Abstract
Background/Objectives: Acute exacerbation of interstitial lung disease (AE-ILD) is a life-threatening complication in lung cancer patients with pre-existing ILD. Anatomical resection is recognized as a significant risk factor for AE-ILD. We investigated the safety and feasibility of wedge resection in lung cancer patients with ILD. Methods: This retrospective study analyzed clinical stage IA–IIIA primary lung cancer patients with ILD, as recorded in the Shizuoka Registry across eight institutions from January 2019 to May 2023. Patients were categorized into a wedge resection group (WG) and an anatomical resection group (AG), which included segmentectomy, lobectomy, and bilobectomy. Perioperative outcomes were compared between the groups. Results: The WG comprised 36 patients, while the AG included 81. The WG had significantly older patients (77 vs. 72 years, p < 0.01) and smaller tumors (18 vs. 24 mm, p < 0.01). Wedge resection was associated with shorter operative time (100 vs. 205 min, p < 0.01) and less blood loss (5 vs. 30 mL, p = 0.02). The incidence of postoperative complications did not differ significantly (p = 0.84). AE-ILD occurred in three patients (8%) in the WG and four patients (4%) in the AG. Perioperative mortality was 0% in the WG and 2% in the AG; both deaths were due to AE-ILD. Marginal recurrence was observed in four patients (11%) in the WG. Conclusions: Although AE-ILD incidence was higher, no deaths due to IP-AE were observed in the WG. While wedge resection cannot completely prevent postoperative AE-ILD, it may reduce perioperative mortality in lung cancer patients with ILD.
1. Introduction
The prognosis of lung cancer patients is influenced not only by tumor characteristics but also by comorbidities. Interstitial lung disease (ILD), a representative diffuse parenchymal lung disease characterized by pulmonary fibrosis, is associated with poorer outcomes [1,2]. Idiopathic pulmonary fibrosis (IPF), a common subtype of ILD, can be life-threatening, with a reported median survival time of 2 to 3 years following diagnosis [3,4]. Additionally, previous studies have demonstrated an increased incidence of lung cancer among patients with ILD. Not only respiratory failure due to the progression of ILD itself but also lung cancer are among the leading causes of death in patients with ILD [5]. In addition to its high incidence, the following treatment challenges pose significant difficulties for clinicians.
Surgical resection provides the highest possibility of cure in early-stage lung cancer. However, acute exacerbation of ILD (AE-ILD) is a fatal complication and the leading cause of perioperative mortality in Japanese patients undergoing pulmonary resection [6]. Reports indicate that AE-ILD occurs in 4.2% to 12.4% of cases, with mortality rates as high as 44% [7,8,9]. Although pirfenidone has shown prophylactic efficacy in a prospective trial (WJOG6711L), no standardized preventative strategy for AE-ILD has been established [10]. To reduce postoperative mortality in lung cancer patients with ILD, it is essential to minimize the occurrence of AE-ILD as much as possible. Safe treatment strategies for lung cancer patients with pre-existing ILD is a clinical need.
According to the Japanese Association for Chest Surgery (JACS), seven risk factors for AE-ILD have been identified. Notably, wedge resection is associated with a lower risk of AE-ILD than more extensive resections, such as lobectomy [7]. In high-risk patients, wedge resection is often chosen, with an emphasis on perioperative safety. Based on this, we evaluated the postoperative outcomes of lung cancer patients with ILD to assess the impact of wedge resection in minimizing AE-ILD risk. The objective of this study was to examine the safety and feasibility of wedge resection in lung cancer patients with ILD.
2. Materials and Methods
This study was approved by the Institutional Review Board of Hamamatsu University School of Medicine (approval number 24-303). The requirement for informed consent was waived due to the retrospective design.
2.1. Shizuoka Registry
We retrospectively reviewed the medical records of patients who underwent operation for diagnosis of primary lung cancer at eight institutions (Hamamatsu University School of Medicine, Seirei Hamamatsu General Hospital, Hamamatsu Medical Center, Iwata city Hospital, Yaizu City Hospital, Fujieda Municipal General Hospital, Japanese Red Cross Shizuoka Hospital, and Fujinomiya City General Hospital) in Shizuoka Prefecture between January 2019 and May 2023. This registry included a total of 1261 patients. Tumors were staged according to the 8th edition of the TNM Classification by the International Union for Cancer Control. Pulmonary function tests were conducted, and the serum Cartino Enbriotic Antigen (CEA) Krebs von den Lungen-6 (KL-6) level was examined preoperatively.
