Retinal Thinning in Attention Deficit Hyperactivity Disorder (ADHD): Structural Changes Detected by Spectral-Domain OCT
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
2.3. Ophthalmic Examination
2.4. OCT Imaging Protocol
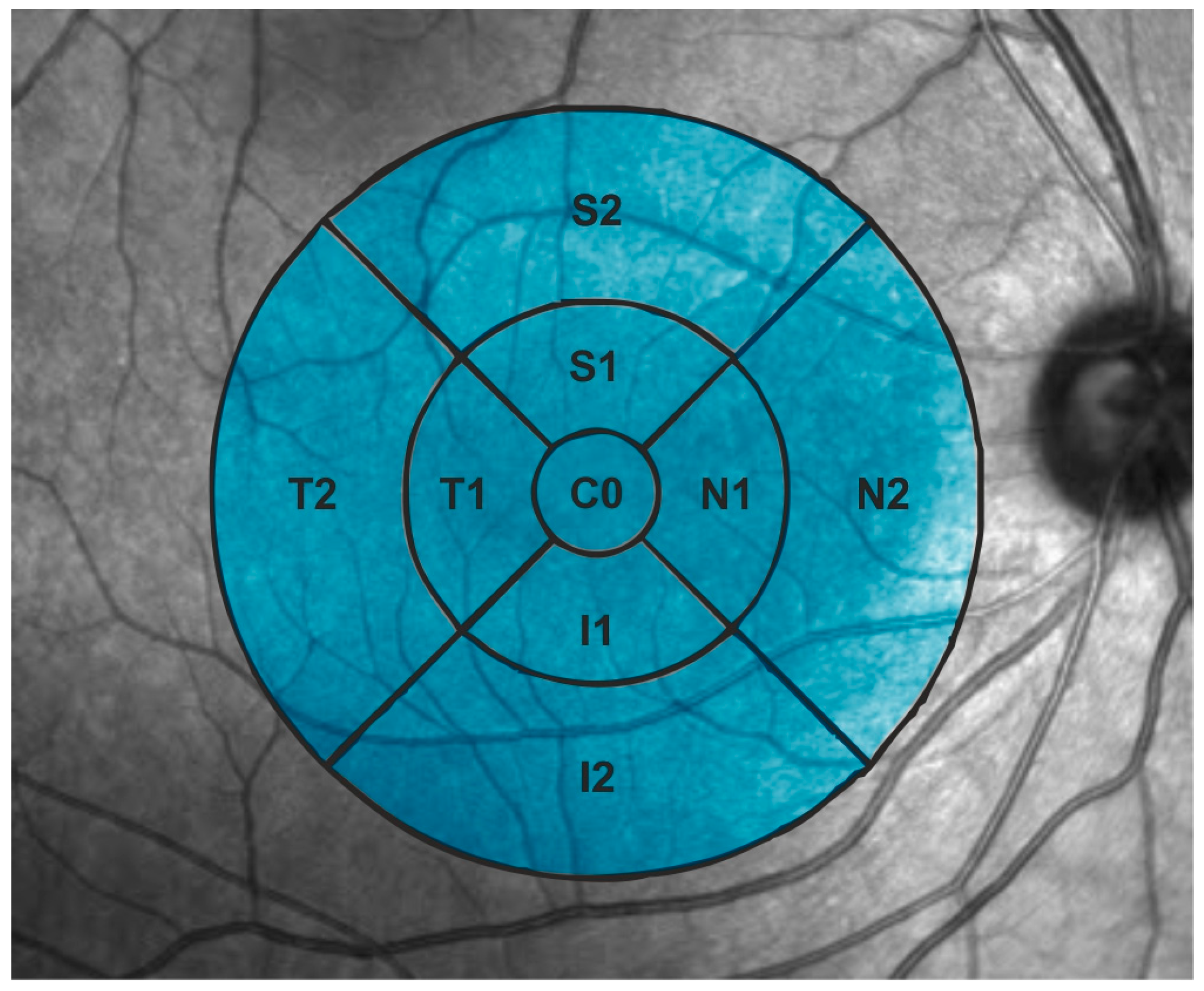
