Contralateral Robotic-Assisted Anatomical Resection for Synchronous or Metachronous Lung Cancer: A Retrospective Case Series
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Surgical Characteristics
3.3. Postoperative Outcomes
3.4. Pathological and Oncological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| ASA | American Society of Anesthesiologists |
| BP | Blood Pressure |
| BMI | Body mass index |
| CCI | Charlson Comorbidity Index |
| CO2 | Carbon dioxide |
| CPB | Cardiopulmonary Bypass |
| CT | Computed tomography |
| DLCO | Diffusing capacity for carbon monoxide |
| ECMO | Extracorporeal membrane oxygenation |
| ESTS | European Society of Thoracic Surgery |
| ETCO2 | End-Tidal Carbon Dioxide |
| FEV1 | Forced expiratory volume in the 1st second |
| FiO2 | Fraction of Inspired Oxygen |
| FVC | Forced vital capacity |
| HPV | Hypoxic pulmonary vasoconstriction |
| IQR | Interquartile range |
| LLL | Left lower lobe |
| LOS | Length of hospital stay |
| LUL | Left upper lobe |
| ML | Middle lobe |
| MRI | Magnetic resonance imaging |
| NSCLC | Non-small cell lung cancer |
| OLV | One-lung ventilation |
| PEEP | Positive End-Expiratory Pressure |
| PROCESS | Preferred Reporting of CasE Series in Surgery |
| RATS | Robotic-assisted thoracic surgery |
| RLL | Right lower lobe |
| RUL | Right upper lobe |
| SaO2 | Arterial Oxygen Saturation |
| SPSS | Statistical Package for the Social Sciences |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [PubMed]
- Ghosh, S.; Mehta, A.C.; Abuquyyas, S.; Raju, S.; Farver, C. Primary lung neoplasms presenting as multiple synchronous lung nodules. Eur. Respir. Rev. 2020, 29, 190142. [Google Scholar] [CrossRef]
- Varlotto, J.; Voland, R.; De Camp, M.; Rava, P.; Fitzgerald, T.; Maxfield, M.; Lou, F.; Oliveira, P.; Sood, R.; Baima, J.; et al. The rates of second lung cancers and the survival of surgically resected second primary lung cancers in patients undergoing resection of an initial primary lung cancer. Lung Cancer 2020, 147, 115–122. [Google Scholar] [CrossRef]
- Campos, J.H.; Feider, A. Hypoxia during one-lung ventilation: A review and update. J. Cardiothorac. Vasc. Anesth. 2018, 32, 2330–2338. [Google Scholar] [CrossRef]
