Lactate in Drainage Fluid to Predict Complications in Robotic Esophagectomies—A Pilot Study in a Matched Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Endpoints
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AL | Anastomotic leakages |
| POD | postoperative days |
| AUC | area under curve |
| ROC | receiver operating characteristic curve |
| CRP | C-reactive protein |
| RAMIE | robot-assisted minimally invasive esophagectomy |
| OE | open esophagectomy |
| PIC | postoperative infectious complications |
| WBC | white blood cell |
| PCT | procalcitonin |
| NUn-score | Noble and Underwood-score |
| COPD | chronic obstructive pulmonary disease |
| BMI | body mass index |
| ICU | intensive care unit |
References
- Bektaş, M.; Burchell, G.L.; Bonjer, H.J.; van der Peet, D.L. Machine learning applications in upper gastrointestinal cancer surgery: A systematic review. Surg. Endosc. 2023, 37, 75–89. [Google Scholar] [CrossRef]
- Fabbi, M.; Hagens, E.R.C.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Anastomotic leakage after esophagectomy for esophageal cancer: Definitions, diagnostics, and treatment. Dis. Esophagus 2021, 34, doaa039. [Google Scholar] [CrossRef]
- Franke, F.; Moeller, T.; Mehdorn, A.-S.; Beckmann, J.H.; Becker, T.; Egberts, J.-H. Ivor-Lewis oesophagectomy: A standardized operative technique in 11 steps. Int. J. Med. Robot. 2021, 17, 1–10. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Sinelnikov, M.Y.; Zhang, X.; Cao, Y.; Lu, P. Robot-Assisted Minimally Invasive Breast Surgery: Recent Evidence with Comparative Clinical Outcomes. J. Clin. Med. 2022, 11, 1827. [Google Scholar] [CrossRef]
- van der Sluis, P.C.; Schizas, D.; Liakakos, T.; van Hillegersberg, R. Minimally Invasive Esophagectomy. Dig. Surg. 2020, 37, 93–100. [Google Scholar] [CrossRef]
- Ajani, J.A.; Barthel, J.S.; Bentrem, D.J.; D’Amico, T.A.; Das, P.; Denlinger, C.S.; Fuchs, C.S.; Gerdes, H.; Glasgow, R.E.; Hayman, J.A.; et al. Esophageal and Esophagogastric Junction Cancers. J. Natl. Compr. Cancer Netw. 2011, 9, 830–887. [Google Scholar] [CrossRef]
- Takeuchi, H.; Miyata, H.; Gotoh, M.; Kitagawa, Y.; Baba, H.; Kimura, W.; Tomita, N.; Nakagoe, T.; Shimada, M.; Sugihara, K.; et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann. Surg. 2014, 260, 259–266. [Google Scholar] [CrossRef]
