Efficacy of Biologic Agents and Small Molecules for Endoscopic Improvement and Mucosal Healing in Patients with Moderate-to-Severe Ulcerative Colitis: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Outcome Definitions
2.3. Data Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- Pillai, N.; Dusheiko, M.; Maillard, M.H.; Rogler, G.; Brüngger, B.; Bähler, C.; Pittet, V.E.H. The Evolution of Health Care Utilisation and Costs for Inflammatory Bowel Disease Over Ten Years. J. Crohn’s Colitis 2019, 13, 744–754. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Calvez, V.; Puca, P.; Di Vincenzo, F.; Del Gaudio, A.; Bartocci, B.; Murgiano, M.; Iaccarino, J.; Parand, E.; Napolitano, D.; Pugliese, D.; et al. Novel Insights into the Pathogenesis of Inflammatory Bowel Diseases. Biomedicines 2025, 13, 305. [Google Scholar] [CrossRef]
- Mousa, R.S.; Invernizzi, P.; Mousa, H.S. Innate immune cells in the pathogenesis of inflammatory bowel disease-from microbial metabolites to immune modulation. Front. Gastroenterol. 2024, 3, 1452430. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Sandborn, W.J.; Panaccione, R.; Domènech, E.; Pouillon, L.; Siegmund, B.; Danese, S.; Ghosh, S. Tumour necrosis factor inhibitors in inflammatory bowel disease: The story continues. Ther. Adv. Gastroenterol. 2021, 14, 17562848211059954. [Google Scholar] [CrossRef] [PubMed]
- Wyant, T.; Fedyk, E.; Abhyankar, B. An Overview of the Mechanism of Action of the Monoclonal Antibody Vedolizumab. J. Crohn’s Colitis 2016, 10, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.D.; Dyer, E.C.; Rubin, D.T. IL-23 Monoclonal Antibodies for IBD: So Many, So Different? J. Crohn’s Colitis 2022, 16 (Suppl. S2), ii42–ii53. [Google Scholar] [CrossRef] [PubMed]
- Honap, S.; Agorogianni, A.; Colwill, M.J.; Mehta, S.K.; Donovan, F.; Pollok, R.; Poullis, A.; Patel, K. JAK inhibitors for inflammatory bowel disease: Recent advances. Frontline Gastroenterol. 2024, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Bencardino, S.; D’amico, F.; Faggiani, I.; Bernardi, F.; Allocca, M.; Furfaro, F.; Parigi, T.L.; Zilli, A.; Fiorino, G.; Peyrin-Biroulet, L.; et al. Efficacy and Safety of S1P1 Receptor Modulator Drugs for Patients with Moderate-to-Severe Ulcerative Colitis. J. Clin. Med. 2023, 12, 5014. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’aMico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Rutgeerts, P.; Reinisch, W.; Esser, D.; Wang, Y.; Lang, Y.; Marano, C.W.; Strauss, R.; Oddens, B.J.; Feagan, B.G.; et al. Early Mucosal Healing with Infliximab Is Associated with Improved Long-term Clinical Outcomes in Ulcerative Colitis. Gastroenterology 2011, 141, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Meucci, G.; Fasoli, R.; Saibeni, S.; Valpiani, D.; Gullotta, R.; Colombo, E.; D’incà, R.; Terpin, M.; Lombardi, G. Prognostic Significance of Endoscopic Remission in Patients with Active Ulcerative Colitis Treated with Oral and Topical Mesalazine: A Prospective, Multicenter Study. Inflamm. Bowel Dis. 2012, 18, 1006–1010. [Google Scholar] [CrossRef]