- Lohser, J.; Slinger, P. Lung injury after one-lung ventilation: A review of the pathophysiologic mechanisms affecting the ventilated and the collapsed lung. Anesth. Analg. 2015, 121, 302–318. [Google Scholar] [CrossRef]
- Zhou, H.; Kang, X.; Dai, L.; Yan, W.; Yang, Y.; Lin, Y.; Chen, K. Efficacy of repeated surgery is superior to that of non-surgery for recurrent/second primary lung cancer after initial operation for primary lung cancer. Thorac. Cancer 2018, 9, 1062–1068. [Google Scholar] [CrossRef]
- Karzai, W.; Schwarzkopf, K. Hypoxemia during one-lung ventilation: Prediction, prevention, and treatment. Anesthesiology 2009, 110, 1402–1411. [Google Scholar] [CrossRef]
- Gu, Y.; Duan, R.; Lv, X.; Song, J. Airway Management of the Right Anterior Segmentectomy through Uniportal video-assisted thoracoscopic surgery (VATS) after left pneumonectomy by an adapted double-lumen endobronchial tube (DLT): A case report. BMC Anesthesiol. 2019, 19, 82. [Google Scholar] [CrossRef]
- Heward, E.; Hayes, T.; Evison, M.; Booton, R.; Barnard, J.; Shah, R. Extracorporeal Membrane Oxygenation Assisted Segmentectomy for Metachronous Lung Cancer After Pneumonectomy. Ann. Thorac. Surg. 2016, 102, e187–e189. [Google Scholar] [CrossRef]
- Lim, E.; Harris, R.A.; McKeon, H.E.; Batchelor, T.J.; Dunning, J.; Shackcloth, M.; Anikin, V.; Naidu, B.; Belcher, E.; Loubani, M.; et al. Impact of video-assisted thoracoscopic lobectomy versus open lobectomy for lung cancer on recovery assessed using self-reported physical function: VIOLET RCT. Health Technol. Assess. 2022, 26, 1–162. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.J.; Ghanim, A.F.; Dylewski, M.; Veronesi, G.; Spaggiari, L.; Park, B.J. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J. Thorac. Cardiovasc. Surg. 2018, 155, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Lumb, A.B.; Slinger, P. Hypoxic pulmonary vasoconstriction: Physiology and anesthetic implications. Anesthesiology 2015, 122, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Byhahn, C.; Mierdl, S.; Meininger, D.; Wimmer-Greinecker, G.; Matheis, G.; Westphal, K. Hemodynamics and gas exchange during carbon dioxide insufflation for totally endoscopic coronary artery bypass grafting. Ann. Thorac. Surg. 2001, 71, 1496–1501. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, X.; Yan, H.; Chen, L.; Mao, Q. Cardiorespiratory impact of intrathoracic pressure overshoot during artificial carbon dioxide pneumothorax: A randomized controlled study. BMC Anesthesiol. 2022, 22, 76. [Google Scholar] [CrossRef]
