Volume, ml [mean ± SD] | 62 ± 24 | 71 ± 24 | 0.20 |
| MR Vmax, m/s [mean ± SD] | 5 ± 1 | 5 ± 1 | 0.50 |
| MR EROA (TEE), mm [mean ± SD] | 0.6 ± 1.0 | 0.5 ± 0.1 | 0.60 |
| MR Volume (TEE), ml [mean ± SD] | 57 ± 26 | 65 ± 23 | 0.20 |
| MR Vmax (TEE), m/s [mean ± SD] | 5 ± 1 | 5 ± 1 | 0.60 |
| Mitral Valve Orifice Area, cm2 [mean ± SD] | 3.6 ± 1.6 | 3.6 ± 2.1 | 0.70 |
| MV MPG, mmHg [mean ± SD] | 4.8 ± 3.6 | 3.8 ± 2.5 | 0.20 |
| Annular Area (measured in 3D), cm2 [mean ± SD] | 14.1 ± 4.2 | 15.0 ± 3.7 | 0.30 |
| Annular Circumference, cm [mean ± SD] | 13.7 ± 2.1 | 14.1 ± 1.9 | 0.30 |
| Aortomitral Angle, ° [mean ± SD] | 120 ± 17 | 121 ± 19 | >0.90 |
| Tenting Height, cm [mean ± SD] | 0.5 ± 0.3 | 0.7 ± 0.4 | 0.021 |
| Tenting Area, cm2 [mean ± SD] | 1.5 ± 0.9 | 2.6 ± 2.5 | 0.004 |
| Tenting Volume, mL [mean ± SD] | 3.4 ± 3.1 | 5.5 ± 4.2 | 0.006 |
| Anterior Billowing Height, mm [mean ± SD] | 0.5 ± 1.3 | 0.4 ± 1.1 | 0.90 |
| Posterior Billowing Height, mm [mean ± SD] | 0.9 ± 1.5 | 0.2 ± 0.5 | 0.08 |
| Characteristic | M-TEER Screened | TMVR Screened | p-Value 1 |
|---|---|---|---|
| n = 189 | n = 104 | ||
| Age, years [median (IQR)] | 79 (73–83) | 80 (76–83) | 0.20 |
| Female [n (%)] | 80 (42%) | 51(50%) | 0.20 |
| BMI, kg/m2 [mean ± SD] | 26.5 ± 6 | 25.8 ± 4 | 0.90 |
| STS Score, % [median (IQR)] | 2.4 (1.3–4.1) | 2.8 (1.7–4.8) | 0.14 |
| EuroSCORE II, % [median (IQR)] | 4.3 (2.5–7.3) | 5.1 (3.1–9.1) | 0.049 |
| Atrial fibrillation [n (%)] | 124 (66%) | 78 (76%) | 0.74 |
| Prior MI [n (%)] | 31 (16%) | 15 (15%) | 0.70 |
| Prior cardiac surgery [n (%)] | 42 (22%) | 82 (80%) | <0.001 |
| NYHA class baseline ≥ 3 [n (%)] | 159 (85%) | 83 (82%) | 0.50 |
| Prior MV intervention | 0.11 | ||
| None [n (%)] | 181 (96%) | 93 (90%) | |
| Prior MV replacement [n (%)] | 2 (1.1%) | 2 (1.9%) | |
| Prior MV repair [n (%)] | 6 (3.2%) | 8 (7.8%) | |
| MV disease | <0.001 | ||
| MV regurgitation [n (%)] | 181 (96%) | 65 (63%) | |
| Combined MV disease [n (%)] | 8 (4.2%) | 38 (37%) | |
| MV stenosis [n (%)] | 0 (0%) | 1 (1%) | |
| MV regurgitation etiology | <0.001 | ||
| Degenerative [n (%)] | 68 (36%) | 48 (46%) | |
| Functional [n (%)] | 100 (53%) | 34 (33%) | |
| Mixed [n (%)] | 21 (11%) | 16 (15%) | |
| Residual [n (%)] | 0 (0%) | 6 (5.8%) | |
| MR severity | <0.001 | ||
| Mild [n (%)] | 1 (0.5%) | 1 (1%) | |
| Mild to moderate [n (%)] | 4 (2.1%) | 1 (1%) | |
| Moderate to severe [n (%)] | 90 (48%) | 26 (25%) | |
| Severe [n (%)] | 94 (50%) | 76 (73%) |
| Characteristic | M-TEER Screened | TMVR Screened | p-Value 1 |
|---|---|---|---|
| n = 189 | n = 104 | ||
| LVEF, % [mean ± SD] | 50 ± 15 | 52 ± 10 | 0.50 |
| LVEDD, mm [median (IQR)] | 57 (51–63) | 55 (48–60) | 0.041 |
| LVEDV, ml [median (IQR)] | 104 (72–154) | 101 (75–137) | 0.70 |
| LVESD, mm [median (IQR)] | 39 (33–50) | 39 (32–48) | 0.40 |
| LVESV, ml [median (IQR)] | 47 (30–84) | 50 (32–72) | 0.90 |