- van Kooten, R.T.; Voeten, D.M.; Steyerberg, E.W.; Hartgrink, H.H.; van Berge Henegouwen, M.I.; van Hillegersberg, R.; Tollenaar, R.A.E.M.; Wouters, M.W.J.M. Patient-Related Prognostic Factors for Anastomotic Leakage, Major Complications, and Short-Term Mortality Following Esophagectomy for Cancer: A Systematic Review and Meta-Analyses. Ann. Surg. Oncol. 2022, 29, 1358–1373. [Google Scholar] [CrossRef] [PubMed]
- Hagens, E.R.C.; Reijntjes, M.A.; Anderegg, M.C.J.; Eshuis, W.J.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Risk Factors and Consequences of Anastomotic Leakage After Esophagectomy for Cancer. Ann. Thorac. Surg. 2021, 112, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Kassis, E.S.; Kosinski, A.S.; Ross, P.; Koppes, K.E.; Donahue, J.M.; Daniel, V.C. Predictors of anastomotic leak after esophagectomy: An analysis of the society of thoracic surgeons general thoracic database. Ann. Thorac. Surg. 2013, 96, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Zehetner, J.; DeMeester, S.R.; Alicuben, E.T.; Oh, D.S.; Lipham, J.C.; Hagen, J.A.; DeMeester, T.R. Intraoperative Assessment of Perfusion of the Gastric Graft and Correlation With Anastomotic Leaks After Esophagectomy. Ann. Surg. 2015, 262, 74–78. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Oka, S.; Gomyo, Y.; Tsujitani, S.; Maeta, M.; Kaibara, N. Postoperative morbidity and mortality after gastrectomy for gastric carcinoma. Hepatogastroenterology 2001, 48, 1517–1520. [Google Scholar]
- Lang, H.; Piso, P.; Stukenborg, C.; Raab, R.; Jähne, J. Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur. J. Surg. Oncol. 2000, 26, 168–171. [Google Scholar] [CrossRef]
- Alanezi, K.; Urschel, J.D. Mortality secondary to esophageal anastomotic leak. Ann. Thorac. Cardiovasc. Surg. 2004, 10, 71–75. [Google Scholar] [PubMed]
- Babic, B.; Tagkalos, E.; Gockel, I.; Corvinus, F.; Hadzijusufovic, E.; Hoppe-Lotichius, M.; Lang, H.; van der Sluis, P.C.; Grimminger, P.P. C-reactive Protein Levels After Esophagectomy Are Associated With Increased Surgical Trauma and Complications. Ann. Thorac. Surg. 2020, 109, 1574–1583. [Google Scholar] [CrossRef]
- Yodying, H.; Matsuda, A.; Miyashita, M.; Matsumoto, S.; Sakurazawa, N.; Yamada, M.; Uchida, E. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2016, 23, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Schlanger, D.; Aiolfi, A.; ElShafei, M.; Triantafyllou, T.; Theodorou, D.; Skrobic, O.; Simic, A.; Al Hajjar, N.; Bonavina, L. Biomarkers associated with anastomotic leakage after esophagectomy: A systematic review. Langenbeck’s Arch. Surg. 2025, 410, 55. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Calvano, S.E.; Lowry, S.F. Inflammatory cytokines and cell response in surgery. Surgery 2000, 127, 117–126. [Google Scholar] [CrossRef]