- Mathew, G.; Sohrabi, C.; Franchi, T.; Nicola, M.; Kerwan, A.; Agha, R.; PROCESS Group. Preferred Reporting Of Case Series in Surgery (PROCESS) 2023 guidelines. Int. J. Surg. 2023, 109, 3760–3769. [Google Scholar] [CrossRef]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; Waller, D.; Passlick, B.; Zielinski, M.; Lerut, T.; Weder, W. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. AJCC Cancer Staging Manual, 8th ed.; Springer: Cham, Switzerland, 2017; pp. 431–455. [Google Scholar]
- Martini, N.; Melamed, M.R. Multiple primary lung cancers. J. Thorac. Cardiovasc. Surg. 1975, 70, 606–612. [Google Scholar] [CrossRef]
- Kozower, B.D.; Larner, J.M.; Detterbeck, F.C.; Jones, D.R. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. 5), e369S–e399S. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, C.; Xie, G.; Wu, F.; Hu, C. Multiple Primary Lung Cancers: A New Challenge in the Era of Precision Medicine. Cancer Manag. Res. 2020, 12, 10361–10374. [Google Scholar] [CrossRef]
- Fourdrain, A.; Bagan, P.; Georges, O.; Lafitte, S.; De Dominicis, F.; Meynier, J.; Berna, P. Outcomes after Contralateral Anatomic Surgical Resection in Multiple Lung Cancer. Thorac. Cardiovasc. Surg. 2021, 69, 373–379. [Google Scholar] [CrossRef]
- Choe, J.K.; Zhu, A.; Byun, A.J.; Zheng, J.; Tan, K.S.; Dycoco, J.; Bains, M.S.; Bott, M.J.; Downey, R.J.; Huang, J.; et al. Brief Report: Contralateral Lobectomy for Second Primary NSCLC: Perioperative and Long-Term Outcomes. JTO Clin. Res. Rep. 2022, 3, 100362. [Google Scholar] [CrossRef]
- Pastene, B.; Labarriere, A.; Lopez, A.; Charvet, A.; Culver, A.; Fiocchi, D.; Cluzel, A.; Brioude, G.; Einav, S.; Tankel, J.; et al. Ultra-early initiation of postoperative rehabilitation in the post-anaesthesia care unit after major thoracic surgery: Case-control study. BJS Open 2022, 6, zrac063. [Google Scholar] [CrossRef]
| Pts = 20 | |
|---|---|
| Sex | |
| Male | 8 (40.0%) |
| Female | 12 (60.0%) |