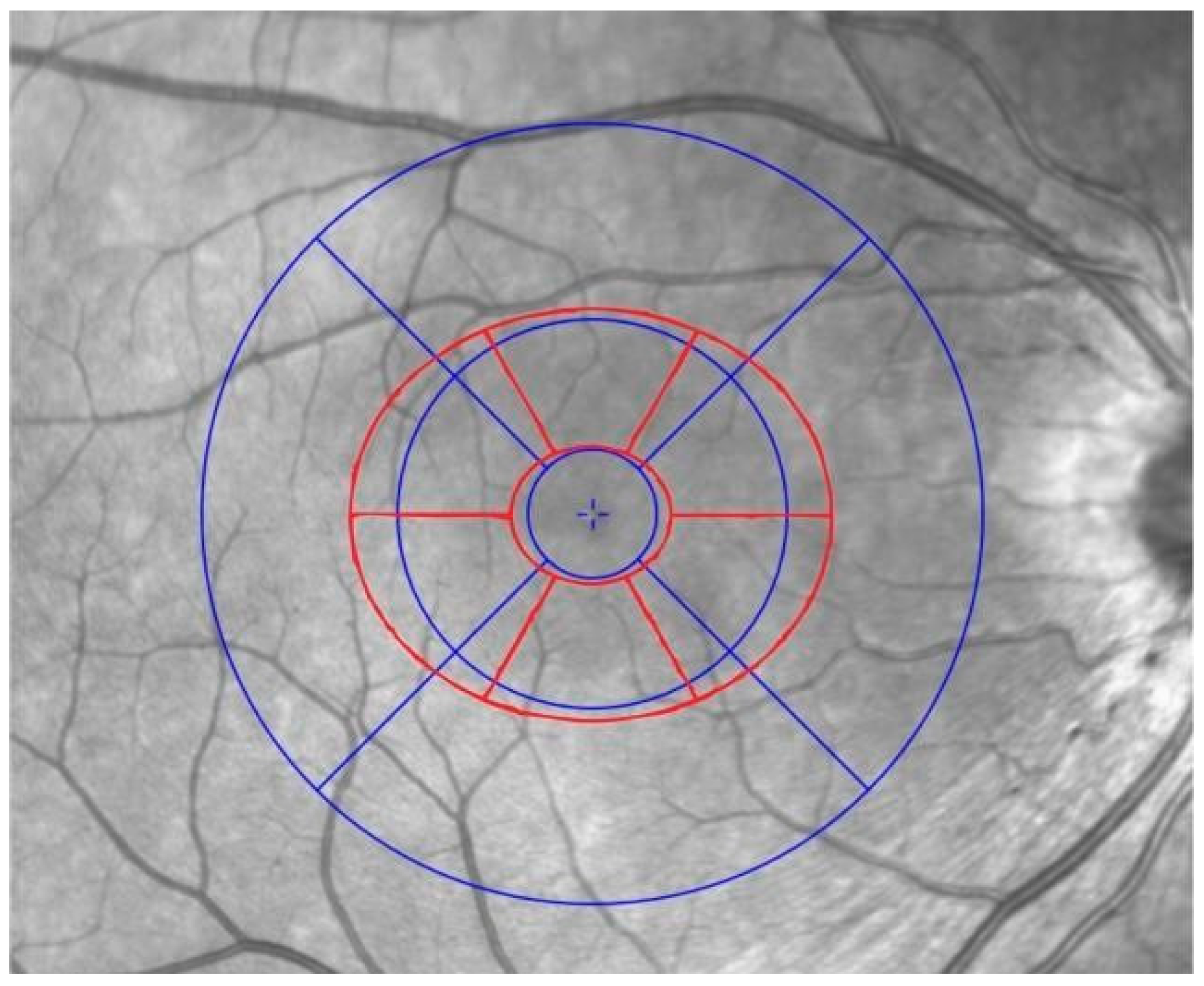
2.5. Retinal Structural Analysis
- -
- Macular RNFL (mRNFL).
- -
- Ganglion cell layer plus inner plexiform layer (GCIPL).
- -
- Outer retinal layers (between inner nuclear layer and Bruch’s membrane).