- Santonocito, C.; de Loecker, I.; Donadello, K.; Moussa, M.D.; Markowicz, S.; Gullo, A.; Vincent, J.-L. C-Reactive Protein Kinetics After Major Surgery. Anesth. Analg. 2014, 119, 624–629. [Google Scholar] [CrossRef]
- Kokotovic, D.; Burcharth, J.; Helgstrand, F.; Gögenur, I. Systemic inflammatory response after hernia repair: A systematic review. Langenbeck’s Arch. Surg. 2017, 402, 1023–1037. [Google Scholar] [CrossRef]
- Gordon, A.C.; Cross, A.J.; Foo, E.W.; Roberts, R.H. C-reactive protein is a useful negative predictor of anastomotic leak in oesophago-gastric resection. ANZ J. Surg. 2018, 88, 223–227. [Google Scholar] [CrossRef]
- Richter, F.; Mehdorn, A.-S.; Fedders, T.; Reichert, B.; Egberts, J.-H.; Becker, T.; Pochhammer, J. C-Reactive Protein as Predictor for Infectious Complications after Robotic and Open Esophagectomies. J. Clin. Med. 2022, 11, 5654. [Google Scholar] [CrossRef]
- Asti, E.; Bonitta, G.; Melloni, M.; Tornese, S.; Milito, P.; Sironi, A.; Costa, E.; Bonavina, L. Utility of C-reactive protein as predictive biomarker of anastomotic leak after minimally invasive esophagectomy. Langenbeck’s Arch. Surg. 2018, 403, 235–244. [Google Scholar] [CrossRef]
- Noble, F.; Curtis, N.; Harris, S.; Kelly, J.J.; Bailey, I.S.; Byrne, J.P.; Underwood, T.J. Risk assessment using a novel score to predict anastomotic leak and major complications after oesophageal resection. J. Gastrointest. Surg. 2012, 16, 1083–1095. [Google Scholar] [CrossRef]
- Adamina, M.; Steffen, T.; Tarantino, I.; Beutner, U.; Schmied, B.M.; Warschkow, R. Meta-analysis of the predictive value of C-reactive protein for infectious complications in abdominal surgery. Br. J. Surg. 2015, 102, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.L.; Alvarez, M.O.; Cuquerella, V.; Miranda, E.; Picó, C.; Flores, R.; Resalt-Pereira, M.; Moya, P.; Pérez, A.; Arroyo, A. Procalcitonin and C-reactive protein as early markers of anastomotic leak after laparoscopic colorectal surgery within an enhanced recovery after surgery (ERAS) program. Surg. Endosc. 2018, 32, 4003–4010. [Google Scholar] [CrossRef] [PubMed]