| LA Diameter, mm [median (IQR)] | 52 (46–58) | 52 (45–58) | 0.90 |
| LA Volume, mL [mean ± SD] | 52 ± 10 | 52 ± 10 | 0.90 |
| RA Area, cm2 [mean ± SD] | 24 ± 10 | 24 ± 11 | 0.80 |
| RV Basal Diameter, mm [mean ± SD] | 37 ± 10 | 36 ± 11 | 0.12 |
| TAPSE, mm [mean ± SD] | 19 ± 5 | 18 ± 5 | 0.028 |
| MR EROA, cm2 [mean ± SD] | 0.4 ± 0.3 | 0.5 ± 0.3 | 0.001 |
| MR Volume, ml [mean ± SD] | 58 ± 34 | 64 ± 24 | 0.003 |
| MR Vmax, m/s [mean ± SD] | 5.3 ± 1 | 5.0 ± 1 | 0.06 |
| MR EROA (TEE), mm [mean ± SD] | 0.4 ± 0.3 | 0.6 ± 1 | <0.001 |
| MR Volume (TEE), ml [mean ± SD] | 53 ± 32 | 59 ± 25 | 0.017 |
| MR Vmax (TEE), m/s [mean ± SD] | 5 ± 1 | 4.7 ± 1 | 0.035 |
| Mitral Valve Orifice Area, cm2 [mean ± SD] | 4.8 ± 2 | 3.6 ± 2 | <0.001 |
| MV MPG, mmHg [mean ± SD] | 2.4 ± 1 | 4.6 ± 3 | <0.001 |
| Annular Area (measured in 3D), cm2 [mean ± SD] | 12 ± 3 | 15 ± 4 | <0.001 |
| Annular Circumference, cm [mean ± SD] | 12 ± 2 | 14 ± 2 | <0.001 |
| Aortomitral Angle, ° [mean ± SD] | 140 ± 13 | 120 ± 17 | <0.001 |
| Tenting Height, cm [mean ± SD] | 0.6 ± 0.4 | 0.6 ± 0.3 | 0.20 |
| Tenting Area, cm2 [mean ± SD] | 1.8 ± 1.4 | 1.8 ± 1.6 | 0.80 |
| Tenting Volume, mL [mean ± SD] | 2.5 ± 2 | 4.0 ± 4 | <0.001 |
| Anterior Billowing Height, mm [mean ± SD] | 0.1 ± 0.5 | 0.6 ± 1 | 0.007 |
| Posterior Billowing Height, mm [mean ± SD] | 1.1 ± 2 | 0.7 ± 1 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Göttsche Esperança Clara, C.; Eustergerling, H.; Pepping, J.I.; Trenkpohl, V.; Friedrichs, K.P.; Ivannikova, M.; Rudolph, T.K.; Bormann, J.; Kirchner, J.; Potratz, M.; et al. Characterization of Patients Unsuited for Transcatheter Mitral Valve Interventions. J. Clin. Med. 2025, 14, 7275. https://doi.org/10.3390/jcm14207275
Göttsche Esperança Clara C, Eustergerling H, Pepping JI, Trenkpohl V, Friedrichs KP, Ivannikova M, Rudolph TK, Bormann J, Kirchner J, Potratz M, et al. Characterization of Patients Unsuited for Transcatheter Mitral Valve Interventions. Journal of Clinical Medicine. 2025; 14(20):7275. https://doi.org/10.3390/jcm14207275
Chicago/Turabian StyleGöttsche Esperança Clara, Carolina, Hannah Eustergerling, Johanna Isabella Pepping, Vanessa Trenkpohl, Kai Peter Friedrichs, Maria Ivannikova, Tanja Katharina Rudolph, Johanna Bormann, Johannes Kirchner, Max Potratz, and et al. 2025. "Characterization of Patients Unsuited for Transcatheter Mitral Valve Interventions" Journal of Clinical Medicine 14, no. 20: 7275. https://doi.org/10.3390/jcm14207275
APA StyleGöttsche Esperança Clara, C., Eustergerling, H., Pepping, J. I., Trenkpohl, V., Friedrichs, K. P., Ivannikova, M., Rudolph, T. K., Bormann, J., Kirchner, J., Potratz, M., Rudolph, V., Kassar, M., Gerçek, M., & Rudolph, F. (2025). Characterization of Patients Unsuited for Transcatheter Mitral Valve Interventions. Journal of Clinical Medicine, 14(20), 7275. https://doi.org/10.3390/jcm14207275