| Age | 71.0 (61.5–74.75) |
| BMI | 24.6 (61.75–80.0) |
| Smoking history | |
| Never | 4 (20.0%) |
| Previous smoker | 12 (60.0%) |
| Current smoker | 4 (20.0%) |
| Previous chest surgery side | |
| Right | 11 (55.0%) |
| Left | 9 (45.0%) |
| Comorbidities (pts) | 19 (95.0%) |
| Comorbidities (number) | 4.0 (2.0–5.0) |
| CCI | 6.0 (5.0–7.0) |
| FEV1 (%) | 75.94 (66.62–89.24) |
| FVC (%) | 81.82 (72.47–89.86) |
| DLCO (%) | 57.35 (52.69–63.40) |
| Timing | |
| Synchronous | 6 (30.0%) |
| Metachronous | 14 (70.0%) |
| Tumor interval detection for synchronous nodules (months) | 1.5 (1.0–2.0) |
| Tumor interval detection for metachronous nodules (months) | 24.00 (13.5–96.50) |
| Location of the second tumor | |
| RUL | 4 (20.0%) |
| ML | 1 (5.0%) |
| RLL | 4 (20.0%) |
| LUL | 4 (20.0%) |
| LLL | 7 (35.0%) |
| Size of the second nodule (mm) | 21.0 (13.5–26.75) |
| Clinical stage of the second tumor | |
| IA2 | 8 (40.0%) |
| IA3 | 3 (15.0%) |
| IB | 6 (30.0%) |
| IIB | 3 (15.0%) |
| Systemic therapy for previous cancer | 4 (20.0%) |
| Histology of the first tumor | |
| Adenocarcinoma | 15 (75.0%) |
| Squamous cell carcinoma | 3 (15.0%) |
| Large cell carcinoma | 1 (5.0%) |
| Typical carcinoid | 1 (5.0%) |
| Type of adenocarcinoma (predominant subtype) | |
| Acinar | 1 (6.3%) |
| Lepidic | 4 (26.7%) |
| Solid | 4 (26.7%) |
| Papillary | 5 (33.3%) |
| Cribriform | 1 (6.3%) |
| ASA score | |
| 2 | 7 (35.0%) |
| 3 | 13 (65.0%) |
| Time from previous surgery (months) | 14.0 (2.0–46.0) |
| Pts = 20 | |
|---|---|
| Segmentectomy | 13 (65.0%) |
| S1 right | 1 (5.0%) |
| S6 right | 2 (10.0%) |
| S8 right | 1 (5.0%) |
| S10 right | 1 (5.0%) |
| S1–3 left | 1 (5.0%) |
| S1/2 left | 1 (5.0%) |
| Lingula (S4–5) | 1 (5.0%) |
| S6 left | 2 (10.0%) |
| S8 left | 1 (5.0%) |
| Basalectomy left | 1 (5.0%) |
| Lobectomy | 7 (35.0%) |
| RUL | 3 (15.0%) |
| ML | 1 (5.0%) |
| LUL | 1 (5.0%) |
| LLL | 2 (10.0%) |
| Operation time (min) | 148.0 (108.0–194.75) |
| Estimated blood loss (mL) | 50.0 (0.0–100.0) |
| Conversion to open (yes) | 0 (0.0%) |
| Necessity for ECMO (yes) | 0 (0.0%) |
| Anesthesiological values before incision | |
| FiO2 | 1.00 (1.00–1.00) |
| SaO2 | 100.0 (100.0–100.0) |
| ETCO2 | 34.0 (32.0–34.75) |
| Plateau pressure | 17.0 (15.0–19.75) |