- Åkesson, O.; Abrahamsson, P.; Johansson, G.; Haney, M.; Falkenback, D.; Hermansson, M.; Jeremiasen, M.; Johansson, J. Surface microdialysis measures local tissue metabolism after Ivor Lewis esophagectomy; an attempt to predict anastomotic defect. Dis. Esophagus 2023, 36, doac111. [Google Scholar] [CrossRef]
- Takahashi, N.; Okamura, A.; Kuriyama, K.; Terayama, M.; Tamura, M.; Kanamori, J.; Imamura, Y.; Watanabe, M. Early Postoperative Serum Lactate Levels Predict Anastomotic Leakage After Minimally Invasive Esophagectomy. Ann. Surg. Oncol. 2025, 32, 834–840. [Google Scholar] [CrossRef]
- Egberts, J.-H.; Biebl, M.; Perez, D.R.; Mees, S.T.; Grimminger, P.P.; Müller-Stich, B.P.; Stein, H.; Fuchs, H.; Bruns, C.J.; Hackert, T.; et al. Robot-Assisted Oesophagectomy: Recommendations Towards a Standardised Ivor Lewis Procedure. J. Gastrointest. Surg. 2019, 23, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Scheufele, F.; Vogel, T.; Gasiorek, M.; Novotny, A.; Friess, H.; Demir, I.E.; Schorn, S. Serum albumin at resection predicts in-hospital death, while serum lactate and aPTT on the first postoperative day anticipate anastomotic leakage after Ivor-Lewis-esophagectomy. Langenbeck’s Arch. Surg. 2022, 407, 2309–2317. [Google Scholar] [CrossRef]
- van Daele, E.; Vanommeslaeghe, H.; Peirsman, L.; van Nieuwenhove, Y.; Ceelen, W.; Pattyn, P. Early postoperative systemic inflammatory response as predictor of anastomotic leakage after esophagectomy: A systematic review and meta-analysis. J. Gastrointest. Surg. 2024, 28, 757–765. [Google Scholar] [CrossRef]
- Cikot, M.; Kones, O.; Gedikbası, A.; Kocatas, A.; Karabulut, M.; Temizgonul, K.B.; Alis, H. The marker C-reactive protein is helpful in monitoring the integrity of anastomosis: Plasma calprotectin. Am. J. Surg. 2016, 212, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, K.W.; Poeze, M.; Hulsewé, K.W.E.; van Acker, B.A.; van Bijnen, A.A.; Hoofwijk, A.G.M.; Stoot, J.H.M.B.; Derikx, J.P.M. Accurate prediction of anastomotic leakage after colorectal surgery using plasma markers for intestinal damage and inflammation. J. Am. Coll. Surg. 2014, 219, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Buscemi, S.; Di Buono, G.; Vidali, M.; Lo Sasso, B.; Agrusa, A.; Ciaccio, M. Drainage fluid LDH and neutrophil to lymphocyte ratio as biomarkers for early detecting anastomotic leakage in patients undergoing colorectal surgery. Clin. Chem. Lab. Med. 2024, 62, 967–978. [Google Scholar] [CrossRef] [PubMed]

| AL (n = 17) | No AL (n = 23) | p-Value | |