- Frøslie, K.F.; Jahnsen, J.; Moum, B.A.; Vatn, M.H. Mucosal Healing in Inflammatory Bowel Disease: Results From a Norwegian Population-Based Cohort. Gastroenterology 2007, 133, 412–422. [Google Scholar] [CrossRef]
- Ardizzone, S.; Cassinotti, A.; Duca, P.; Mazzali, C.; Penati, C.; Manes, G.; Marmo, R.; Massari, A.; Molteni, P.; Maconi, G.; et al. Mucosal Healing Predicts Late Outcomes After the First Course of Corticosteroids for Newly Diagnosed Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2011, 9, 483–489.e3. [Google Scholar] [CrossRef]
- Saxena, A.P.; Limdi, J.K.; Farraye, F.A. Zeroing in on endoscopic and histologic mucosal healing to reduce the risk of colorectal neoplasia in inflammatory bowel disease. Gastrointest. Endosc. 2017, 86, 1012–1014. [Google Scholar] [CrossRef]
- Walsh, A.; Palmer, R.; Travis, S. Mucosal Healing As a Target of Therapy for Colonic Inflammatory Bowel Disease and Methods to Score Disease Activity. Gastrointest. Endosc. Clin. N. Am. 2014, 24, 367–378. [Google Scholar] [CrossRef]
- Buda, A.; Pessarelli, T.; Aldinio, G.; De Bona, M.; Iacucci, M.; Tontini, G.E. Endoscopic healing in IBD: Still the target to achieve? Dig. Liver Dis. 2025, 57, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Buchner, A.M.; Farraye, F.A.; Iacucci, M. AGA Clinical Practice Update on Endoscopic Scoring Systems in Inflammatory Bowel Disease: Commentary. Clin. Gastroenterol. Hepatol. 2024, 22, 2188–2196. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Arenson, E.; Rubin, D.T.; Siegel, C.A.; Lee, S.; Laroux, F.S.; Zhou, W.; Finney-Hayward, T.; Gonzalez, Y.S.; Shields, A.L. A Comparative Evaluation of the Measurement Properties of Three Histological Indices of Mucosal Healing in Ulcerative Colitis: Geboes Score, Robarts Histopathology Index and Nancy Index. J. Crohn’s Colitis 2023, 17, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; van Assche, G.; Reinisch, W.; Colombel, J.; D’hAens, G.; Wolf, D.C.; Kron, M.; Tighe, M.B.; Lazar, A.; Thakkar, R.B. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012, 142, 257–265.e3. [Google Scholar] [CrossRef]
- RReinisch, W.; Sandborn, W.J.; Hommes, D.W.; D’Haens, G.; Hanauer, S.; Schreiber, S.; Panaccione, R.; Fedorak, R.N.; Tighe, M.B.; Huang, B.; et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: Results of a randomised controlled trial. Gut 2011, 60, 780–787. [Google Scholar] [CrossRef]