2.6. Statistical Analysis
3. Results
3.1. Demographic and Ophthalmic Data
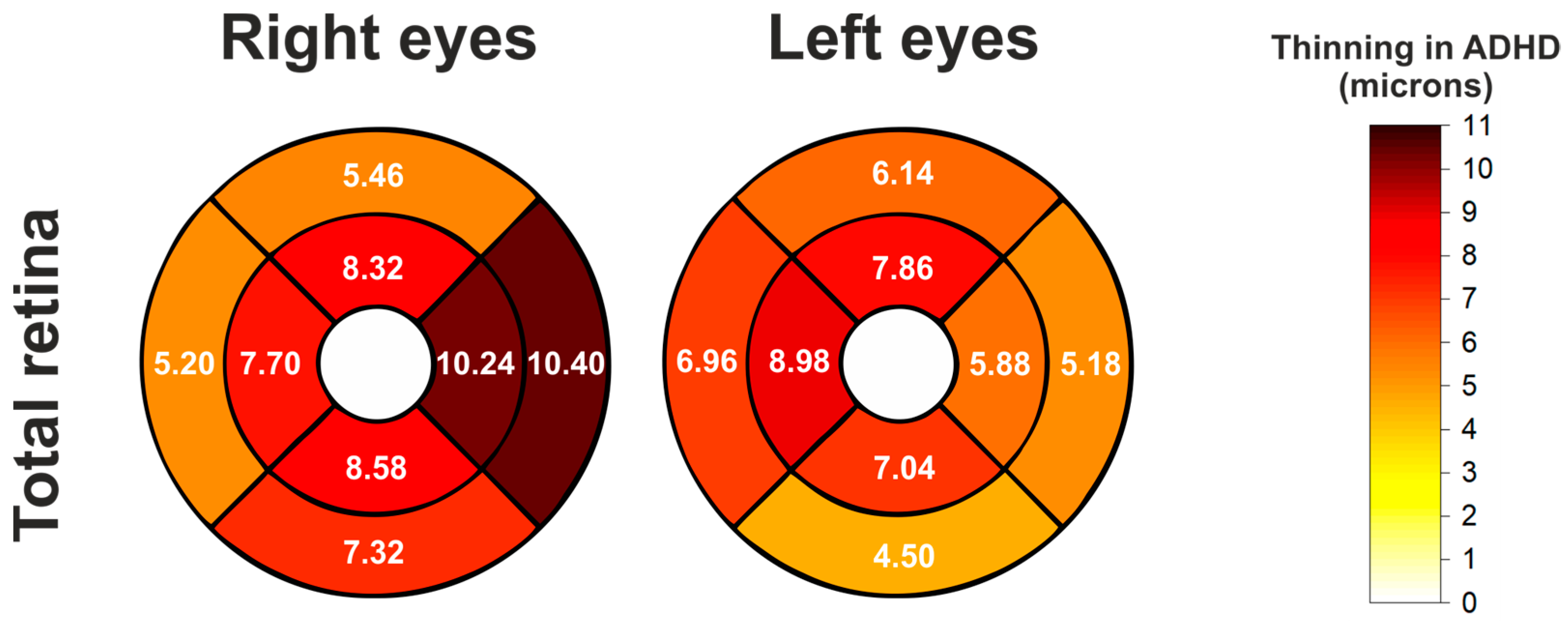
3.2. Comparisons of Total Retina Thickness in ETDRS Pattern
3.3. AUC for Total Retinal Thickness in the ETDRS Pattern
3.4. Comparisons of Outer Retinal Layer Thickness in Elliptical Pattern
3.5. AUC for Outer Retina in the Elliptical Pattern
3.6. Comparisons of mRNFL, GCIPL, and mRNFL + GCIPL Thickness (Inner Retina) in Elliptical Pattern
3.7. Comparisons of Inner Retina and Outer Retina in the Elliptical Pattern
3.8. AUC for Inner Retina and Outer Retina in Elliptical Pattern
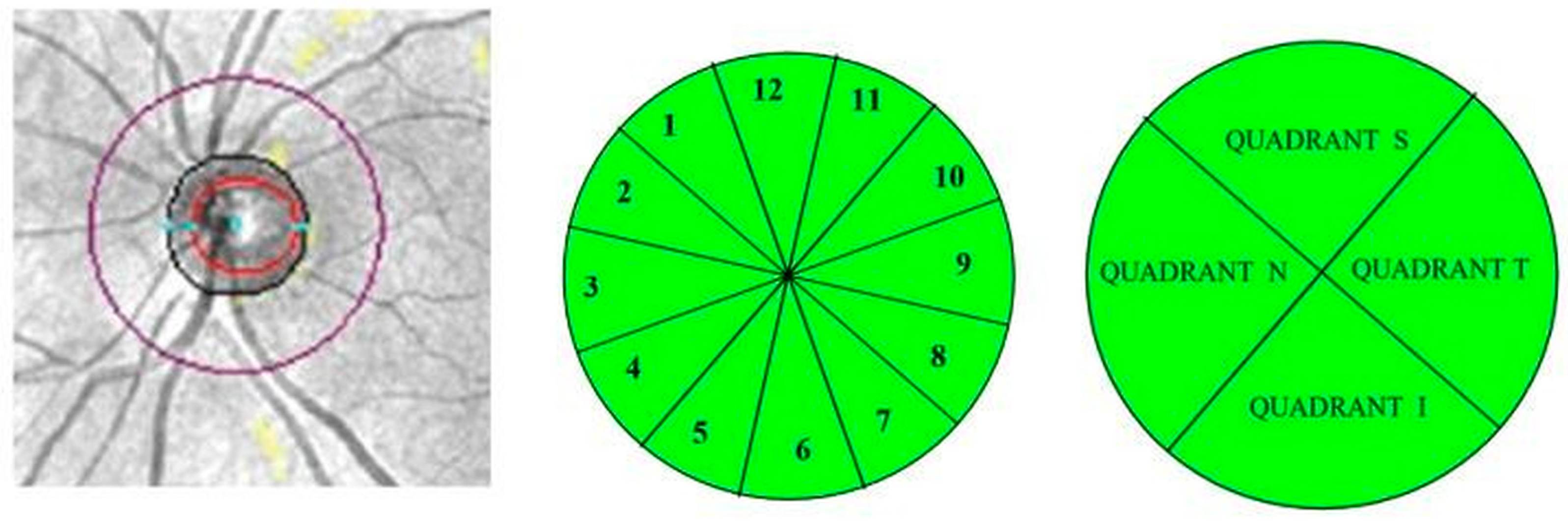
3.9. Comparisons of Peripapillary Retinal Nerve Fiber Layer (pRNFL) Thickness
3.10. Other Structural Parameters of the Optic Nerve
4. Discussion
4.1. Comparison with the Previous Literature
4.2. Clinical Implications
4.3. Pathophysiological Insights
4.4. Limitations
4.5. Strengths
4.6. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faraone, S.V.; Bellgrove, M.A.; Brikell, I.; Hartman, C.A.; Cortese, S.; Newcorn, J.H.; Hollis, C.; Polanczyk, G.V.; Philipson, A.; Rubia, K.; et al. Attention-Deficit/Hyperactivity Disorder. Nat. Rev. Dis. Primers 2024, 10, 29. [Google Scholar]
- Polanczyk, G.V.; Willcutt, E.G.; Salum, G.A.; Kieling, C.; Rohde, L.A. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int. J. Epidemiol. 2014, 43, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Petty, C.R.; Clarke, A. Predictors of persistence in preschool children with attention-deficit/hyperactivity disorder. Pediatrics 2011, 128, 2099–2109. [Google Scholar]
- Drechsler, R.; Brem, S.; Brandeis, D.; Grünblatt, E.; Berger, G.; Walitza, S. ADHD: Current concepts and treatments in children and adolescents. Neuropediatrics 2020, 51, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, I.; Almorín, I.; Fernández, J.I.; De Pablo, L.; Kudsieh, B.; Fernández, J.A. Assessment of changes in the macula and optic nerve head using optical coherence tomography in patients with attention deficit hyperactivity disorder. Arch. Soc. Esp. Oftalmol. (Engl. Ed.) 2020, 95, 271–278. [Google Scholar]