|---|---|---|---|
| Sex (male) | 16 (94.1) | 17 (73.9) | 0.10 |
| Age (years) | 66.1 ± 8.8 | 65.8 ± 10.2 | 0.91 |
| BMI (kg/m2) | 27.5 ± 3.2 | 28.8 ± 4.7 | 0.33 |
| Preoperative weight loss (%) | 2.4 (0–12.8) | 4.2 (0–16) | 0.83 |
| ASA-Score 2 | 4 (23.5) | 10 (43.5) | |
| 3 | 13 (76.5) | 10 (43.5) | |
| 4 | 0 | 3 (13.0) | 0.08 |
| Risk factors | |||
| Smoking | 3 (17.7) | 3 (13.0) | 0.69 |
| Arterial hypertension | 12 (70.6) | 16 (69.6) | 0.94 |
| Coronary heart disease | 5 (29.4) | 1 (4.4) | 0.03 |
| Diabetes mellitus | 1 (5.6) | 3 (13.0) | 0.46 |
| COPD | 4 (23.5) | 0 | 0.01 |
| Alcohol misuse | 1 (5.9) | 1 (4.4) | 0.83 |
| Entity | |||
| Adenocarcinoma | 13 (38.2) | 21 (61.8) | |
| Squamous cell carcinoma | 4 (80.0) | 1 (20.0) | 0.14 |
| Neoadjuvant Treatment | |||
| Chemotherapy | 12 (46.2) | 14 (53.9) | |
| Chemoradiotherapy | 2 (40.0) | 3 (60.0) | 0.79 |
| Adjuvant treatment | |||
| Chemotherapy | 1 (25.0) | 3 (75.0) | |
| Radiotherapy | 0 | 1 (100) | 0.50 |
| AL (n = 17) | No AL (n = 23) | p-Value | |
|---|---|---|---|
| Procedure | |||
| Abdomino-thoracal esophagectomy (Ivor-Lewis) | 16 (50.0) | 16 (50.0) | |
| transhiatal esophagectomy | 1 (12.5) | 7 (87.5) | 0.06 |
| Anastomosis | |||
| End-to-side | 14 (45.2) | 17 (54.8) | |
| Side-to-side | 1 (20.0) | 4 (80.0) | |
| Side-to-end | 0 | 2 (100.0) | |
| End-to-end | 2 (100.0) | 0 | 0.15 |
| Conversion | 1 (100.0) | 0 | 0.24 |
| Length of surgery (min) | 350.3 ± 59.6 | 312.2 ± 56.7 | <0.05 |
| Unplanned ICU-treatment | 17 (47.2) | 19 (52.8) | 0.07 |
| Length of ICU stay | 3 (1–49) | 2 (1–11) | <0.05 |
| Length of hospital stay | 39 (14–77) | 14 (5–30) | <0.01 |
| Complications | |
|---|---|
| 30 d-morbidity | 26 (65.0) |
| Anastomotic Leakage | |
| Day of clinical occurrence | 8 (2–48) |
| Endosponge treatment | 17 (100.0) |
| Duration of endosponge treatment (d) | 22 (3–55) |
| Re-operation | 2 (5.0) |
| Pneumonia | 5 (12.5) |
| Pulmonary artery embolism | 2 (5.0) |
| Pancreatitis | 1 (2.5) |
| Postoperative bleeding | 4 (10.0) |
| Esophagobronchial Fistula | 1 (2.5) |
| Postoperative delirium | 2 (5.0) |
| Surgical Site Infection | 3 (7.5) |
| Superficial | 1 (2.5) |
| Organ–space | 2 (5.0) |
| Anastomotic stenosis | 2 (5.0) |
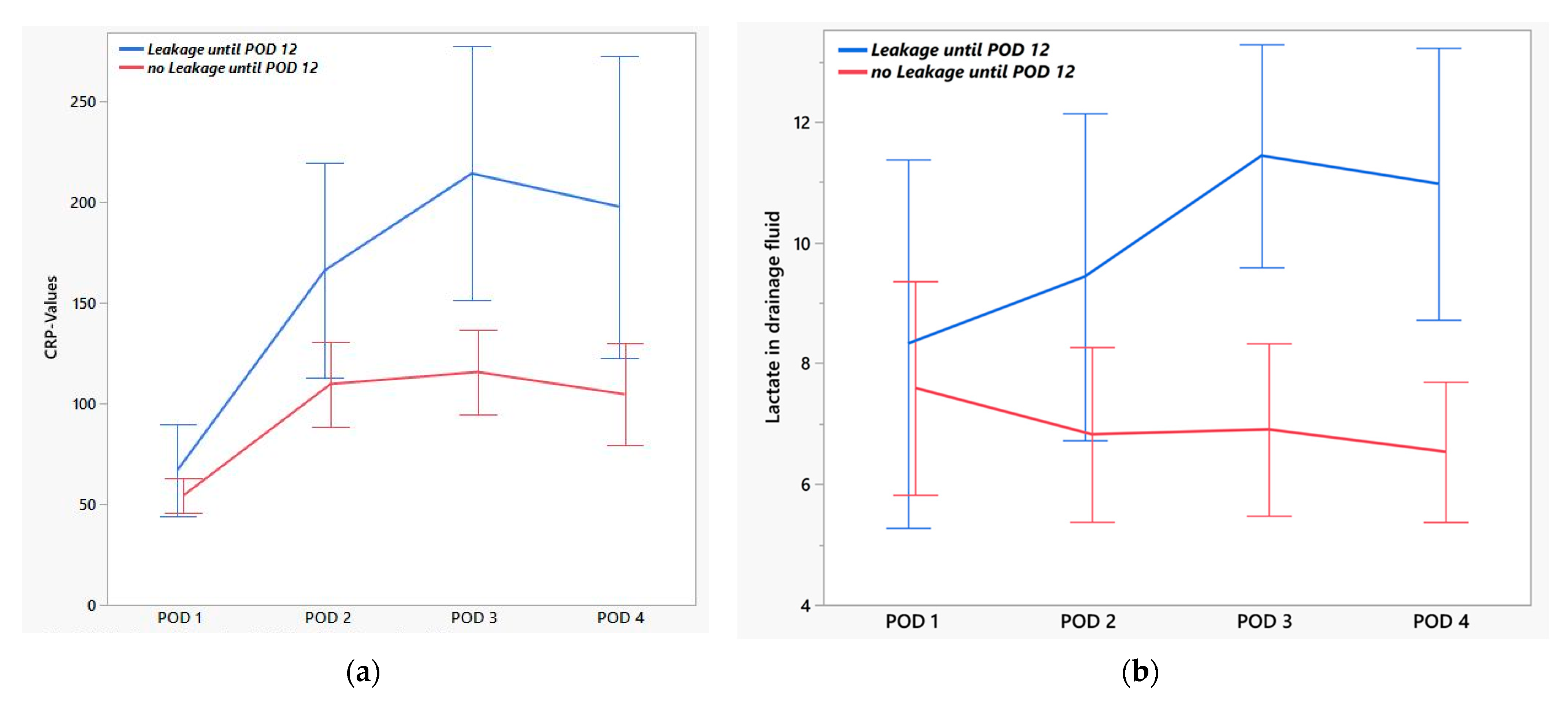
| EAL (n = 13) | No EAL (n = 27) | p-Value | AUC | |
|---|---|---|---|---|
| Lactate [mmol/L] | ||||
| POD 1 | 7.7 (2.3–15.0) | 5.6 (2.0–15.0) | 0.68 | 0.54 |