- Suzuki, Y.; Motoya, S.; Hanai, H.; Matsumoto, T.; Hibi, T.; Robinson, A.M.; Mostafa, N.M.; Chao, J.; Arora, V.; Camez, A.; et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J. Gastroenterol. 2014, 49, 283–294. [Google Scholar] [CrossRef]
- Jiang, X.L.; Cui, H.F.; Gao, J.; Fan, H. Low-dose Infliximab for Induction and Maintenance Treatment in Chinese Patients with Moderate to Severe Active Ulcerative Colitis. J. Clin. Gastroenterol. 2015, 49, 582–588. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef]
- NCT01551290. A Study to Evaluate the Effectiveness and Safety of Infliximab in Chinese Patients with Active Ulcerative Colitis 2012. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01536077/full (accessed on 7 July 2025).
- Kobayashi, T.; Suzuki, Y.; Motoya, S.; Hirai, F.; Ogata, H.; Ito, H.; Sato, N.; Ozaki, K.; Watanabe, M.; Hibi, T. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis-results from a multicenter prospective randomized controlled trial and its post hoc analysis. J. Gastroenterol. 2015, 51, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Peyrin-Biroulet, L.; Zhang, J.; Chiorean, M.; Vermeire, S.; Lee, S.D.; Kühbacher, T.; Yacyshyn, B.; Cabell, C.H.; Naik, S.U.; et al. Efficacy and Safety of Etrasimod in a Phase 2 Randomized Trial of Patients with Ulcerative Colitis. Gastroenterology 2020, 158, 550–561. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Vermeire, S.; Peyrin-Biroulet, L.; Dubinsky, M.C.; Panes, J.; Yarur, A.; Ritter, T.; Baert, F.; Schreiber, S.; Sloan, S.; et al. Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): Two randomised, double-blind, placebo-controlled, phase 3 studies. Lancet 2023, 401, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Hisamatsu, T.; Nakase, H.; Matsuoka, K.; Keating, M.; Yuasa, H.; Oe, M.; Arai, S.; Mazur, R.; Hibi, T. Efficacy and Safety of Etrasimod in Patients with Ulcerative Colitis in Japan: Data from the Phase 3 ELEVATE UC 12 and ELEVATE UC 40 JAPAN Trials. Digestion 2024, 106, 167–175. [Google Scholar] [CrossRef]
- Wu, K.; Zheng, C.; Cao, Q.; Gao, X.; Ding, Y.; Zhong, J.; Zhang, H.; Wang, X.; Wang, B.; Zhang, Z.; et al. DOP052 Etrasimod as induction and maintenance therapy in Asian patients with moderately to severely active Ulcerative Colitis: Results from the maintenance period of a randomised, double-blind, placebo-controlled, multi-centre Phase 3 study (ES101002). J. Crohn’s Colitis 2025, 19 (Suppl. S1), i182–i184. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.-F.; Reinisch, W.; et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 85–95. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Ferrante, M.; Bhandari, B.R.; Berliba, E.; Feagan, B.G.; Hibi, T.; Tuttle, J.L.; Klekotka, P.; Friedrich, S.; Durante, M.; et al. Efficacy and Safety of Mirikizumab in a Randomized Phase 2 Study of Patients with Ulcerative Colitis. Gastroenterology 2020, 158, 537–549.e10. [Google Scholar] [CrossRef] [PubMed]