- Erdogan, E.; Delibas, D.H.; Karti, Ö. Assessment of optical coherence tomography findings in adults with attention deficit hyperactivity disorder: A case-control study. Psychiatry Clin. Psychopharmacol. 2021, 31, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Ulucan, P.B.; Ceylan, O.M.; Donmez, Y.E.; Ozel, O. Ocular findings in patients with attention deficit and hyperactivity. Int. Ophthalmol. 2020, 40, 3105–3113. [Google Scholar] [CrossRef] [PubMed]
- Sujin, B.; Jee, K.; Jung, H.; Doug, H. Pilot study: An ocular biomarker for diagnosis of attention deficit hyperactivity disorder. Psychiatry Investig. 2019, 16, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Tünel, M.; Nedime, K. Retinal scan with optical coherence tomography in adult attention deficit hyperactivity disorder. Turk. Psikiyatri Derg. 2021, 32, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Tonkaz, G.Y.; Gonca, Ö.; Ali, C.; Bahadir, T.; Bahadir, U.; Ahmet, Ö. Evaluation of retinal nerve fiber layer, ganglion cell thickness, and macular thickness in children with comorbid specific learning disorder and attention-deficit hyperactivity disorder. J. Pediatr. Ophthalmol. Strabismus 2024, 61, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Bellato, A.; Perna, J.; Ganapathy, P.S. Association between ADHD and vision problems: A systematic review and meta-analysis. Mol. Psychiatry 2023, 28, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, D.; Gündoğmuş, İ.; Dalkıran, M.; Küçükevcilioğlu, M.; Uzun, Ö. Retinal nerve fiber layer thickness and its relationship with executive functions in adult attention deficit hyperactivity disorder patients. Psychiatry Investig. 2021, 18, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5); American Psychiatric Association: Washington, DC, USA, 2013; pp. 1–991. [Google Scholar]