2.2. Patient Selection and Diagnosis of Interstitial Lung Disease
We selected patients with ILD who underwent R0 resection for clinical stage IA–IIIA primary lung cancer. R0 resection was defined as complete macroscopic and microscopic tumor removal. Smoking history was defined as habitual smoking for any interval that was carefully evaluated at the preoperative outpatient department and the ward at the time of admission. This study included two surgical approaches, thoracotomy and thoracoscopic surgery. Thoracotomy was performed laterally and assisted by a thoracoscope with a maximum skin incision of 8–10 cm. Four-port thoracoscopic surgery was performed with a maximum skin incision of about 3–4 cm. Patients were categorized into two groups based on the surgical procedure: the wedge resection group (WG) and the anatomical resection group (AG), which included segmentectomy, lobectomy, and bilobectomy. ILD was diagnosed in consultation with internal medicine at each institution based on imaging examination on high-resolution computed tomography (HRCT), pulmonary function tests, and preoperative measurements of the serum KL-6 level. Radiological assessments were reviewed retrospectively and categorized according to the 2018 ATS guidelines [11]. UIP patterns were defined as characterized by a subpleural and basal predominant distribution of honeycomb lesions, which have multiple, similarly sized cystic lesions of 2–10 mm diameter with a thick wall.
2.3. Postoperative Acute Exacerbation and Other Complications
Postoperative complications within 30 days of surgery were assessed using the Clavien–Dindo classification [12]; grade III or higher was considered severe. AE-ILD was diagnosed when a patient with ILD acutely developed subjective worsening of dyspnea and met the following criteria: (i) new bilateral radiological opacities on HRCT that were not observed preoperatively; (ii) no evidence of infection, and the possibility of infection was excluded using the laboratory data, radiological findings, and sputum culture; (iii) no evidence of the alternative cause of dyspnea, including left heart failure, pulmonary embolism, or an identifiable cause of acute lung injury; and (iv) a decrease of more than 10 mm Hg in arterial oxygen tension [7,11,13].
2.4. Statistical Analyses
Categorical variables were analyzed using Fisher’s exact test and continuous variables using the t-test. Kaplan–Meier survival curves were used to estimate overall survival (OS), with group comparisons made using the log-rank test. Univariate and multivariate Cox proportional hazards models were used to identify prognostic factors. OS was defined as the time from surgery to death from any cause or last follow-up. Statistical significance was set at p < 0.05. Analyses were conducted using R version 4.3.0 (R Development Core Team 2013, A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria. URL: http://www.r-project.org).
3. Results
3.1. Patient Characteristics
A total of 117 patients with ILD were included in this study. Table 1 summarizes their clinical characteristics.

Table 1.
Clinicopathological characteristics of patients with interstitial lung disease.
The incidence of ILD among all patients in the Shizuoka Registry was 11%. The majority were male (n = 73, 92%), with a median age of 73 years (range: 58–86). The median Brinkman index was 1020 (range: 0–4000). The median % vital capacity (%VC) and % diffusing capacity for carbon monoxide (%DLco) were 108% (range: 67–143%) and 84% (range: 42–223%), respectively. The median serum KL-6 level was 574 U/mL. Radiological UIP patterns were observed in 61% of cases. Clinical stages I, II, and III were found in 78%, 18%, and 4% of patients, respectively. Surgical procedures included wedge resection (31%), segmentectomy (8%), lobectomy (60%), and bilobectomy (1%). Surgical approaches included thoracotomy (95%) and thoracoscopic surgery (5%). The median operative time was 171 min (range: 42–553), with a median blood loss of 20 mL (range: 0–789). No significant difference was shown in operation time between surgical approaches (p = 0.56). Histologically, 50% had squamous cell carcinoma, 37% adenocarcinoma, 5% large cell neuroendocrine carcinoma (LCNEC), and 8% other types. Pathological UIP patterns were confirmed in 25% of cases.
3.2. Comparison Between WG and AG
Table 2 presents the comparison between the WG (n = 37) and AG (n = 82).

Table 2.
Clinicopathological characteristics of wedge resection group and anatomical resection group.
The WG included significantly older patients (median age 77 vs. 72 years, p < 0.01) and had smaller tumors (17 mm vs. 24 mm, p < 0.01). There were no significant differences in smoking history (Brinkman index: 1100 vs. 990; p = 0.48), serum KL-6 levels (586 vs. 574 U/mL; p = 0.30), radiological UIP patterns (64% vs. 59%; p = 0.67), or pulmonary function (%VC: 99% vs. 104%, p = 0.08; %DLco: 81% vs. 85%, p = 0.86). WG patients were more likely to have clinical stage I disease (97% vs. 70%, p < 0.01) and had significantly shorter operative times (99 vs. 203 min, p < 0.01) and lower intraoperative blood loss (5 vs. 32 mL, p = 0.02). Surgical approaches did not show significant difference, and the majority of surgical approaches were thoracotomies in both groups (92 vs. 96%, p = 0.37). Even when analyzed separately by surgical procedure, the operative time was significantly shorter in the WG (p < 0.01 in both). The WG had significantly shorter postoperative hospital stay (6 vs. 8 days, p = 0.01). The histological distribution and pathological UIP pattern did not significantly differ between the groups. The histological types of two cases recorded as “Others” in Table 1 were mucoepidermoid carcinoma in the WG and large cell lung carcinoma in the AG.