| PEEP | 5.0 (5.0–7.0) |
| Systolic BP | 127.50 (120.0–133.75) |
| Mean BP | 95.0 (87.0–100.0) |
| Diastolic BP | 60.0 (60.0–70.0) |
| Necessity of catecholamines | 5 (25.0%) |
| Anesthesiological values during surgery (lowest value) | |
| FiO2 | 0.8 (0.60–0.95) |
| SaO2 | 92.0 (86.50–96.50) |
| ETCO2 | 33.0 (32.0–34.75) |
| Plateau pressure | 28.0 (24.25–34.0) |
| PEEP | 8.0 (7.0–10.0) |
| Systolic BP | 105.0 (96.25–110.0) |
| Mean BP | 78.0 (73.50–82.25) |
| Diastolic BP | 50.0 (41.25–60.0) |
| Necessity of catecholamines | 9 (45.0%) |
| Postoperative necessity of catecholamines (yes) | 1 (5.0%) |
| Pts = 20 | |
|---|---|
| Unplanned reoperation (yes) | 0 (0.0%) |
| Chest drainage duration (days) | 4.0 (3.25–6.0) |
| LOS (days) | 8.0 (7.0–11.75) |
| In-hospital mortality | 0 (0.0%) |
| 30-day mortality | 0 (0.0%) |
| 90-day mortality | 0 (0.0%) |
| Complications (pts) | 5 (25.0%) |
| Complications (type) | |
| Respiratory insufficiency | 1 (5.0%) |
| Tachyarrhythmia | 1 (5.0%) |
| Hypertension | 1 (5.0%) |
| Pleural effusion | 1 (5.0%) |
| Chylothorax | 1 (5.0%) |
| Clavien–Dindo classification | |
| Grade 1 | 0 (0.0%) |
| Grade 2 | 4 (20.0%) |
| Grade 3A | 0 (0.0%) |
| Grade 3B | 0 (0.0%) |
| Grade IVA | 1 (5.0%) |
| Grade IVB | 0 (0.0%) |
| Grade V | 0 (0.0%) |
| Histology (current) | |
| Adenocarcinoma | 16 (80.0%) |
| Squamous cell carcinoma | 2 (10.0%) |
| Large cell carcinoma | 1 (5.0%) |
| Adenoid cystic carcinoma | 1 (5.0%) |
| Type of adenocarcinoma (predominant subtype) | |
| Acinar | 12 (75.0%) |
| Lepidic | 2 (12.5%) |
| Solid | 1 (6.3%) |
| Papillary | 1 (6.3%) |
| Tumor size (mm) | 18.50 (15.0–26.50) |
| Tumor status | |
| pT1a | 0 (0.0%) |
| pT1b | 8 (40.0%) |
| pT1c | 4 (20.0%) |
| pT2a | 6 (30.0%) |
| pT2b | 0 (0.0%) |
| pT3 | 2 (10.0%) |
| pT4 | 0 (0.0%) |
| Lymph node status | |
| N0 | 18 (90.0%) |
| N1 | 2 (10.0%) |
| N2 | 0 (0.0%) |
| R status | |
| R0 | 20 (100%) |
| TNM staging (8th edition) | |
| IA | 11 (55.0%) |
| IB | 5 (25.0%) |
| IIA | 0 (0.0%) |
| IIB | 4 (20.0%) |
| IIIA | 0 (0.0%) |
| IIIB | 0 (0.0%) |
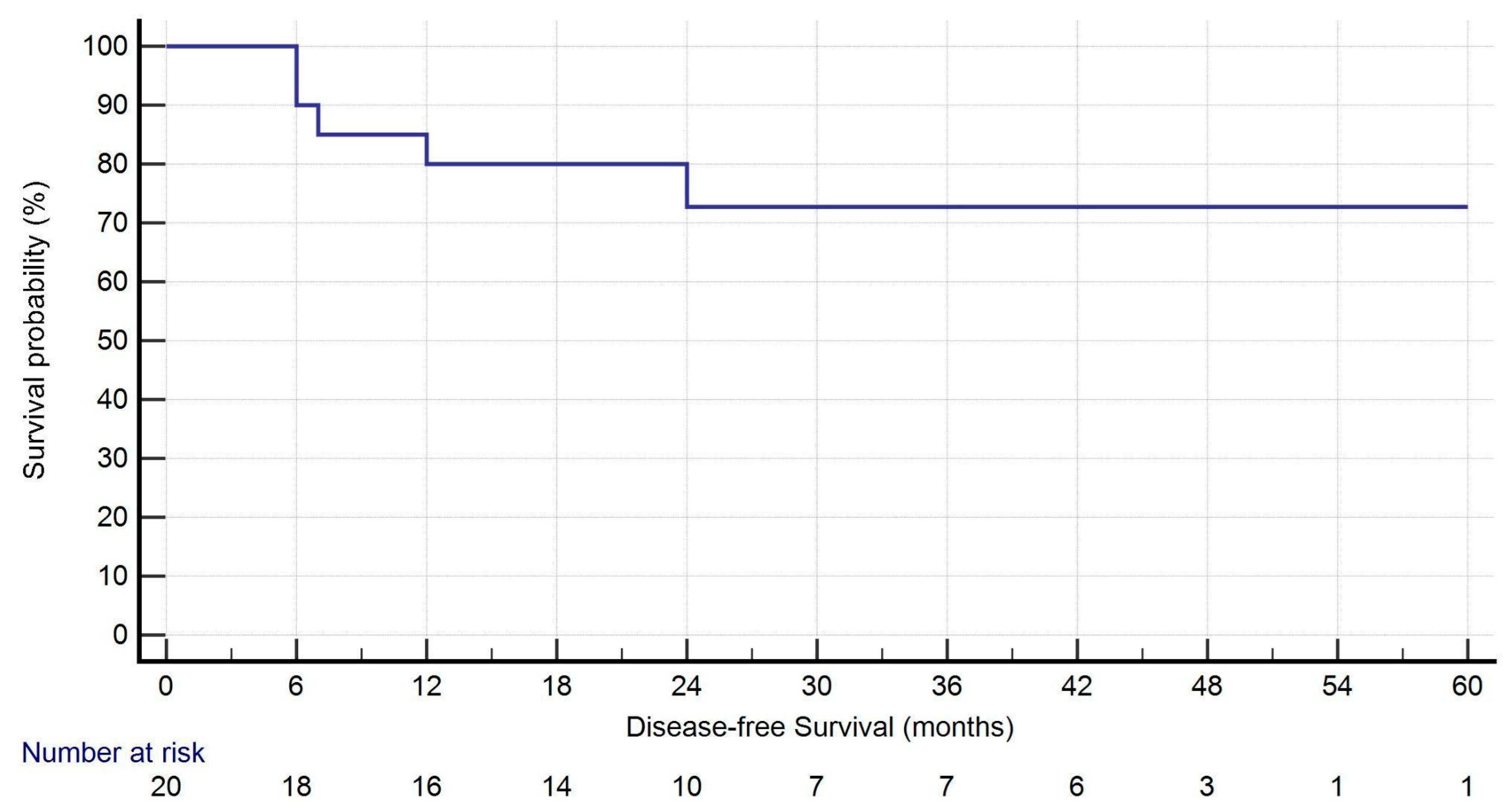
| Recurrence (pts) | 5 (25.0%) |
| Type of recurrence | |
| Local (lymph nodes) | 2 (10.0%) |
| Local + systemic | 3 (15.0%) |