- Kotowski, J.; Folio, L.S.; Wollstein, G.; Ishikawa, H.; Ling, Y.; Bilonick, R.A.; Kagemann, L.; Schuman, J.S. Glaucoma discrimination of segmented Cirrus spectral domain optical coherence tomography (SD-OCT) macular scans. Br. J. Ophthalmol. 2012, 96, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach in multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O.L. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.; Zhang-James, Y.; Perl, A.; Faraone, S.V. Oxidative Stress and ADHD: A Meta-Analysis. J. Atten. Disord. 2015, 19, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Paliwal, R.O.; Mishra, R.Y. Reproducibility of Retinal Nerve Fiber Layer and Macular Thickness Measurements Using Spectral Domain Optical Coherence Tomography. Galician Med. J. 2021, 28, E202147. Available online: https://ifnmujournal.com/gmj/article/view/E202147 (accessed on 1 August 2025). [CrossRef]
| Right Eyes | Control Group | ADHD Group | Mean Dif. | p (T-Test) |
|---|---|---|---|---|
| BCVA | 0.98 ± 0.06 | 0.95 ± 0.13 | 0.04 | 0.533 |
| SE pre cyclo | −1.47 ± 2.52 | −1.47 ± 2.78 | 0.01 | 0.996 |
| SE post cyclo | −1.20 ± 2.56 | −0.81 ± 2.51 | 0.38 | 0.445 |
| Axial length | 24.24 ± 1.05 | 24.12 ± 1.67 | 0.71 | 0.104 |
| K1 | 42.08 ± 4.66 | 42.44 ± 1.57 | −0.36 | 0.602 |
| K2 | 43.68 ± 1.31 | 43.52 ± 1.71 | 0.59 | 0.163 |
| Left eyes | Control group | ADHD group | Mean dif. | p (T-Test) |
| BCVA | 0.92 ± 0.08 | 0.96 ± 0.10 | 0.01 | 0.533 |
| SE pre cyclo | −1.73 ± 2.46 | −1.42 ± 2.97 | 0.30 | 0.579 |
| SE post cyclo | −1.03 ± 2.47 | −0.96 ± 3.05 | 4.07 | 0.251 |
| Axial length | 24.24 ± 1.02 | 24.12 ± 1.73 | 0.11 | 0.683 |
| K1 | 42.65 ± 1.28 | 42.27 ± 1.75 | 0.37 | 0.227 |
| K2 | 43.78 ± 1.40 | 43.54 ± 1.81 | 0.23 | 0.468 |
| Total Retinal Thickness (ETDRS Pattern) | ||||||
|---|---|---|---|---|---|---|
| Eye | Location | Control Mean ± SD | ADHD Mean ± SD | Mean Diff. (Control-ADHD) | p-Value ANCOVA | p-Value ANCOVA + BH |
| Right | C0 | 265.96 ± 22.55 | 259.38 ± 22.04 | 6.58 | 0.1380 | 0.1380 |
| N1 | 332.70 ± 14.60 | 322.46 ± 16.00 | 10.24 | 0.0006 | 0.0025 | |
| S1 | 329.34 ± 13.67 | 321.02 ± 14.16 | 8.32 | 0.0010 | 0.0028 | |