| POD 2 | 8.0 (4.1–15.0) | 6.0 (2.2–15.2) | 0.09 | 0.67 |
| POD 3 | 11.2 (7.3–15.0) | 6.3 (1.7–15.0) | <0.01 | 0.85 |
| POD 4 | 9.7 (6.3–15.0) | 6.3 (2.1–11.5) | <0.01 | 0.85 |
| CRP [mg/L] | ||||
| POD 1 | 49.4 (24.1–148) | 59.5 (9.7–90.5) | 0.54 | 0.56 |
| POD 2 | 131.0 (67.1–375.0) | 109.0 (30.6–227.0) | 0.04 | 0.70 |
| POD 3 | 223.5 (85.0–389.0) | 113.5 (20.5–230.0) | <0.01 | 0.79 |
| POD 4 | 143.0 (49.2–414.0) | 120.5 (13.2–217.0) | 0.03 | 0.72 |
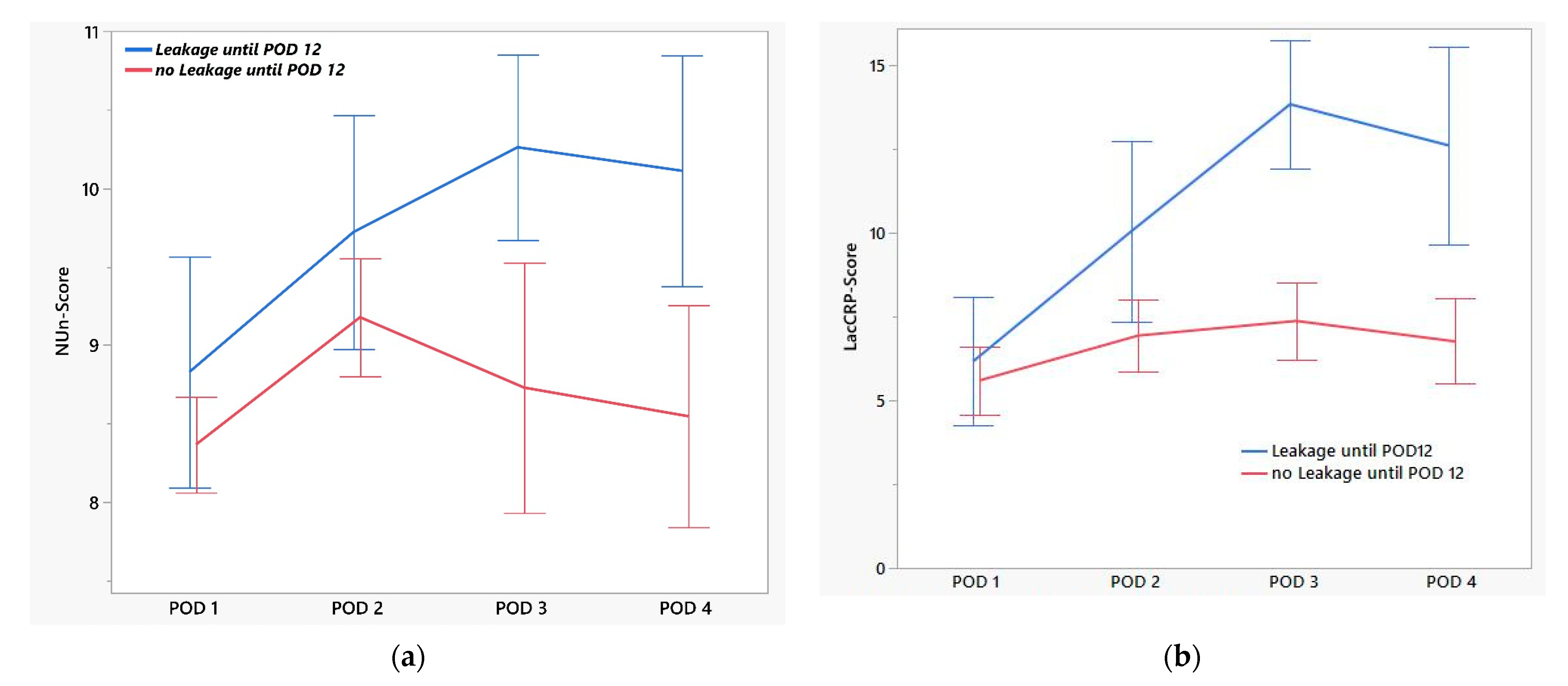
| EAL (n = 13) | No EAL (n = 27) | p-Value | AUC | |
|---|---|---|---|---|
| NUn-Score | ||||
| POD 1 | 8.6 (7.2–11.2) | 8.4 (6.5–9.9) | 0.30 | 0.60 |
| POD 2 | 9.3 (8.1–11.8) | 9.1 (8.1–11.0) | 0.22 | 0.64 |
| POD 3 | 10.3 (9.0–11.5) | 9.1 (6.5–10.1) | <0.01 | 0.89 |
| POD 4 | 10.6 (8.7–11.3) | 8.5 (7.7–9.1) | 0.01 | 0.91 |
| LacCRP-Score | ||||
| POD 1 | 6.1 (2.3–11.3) | 5.4 (2.3–10.1) | 0.58 | 0.55 |
| POD 2 | 9.4 (4.3–20.0) | 7.2 (2.4–11.6) | 0.02 | 0.74 |
| POD 3 | 14.0 (9.6–18.0) | 7.5 (1.5–11.4) | <0.01 | 0.96 |
| POD 4 | 11.1 (6.5–18.5) | 6.7 (3.1–10.8) | <0.01 | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pochhammer, J.; Kiani, S.; Hobbensiefken, H.; Hobbensiefken, H.; Reichert, B.; Taivankhuu, T.; Becker, T.; Gundlach, J.-P. Lactate in Drainage Fluid to Predict Complications in Robotic Esophagectomies—A Pilot Study in a Matched Cohort. J. Clin. Med. 2025, 14, 6190. https://doi.org/10.3390/jcm14176190
Pochhammer J, Kiani S, Hobbensiefken H, Hobbensiefken H, Reichert B, Taivankhuu T, Becker T, Gundlach J-P. Lactate in Drainage Fluid to Predict Complications in Robotic Esophagectomies—A Pilot Study in a Matched Cohort. Journal of Clinical Medicine. 2025; 14(17):6190. https://doi.org/10.3390/jcm14176190
Chicago/Turabian StylePochhammer, Julius, Sarah Kiani, Henning Hobbensiefken, Hilke Hobbensiefken, Benedikt Reichert, Terbish Taivankhuu, Thomas Becker, and Jan-Paul Gundlach. 2025. "Lactate in Drainage Fluid to Predict Complications in Robotic Esophagectomies—A Pilot Study in a Matched Cohort" Journal of Clinical Medicine 14, no. 17: 6190. https://doi.org/10.3390/jcm14176190
APA StylePochhammer, J., Kiani, S., Hobbensiefken, H., Hobbensiefken, H., Reichert, B., Taivankhuu, T., Becker, T., & Gundlach, J.-P. (2025). Lactate in Drainage Fluid to Predict Complications in Robotic Esophagectomies—A Pilot Study in a Matched Cohort. Journal of Clinical Medicine, 14(17), 6190. https://doi.org/10.3390/jcm14176190