- D’hAens, G.; Dubinsky, M.; Kobayashi, T.; Irving, P.M.; Howaldt, S.; Pokrotnieks, J.; Krueger, K.; Laskowski, J.; Li, X.; Lissoos, T.; et al. Mirikizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2023, 388, 2444–2455. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Wolf, D.C.; D’hAens, G.; Vermeire, S.; Hanauer, S.B.; Ghosh, S.; Smith, H.; Cravets, M.; Frohna, P.A.; et al. Ozanimod Induction and Maintenance Treatment for Ulcerative Colitis. N. Engl. J. Med. 2016, 374, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; D’hAens, G.; Wolf, D.C.; Jovanovic, I.; Hanauer, S.B.; Ghosh, S.; Petersen, A.; Hua, S.Y.; Lee, J.H.; et al. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2021, 385, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’hAens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Ghosh, S.; Panes, J.; Schreiber, S.; D’hAens, G.; Tanida, S.; Siffledeen, J.; Enejosa, J.; Zhou, W.; Othman, A.A.; et al. Efficacy of Upadacitinib in a Randomized Trial of Patients with Active Ulcerative Colitis. Gastroenterology 2020, 158, 2139–2149.e14. [Google Scholar] [CrossRef]
- Danese, S.; Vermeire, S.; Zhou, W.; Pangan, A.L.; Siffledeen, J.; Greenbloom, S.; Hébuterne, X.; D’Haens, G.; Nakase, H.; Panés, J.; et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomised trials. Lancet 2022, 399, 2113–2128. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’bRien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Motoya, S.; Watanabe, K.; Ogata, H.; Kanai, T.; Matsui, T.; Suzuki, Y.; Shikamura, M.; Sugiura, K.; Oda, K.; Hori, T.; et al. Vedolizumab in Japanese patients with ulcerative colitis: A Phase 3, randomized, double-blind, placebo-controlled study. Green J, editor. PLoS ONE 2019, 14, e0212989. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Sands, B.E.; Sandborn, W.J.; Germinaro, M.; Vetter, M.; Shao, J.; Sheng, S.; Johanns, J.; Panés, J.; Tkachev, A.; et al. Guselkumab plus golimumab combination therapy versus guselkumab or golimumab monotherapy in patients with ulcerative colitis (VEGA): A randomised, double-blind, controlled, phase 2, proof-of-concept trial. Lancet Gastroenterol. Hepatol. 2023, 8, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Allegretti, J.R.; Rubin, D.T.; Bressler, B.; Germinaro, M.; Huang, K.-H.; Shipitofsky, N.; Zhang, H.; Wilson, R.; Han, C.; et al. Guselkumab in Patients with Moderately to Severely Active Ulcerative Colitis: QUASAR Phase 2b Induction Study. Gastroenterology 2023, 165, 1443–1457. [Google Scholar] [CrossRef]