| T1 | 316.38 ± 14.01 | 308.68 ± 14.89 | 7.7 | 0.0034 | 0.0061 | |
| I1 | 326.74 ± 13.43 | 318.16 ± 15.86 | 8.58 | 0.0012 | 0.0028 | |
| N2 | 302.46 ± 12.22 | 292.06 ± 20.77 | 10.4 | 0.0040 | 0.0061 | |
| S2 | 282.16 ± 12.91 | 276.70 ± 13.03 | 5.46 | 0.0188 | 0.0212 | |
| T2 | 264.38 ± 12.66 | 259.18 ± 14.22 | 5.2 | 0.0117 | 0.0150 | |
| I2 | 271.24 ± 8.92 | 263.92 ± 14.13 | 7.32 | 0.0004 | 0.0025 | |
| Left | C0 | 265.18 ± 22.63 | 260.40 ± 22.13 | 4.78 | 0.2322 | 0.2322 |
| T1 | 315.14 ± 13.63 | 309.26 ± 15.05 | 5.88 | 0.0168 | 0.0251 | |
| S1 | 330.30 ± 13.50 | 322.44 ± 14.55 | 7.86 | 0.0020 | 0.0101 | |
| N1 | 332.96 ± 14.95 | 323.98 ± 16.42 | 8.98 | 0.0022 | 0.0101 | |
| I1 | 325.18 ± 12.75 | 318.14 ± 16.43 | 7.04 | 0.0046 | 0.0104 | |
| T2 | 263.70 ± 11.26 | 258.52 ± 14.96 | 5.18 | 0.0395 | 0.0444 | |
| S2 | 282.62 ± 10.40 | 276.48 ± 13.80 | 6.14 | 0.0042 | 0.0104 | |
| N2 | 302.44 ± 11.75 | 295.48 ± 16.41 | 6.96 | 0.0085 | 0.0152 | |
| I2 | 269.70 ± 9.84 | 265.20 ± 14.72 | 4.5 | 0.0212 | 0.0273 | |
| Total Retina (ETDRS Pattern) | ||
|---|---|---|
| Sector | AUC Right Eyes | AUC Left Eyes |
| C0 | 0.58 | 0.58 |
| N1 | 0.68 | 0.68 |
| N2 | 0.66 | 0.66 |
| S1 | 0.66 | 0.66 |
| S2 | 0.61 | 0.61 |
| T1 | 0.65 | 0.65 |
| T2 | 0.61 | 0.61 |
| I1 | 0.67 | 0.67 |
| I2 | 0.69 | 0.69 |
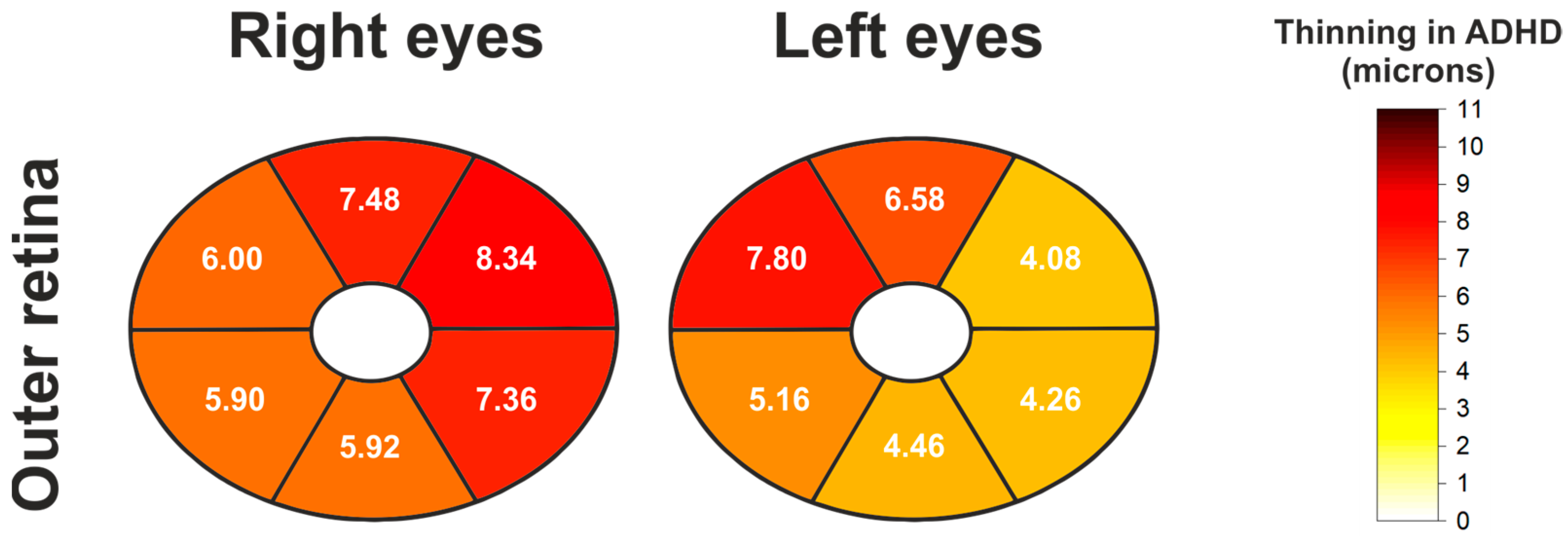
| Outer Retinal Thickness (Elliptical Pattern) | ||||||
|---|---|---|---|---|---|---|
| Eye | Location | Control Mean ± SD | ADHD Mean ± SD | Mean Diff. (Control-ADHD) | p-Value ANCOVA | p-Value ANCOVA + BH |
| Right | Global | 128.74 ± 6.60 | 121.98 ± 8.67 | 6.76 | <0.0001 | <0.0001 |
| Superotemporal | 129.14 ± 7.33 | 123.14 ± 8.85 | 6.00 | 0.0003 | 0.0004 | |
| Superior | 133.46 ± 7.84 | 125.98 ± 9.79 | 7.48 | 0.0001 | 0.0001 | |
| Superonasal | 130.72 ± 9.36 | 122.38 ± 10.80 | 8.34 | 0.0002 | 0.0002 | |
| Inferonasal | 127.30 ± 8.63 | 119.94 ± 8.89 | 7.36 | <0.0001 | <0.0001 | |
| Inferior | 125.10 ± 6.90 | 119.18 ± 9.07 | 5.92 | <0.0001 | <0.0001 | |
| Inferotemporal | 127.10 ± 7.26 | 121.20 ± 9.44 | 5.90 | 0.0001 | 0.0002 | |