| Status (alive) | 19 (95.0%) |
| Status (alive) | 19 (95.0%) |
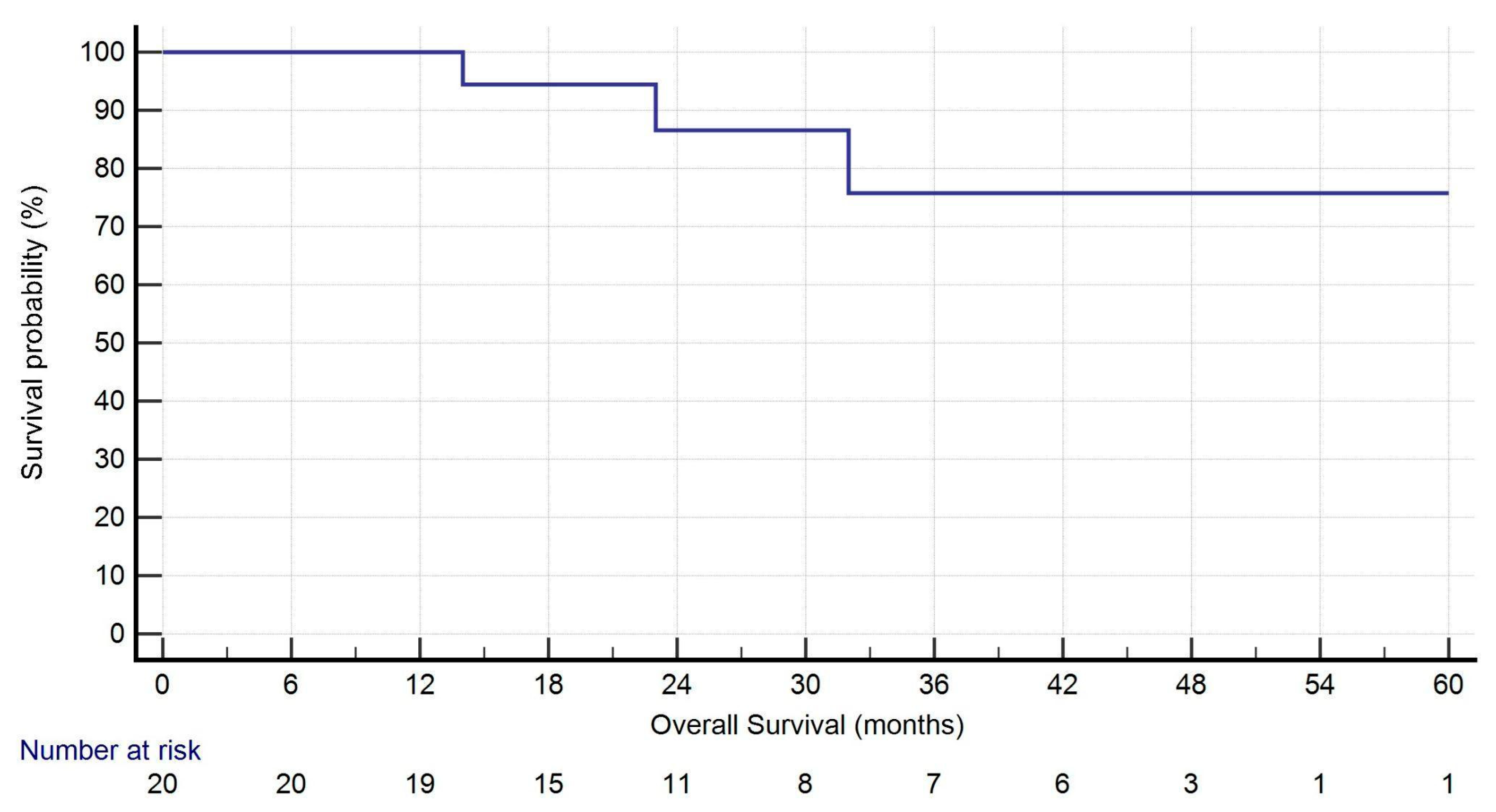
| 1-year Survival | 19 (95.0%) |
| Follow-up (months) | 27.5 (16.75–44.50) |
| OS (months) | 27.5 (16.75–44.50) |
| DFS (months) | 25.5 (14.50–44.50) |
| Patient ID | Previous Cancer | Current Cancer |
|---|---|---|
| N.5 | Solid Adenocarcinoma | Acinar Adenocarcinoma |
| N.10 | Lepidic Adenocarcinoma | Acinar Adenocarcinoma |
| N.11 | Papillary Adenocarcinoma | Cribriform Adenocarcinoma |
| N.12 | Typical Carcinoid | Large Cell Carcinoma |
| N.15 | Lepidic Adenocarcinoma | Acinar Adenocarcinoma |
| N.23 | Squamous Cell Carcinoma | Squamous Cell Carcinoma |
| Univariate Analysis | |
|---|---|
| Variable | p-Value |
| Synchronous/Metachronous | 0.597 |
| Pathological Stage | 0.884 |
| Histology First Tumor | 0.277 |
| Histology Current Tumor | 0.303 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campisi, A.; Khan, N.; Pinna, F.; Aliev, D.; Griffo, R.; Baum, P.; Schmidt, W.; Winter, H.; Eichhorn, M. Contralateral Robotic-Assisted Anatomical Resection for Synchronous or Metachronous Lung Cancer: A Retrospective Case Series. J. Clin. Med. 2025, 14, 5786. https://doi.org/10.3390/jcm14165786
Campisi A, Khan N, Pinna F, Aliev D, Griffo R, Baum P, Schmidt W, Winter H, Eichhorn M. Contralateral Robotic-Assisted Anatomical Resection for Synchronous or Metachronous Lung Cancer: A Retrospective Case Series. Journal of Clinical Medicine. 2025; 14(16):5786. https://doi.org/10.3390/jcm14165786
Chicago/Turabian StyleCampisi, Alessio, Nabil Khan, Federica Pinna, Dennis Aliev, Raffaella Griffo, Philip Baum, Werner Schmidt, Hauke Winter, and Martin Eichhorn. 2025. "Contralateral Robotic-Assisted Anatomical Resection for Synchronous or Metachronous Lung Cancer: A Retrospective Case Series" Journal of Clinical Medicine 14, no. 16: 5786. https://doi.org/10.3390/jcm14165786
APA StyleCampisi, A., Khan, N., Pinna, F., Aliev, D., Griffo, R., Baum, P., Schmidt, W., Winter, H., & Eichhorn, M. (2025). Contralateral Robotic-Assisted Anatomical Resection for Synchronous or Metachronous Lung Cancer: A Retrospective Case Series. Journal of Clinical Medicine, 14(16), 5786. https://doi.org/10.3390/jcm14165786