- Rubin, D.T.; Allegretti, J.R.; Panés, J.; Shipitofsky, N.; Yarandi, S.S.; Huang, K.G.; Germinaro, M.; Wilson, R.; Zhang, H.; Johanns, J.; et al. Guselkumab in patients with moderately to severely active ulcerative colitis (QUASAR): Phase 3 double-blind, randomised, placebo-controlled induction and maintenance studies. Lancet 2025, 405, 33–49. [Google Scholar] [CrossRef]
- Louis, E.; Schreiber, S.; Panaccione, R.; Bossuyt, P.; Biedermann, L.; Colombel, J.F.; Parkes, G.; Peyrin-Biroulet, L.; D’Haens, G.; Hisamatsu, T.; et al. Risankizumab for Ulcerative Colitis: Two Randomized Clinical Trials. JAMA 2024, 332, 881–897. [Google Scholar] [CrossRef]
- Feagan, B.G.; Danese, S.; Loftus, E.V.; Vermeire, S.; Schreiber, S.; Ritter, T.; Fogel, R.; Mehta, R.; Nijhawan, S.; Kempiński, R.; et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): A phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet 2021, 397, 2372–2384. [Google Scholar] [CrossRef]
- Probert, C.S.J.; Hearing, S.D.; Schreiber, S.; Kühbacher, T.; Ghosh, S.; Arnott, I.D.R.; Forbes, A. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: A randomised controlled trial. Gut 2003, 52, 998–1002. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Ghosh, S.; Panes, J.; Vranic, I.; Su, C.; Rousell, S.; Niezychowski, W. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N. Engl. J. Med. 2012, 367, 616–624. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.; Reinisch, W.; et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 96–109.e1. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Baert, F.; Danese, S.; Krznarić, Ž.; Kobayashi, T.; Yao, X.; Chen, J.; Rosario, M.; Bhatia, S.; Kisfalvi, K.; et al. Efficacy and Safety of Vedolizumab Subcutaneous Formulation in a Randomized Trial of Patients with Ulcerative Colitis. Gastroenterology 2020, 158, 562–572.e12. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Hanauer, S.B.; Colombel, J.F.; Sandborn, W.; Schreiber, S.; Danese, S.; Klopocka, M.; Kulynych, R.; Kierkus, J.; Soltysiak, A.; et al. P492 Subcutaneous infliximab (CT-P13 SC) as maintenance therapy for ulcerative colitis: A Phase 3, randomized, placebo-controlled study: Results of the LIBERTY-UC study. J. Crohn’s Colitis 2023, 17 (Suppl. S1), i623–i624. [Google Scholar] [CrossRef]
- Hibi, T.; Imai, Y.; Senoo, A.; Ohta, K.; Ukyo, Y. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: A phase 3, double-blind, randomized, placebo-controlled study-(PURSUIT-J study). J. Gastroenterol. 2017, 52, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Peyrin-Biroulet, L.; Loftus, E.V., Jr.; Danese, S.; Colombel, J.-F.; Törüner, M.; Jonaitis, L.; Abhyankar, B.; Chen, J.; Rogers, R.; et al. Vedolizumab versus Adalimumab for Moderate-to-Severe Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1215–1226. [Google Scholar] [CrossRef]