| Left | Global | 128.42 ± 6.37 | 122.98 ± 8.74 | 5.44 | 0.0002 | 0.0012 |
| Superotemporal | 129.24 ± 6.70 | 125.16 ± 9.93 | 4.08 | 0.0082 | 0.0082 | |
| Superior | 133.28 ± 7.81 | 126.70 ± 10.30 | 6.58 | 0.0005 | 0.0012 | |
| Superonasal | 130.50 ± 10.43 | 122.70 ± 10.62 | 7.80 | 0.0005 | 0.0012 | |
| Inferonasal | 126.16 ± 8.49 | 120.98 ± 8.72 | 5.18 | 0.0011 | 0.0018 | |
| Inferior | 124.50 ± 6.00 | 120.04 ± 9.49 | 4.46 | 0.0006 | 0.0012 | |
| Inferotemporal | 126.80 ± 6.09 | 122.54 ± 9.63 | 4.26 | 0.0046 | 0.0053 | |
| Outer Retina (Elliptical Pattern). | ||
|---|---|---|
| Sector | AUC Right Eyes | AUC Left Eyes |
| Global | 0.73 | 0.71 |
| Superior | 0.72 | 0.70 |
| Superotemporal | 0.69 | 0.62 |
| Inferotemporal | 0.69 | 0.65 |
| Inferior | 0.70 | 0.68 |
| Inferonasal | 0.73 | 0.66 |
| Superonasal | 0.73 | 0.72 |
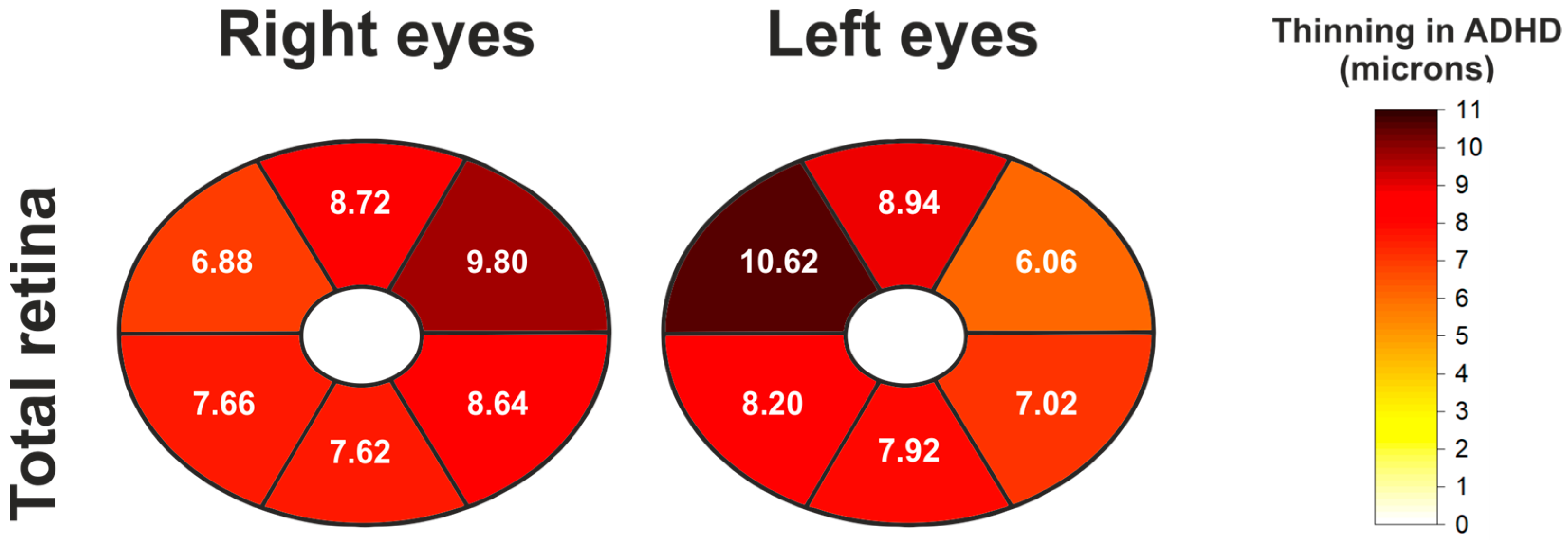
| Total Retinal Thickness (Elliptical Pattern) | ||||||
|---|---|---|---|---|---|---|
| Eye | Location | Control Mean ± SD | ADHD Mean ± SD | Mean Diff. (Control-ADHD) | p-Value ANCOVA | p-Value ANCOVA + BH |
| Right | Global | 244.76 ± 10.54 | 236.62 ± 12.17 | 8.14 | 0.0005 | 0.0020 |
| Superotemporal | 233.0 ± 9.75 | 226.12 ± 11.65 | 6.88 | 0.0028 | 0.0031 | |
| Superior | 251.98 ± 10.79 | 243.26 ± 13.1 | 8.72 | 0.0016 | 0.0025 | |
| Superonasal | 253.74 ± 11.55 | 243.94 ± 13.47 | 9.8 | 0.0005 | 0.0020 | |
| Inferonasal | 251.1 ± 12.72 | 242.46 ± 12.91 | 8.64 | 0.0008 | 0.0020 | |
| Inferior | 243.96 ± 15.86 | 236.34 ± 13.66 | 7.62 | 0.0016 | 0.0025 | |
| Inferotemporal | 234.7 ± 11.01 | 227.04 ± 14.69 | 7.66 | 0.0025 | 0.0031 | |
| Left | Global | 245.4 ± 8.64 | 237.18 ± 12.94 | 8.22 | 0.0002 | 0.0008 |
| Superotemporal | 234.74 ± 9.14 | 228.68 ± 13.17 | 6.06 | 0.0036 | 0.0041 | |
| Superior | 252.64 ± 9.81 | 243.7 ± 14.58 | 8.94 | 0.0005 | 0.0008 | |
| Superonasal | 253.86 ± 12.1 | 243.24 ± 14.65 | 10.62 | 0.0003 | 0.0008 | |
| Inferonasal | 250.86 ± 10.57 | 242.66 ± 13.22 | 8.2 | 0.0006 | 0.0009 | |
| Inferior | 244.18 ± 9.47 | 236.26 ± 14.69 | 7.92 | 0.0003 | 0.0008 | |
| Inferotemporal | 235.16 ± 8.7 | 228.14 ± 15.01 | 7.02 | 0.0043 | 0.0043 | |
| Total Retina (Elliptical Pattern) | ||
|---|---|---|
| Sector | AUC Right Eyes | AUC Left Eyes |
| Global | 0.70 | 0.70 |
| Superior | 0.69 | 0.69 |
| Superotemporal | 0.67 | 0.65 |
| Inferotemporal | 0.66 | 0.65 |
| Inferior | 0.70 | 0.68 |
| Inferonasal | 0.69 | 0.68 |
| Superonasal | 0.69 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miquel-Lopez, C.; Garcia-Medina, J.J.; Lopez-Hernandez, A.E.; Garcia-Ayuso, D.; De-Paco-Matallana, M.; Hernandez-Olivares, J.; Pinazo-Duran, M.D.; Del-Rio-Vellosillo, M. Retinal Thinning in Attention Deficit Hyperactivity Disorder (ADHD): Structural Changes Detected by Spectral-Domain OCT. J. Clin. Med. 2025, 14, 5723. https://doi.org/10.3390/jcm14165723
Miquel-Lopez C, Garcia-Medina JJ, Lopez-Hernandez AE, Garcia-Ayuso D, De-Paco-Matallana M, Hernandez-Olivares J, Pinazo-Duran MD, Del-Rio-Vellosillo M. Retinal Thinning in Attention Deficit Hyperactivity Disorder (ADHD): Structural Changes Detected by Spectral-Domain OCT. Journal of Clinical Medicine. 2025; 14(16):5723. https://doi.org/10.3390/jcm14165723
Chicago/Turabian StyleMiquel-Lopez, Carmen, Jose Javier Garcia-Medina, A. Eusebio Lopez-Hernandez, Diego Garcia-Ayuso, Maravillas De-Paco-Matallana, Javier Hernandez-Olivares, Maria Dolores Pinazo-Duran, and Monica Del-Rio-Vellosillo. 2025. "Retinal Thinning in Attention Deficit Hyperactivity Disorder (ADHD): Structural Changes Detected by Spectral-Domain OCT" Journal of Clinical Medicine 14, no. 16: 5723. https://doi.org/10.3390/jcm14165723
APA StyleMiquel-Lopez, C., Garcia-Medina, J. J., Lopez-Hernandez, A. E., Garcia-Ayuso, D., De-Paco-Matallana, M., Hernandez-Olivares, J., Pinazo-Duran, M. D., & Del-Rio-Vellosillo, M. (2025). Retinal Thinning in Attention Deficit Hyperactivity Disorder (ADHD): Structural Changes Detected by Spectral-Domain OCT. Journal of Clinical Medicine, 14(16), 5723. https://doi.org/10.3390/jcm14165723