- Mohammed Vashist, N.; Samaan, M.; Mosli, M.H.; Parker, C.E.; MacDonald, J.K.; Nelson, S.A.; Zou, G.Y.; Feagan, B.G.; Khanna, R.; Jairath, V. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst. Rev. 2018, 2018, CD011450. [Google Scholar] [CrossRef]
- Estevinho, M.M.; Sousa-Pinto, B.; Moreira, P.L.; Solitano, V.; Mesquita, P.; Costa, C.; Peyrin-Biroulet, L.; Danese, S.; Jairath, V.; Magro, F. Network Meta-Analysis: Histologic and Histo-Endoscopic Improvement and Remission with Advanced Therapy in Ulcerative Colitis. Aliment. Pharmacol. Ther. 2024, 60, 1276–1292. [Google Scholar] [CrossRef]
- Shehab, M.; Alrashed, F.; Alsayegh, A.; Aldallal, U.; Ma, C.; Narula, N.; Jairath, V.; Singh, S.; Bessissow, T. Comparative Efficacy of Biologics and Small Molecule in Ulcerative Colitis: A Systematic Review and Network Meta-analysis. Clin. Gastroenterol. Hepatol. 2025, 23, 250–262. [Google Scholar] [CrossRef]
- St-Pierre, J.; Choi, D.; Fear, E.; Choi, N.K.; Mathew, A.J.; Cohen, R.D.; Dalal, S.R.; Pekow, J.; Cleveland, N.K.; Rubin, D.T. Mirikizumab in the Treatment of Ulcerative Colitis: Initial Real-World Data in a Population from a Large Tertiary Center. Dig. Dis. Sci. 2025, 70, 1864–1872. [Google Scholar] [CrossRef]
- Alsoud, D.; Sabino, J.; Franchimont, D.; Cremer, A.; Busschaert, J.; D’hEygere, F.; Bossuyt, P.; Vijverman, A.; Vermeire, S.; Ferrante, M. Real-world Effectiveness and Safety of Risankizumab in Patients with Moderate to Severe Multirefractory Crohn’s Disease: A Belgian Multicentric Cohort Study. Inflamm. Bowel Dis. 2024, 30, 2289–2296. [Google Scholar] [CrossRef]

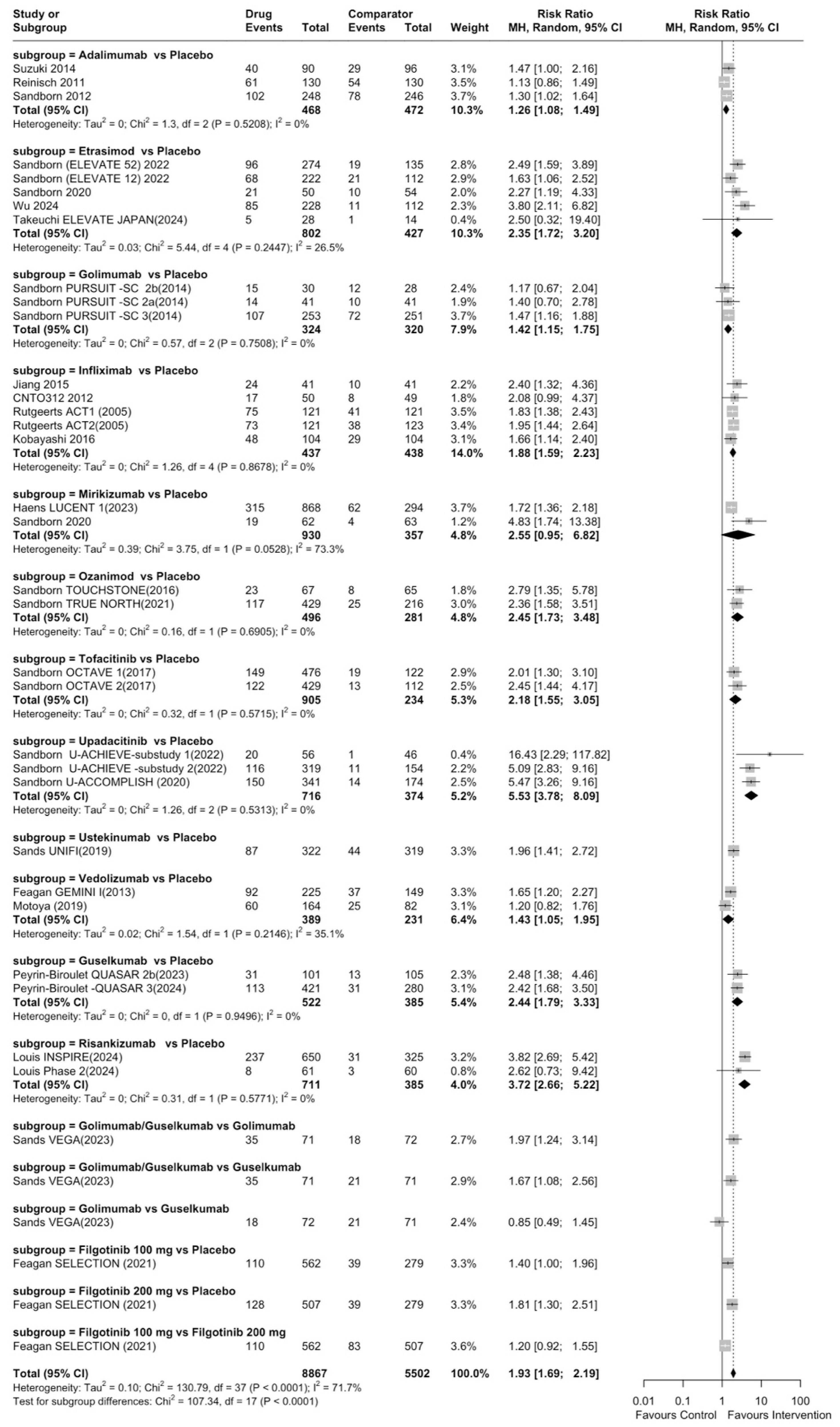
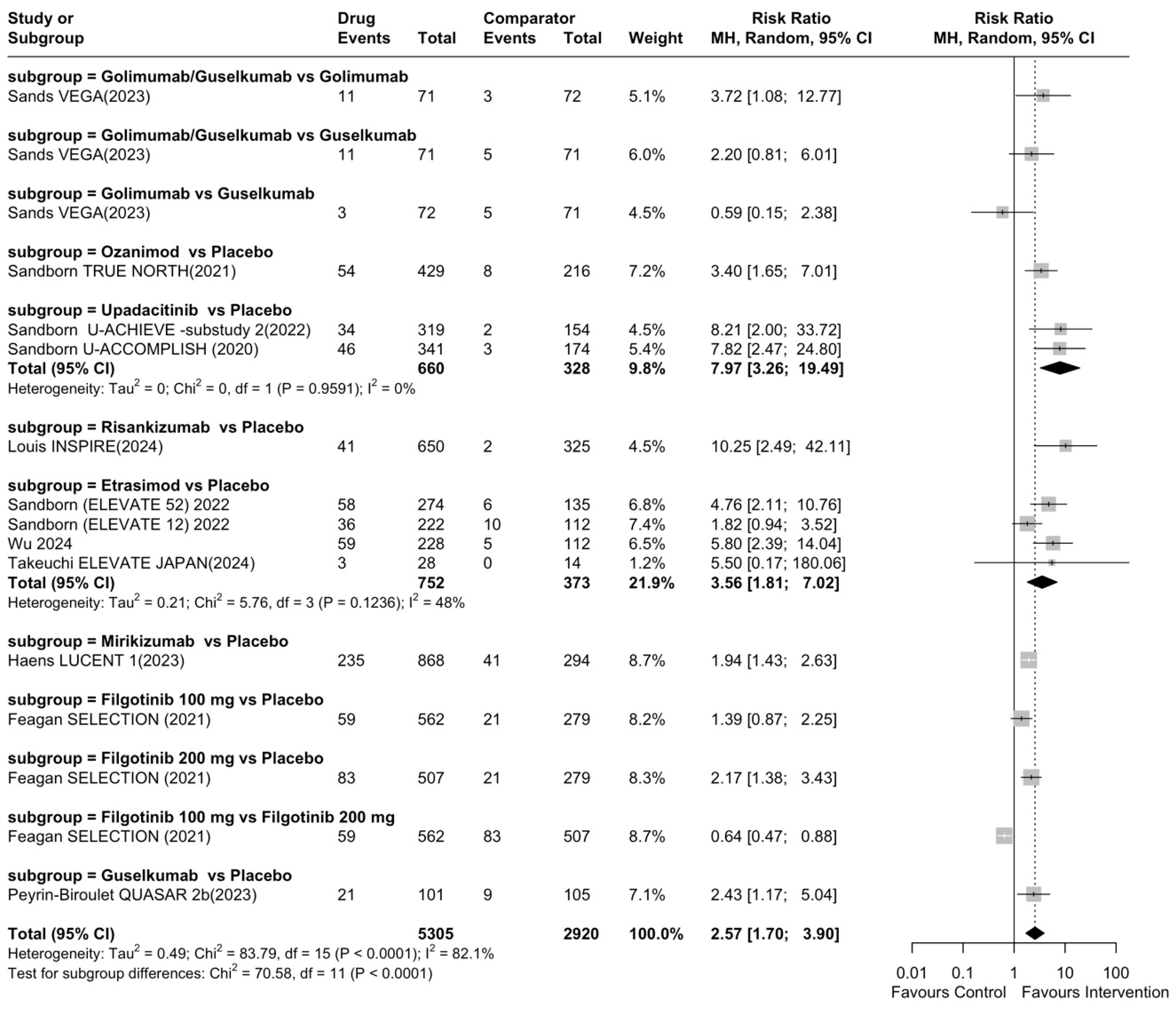
| Rank | Drug | Comparator | Total RR (95% CI) | Category |
|---|---|---|---|---|
| 1 | Upadacitinib | Placebo | 5.53 (95% CI: 3.78–8.09) | Highly efficacious |
| 2 | Risankizumab | Placebo | 3.72 (95% CI: 2.65–5.22) | Highly efficacious |
| 3 | Tofacitinib | Placebo | 2.45 (95% CI: 1.73–3.48) | Highly efficacious |
| 4 | Ozanimod | Placebo | 2.45 (95% CI: 1.73–3.48) | Highly efficacious |
| 5 | Guselkumab | Placebo | 2.44 (95% CI: 1.79–3.32) | Highly efficacious |
| 6 | Etrasimod | Placebo | 2.35 (95% CI: 1.72–3.20) | Highly efficacious |
| 7 | Ustekinumab | Placebo | 1.96 (95% CI: 1.41–2.72) | Moderately efficacious |
| 8 | Infliximab | Placebo | 1.88 (95% CI: 1.59–2.23) | Moderately efficacious |
| 9 | Filgotinib 200 mg | Placebo | 1.81 (95% CI: 1.30–2.51) | Moderately efficacious |
| 10 | Vedolizumab | Placebo | 1.43 (95% CI: 1.05–1.96) | Moderately efficacious |
| 11 | Golimumab | Placebo | 1.42 (95% CI: 1.15–1.75) | Moderately efficacious |
| 12 | Adalimumab | Placebo | 1.26 (95% CI: 1.08–1.49) | Moderately efficacious |
| 13 | Mirikizumab | Placebo | 2.55 (95% CI: 0.95–6.82) | Not efficacious (CI includes 1) |
| 14 | Filgotinib 100 mg | Placebo | 1.40 (95% CI: 1.00–1.94) | Not efficacious (CI includes 1) |
| Rank | Drug | Comparator | Total RR (95% CI) | Category |
|---|---|---|---|---|
| 1 | Risankizumab | Placebo | 10.25 (95% CI: 2.49–42.11) | Highly efficacious |
| 2 | Upadacitinib | Placebo | 7.97 (95% CI: 3.26–19.49) | Highly efficacious |
| 3 | Etrasimod | Placebo | 3.56 (95% CI: 1.81–7.02) | Highly efficacious |
| 4 | Ozanimod | Placebo | 3.40 (95% CI: 1.65–7.01) | Highly efficacious |
| 5 | Guselkumab | Placebo | 2.43 (95% CI: 1.17–5.04) | Highly efficacious |
| 6 | Filgotinib 200 mg | Placebo | 2.17 (95% CI: 1.38–3.43) | Highly efficacious |
| 7 | Mirikizumab | Placebo | 1.94 (95% CI: 1.43–2.63) | Moderately efficacious |
| 8 | Filgotinib 100 mg | Placebo | 1.39 (95% CI: 0.87–2.25) | Not efficacious |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mademlis, C.; Katsoula, A.; Koufakis, T.; Paschos, P.; Kefas, A.; Teperikidis, L.; Theodoridou, N.; Giouleme, O. Efficacy of Biologic Agents and Small Molecules for Endoscopic Improvement and Mucosal Healing in Patients with Moderate-to-Severe Ulcerative Colitis: Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 5789. https://doi.org/10.3390/jcm14165789
Mademlis C, Katsoula A, Koufakis T, Paschos P, Kefas A, Teperikidis L, Theodoridou N, Giouleme O. Efficacy of Biologic Agents and Small Molecules for Endoscopic Improvement and Mucosal Healing in Patients with Moderate-to-Severe Ulcerative Colitis: Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(16):5789. https://doi.org/10.3390/jcm14165789
Chicago/Turabian StyleMademlis, Christos, Anastasia Katsoula, Theocharis Koufakis, Paschalis Paschos, Aristeidis Kefas, Lefteris Teperikidis, Niki Theodoridou, and Olga Giouleme. 2025. "Efficacy of Biologic Agents and Small Molecules for Endoscopic Improvement and Mucosal Healing in Patients with Moderate-to-Severe Ulcerative Colitis: Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 16: 5789. https://doi.org/10.3390/jcm14165789
APA StyleMademlis, C., Katsoula, A., Koufakis, T., Paschos, P., Kefas, A., Teperikidis, L., Theodoridou, N., & Giouleme, O. (2025). Efficacy of Biologic Agents and Small Molecules for Endoscopic Improvement and Mucosal Healing in Patients with Moderate-to-Severe Ulcerative Colitis: Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(16), 5789. https://doi.org/10.3390/jcm14165789