3.3. Postoperative Complications
Postoperative complications are shown in Table 3.

Table 3.
The comparison of postoperative complication and mortality between the wedge resection group and anatomical resection group.
Overall, 46 patients (39%) experienced complications—11 (30%) in the WG and 35 (43%) in the AG (p = 0.84). No 30-day postoperative deaths occurred in the WG, while two deaths (2%) occurred in the AG, both attributed to AE-ILD. AE-ILD developed in three patients (8%) in the WG and in four (4%) in the AG. Other severe complications were observed only in the AG, including prolonged air leak (n = 5), postoperative bleeding (n = 1), empyema (n = 1), and bronchopleural fistula (n = 1).
3.4. Overall Survival and Multivariant Analysis
The median follow-up period was 14 months. One-year overall survival (OS) was significantly lower in the ILD group compared to the non-ILD group (85% vs. 96%, p < 0.01) (Figure 1).
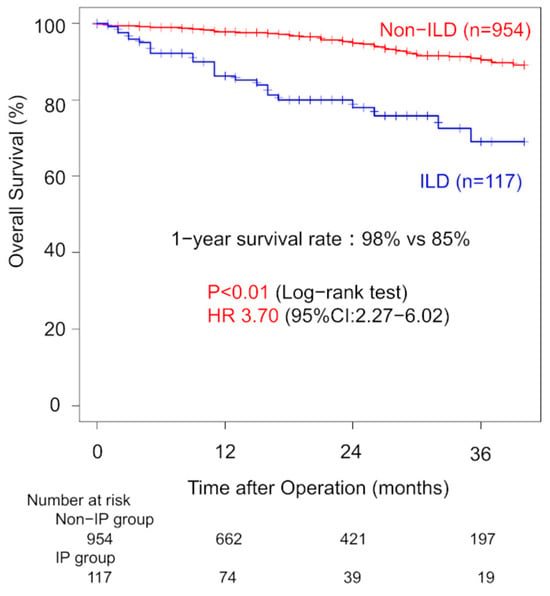
Figure 1.
Comparison of overall survival between patients with and without interstitial lung disease.
Three-year OS was 69% for ILD patients and 90% for non-ILD patients. Univariate analysis showed that ILD was associated with worse OS (HR: 3.70; 95% CI: 2.27–6.02). Among ILD patients with clinical stage I disease, one-year OS did not significantly differ between the WG and AG (96% vs. 79%, p = 0.40). Three-year OS rates were 60% (WG) and 68% (AG). The univariate HR for the surgical procedure was 1.53 (95% CI: 0.58–4.06). Margin recurrence occurred in four patients (11%) in the WG during follow-up (Figure 2). Thirteen patients (36%) in the WG developed recurrence. In the AG, nine patients (11%) developed recurrence with significant lower incidence (p < 0.01).
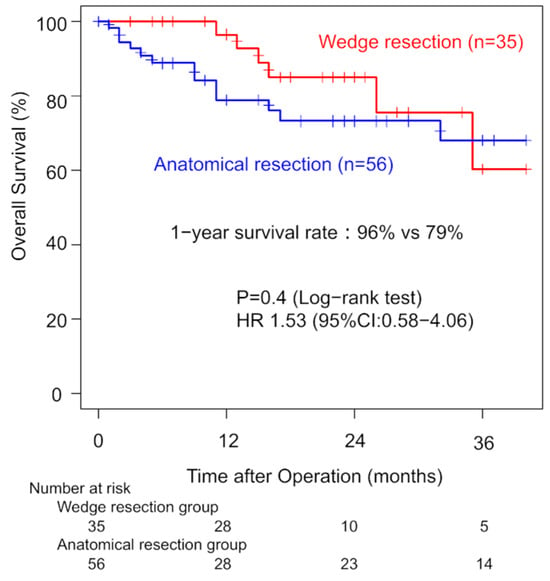
Figure 2.
Comparison of overall survival in clinical stage I patients between the wedge resection group and the anatomical resection group.
Among ILD patients with pathological stage I disease, the analysis showed that one-year OS did not significantly differ between the WG and AG (96% vs. 79%, p = 0.40). Three-year OS rates were 82% (WG) and 78% (AG). The univariate HR for the surgical procedure was 1.60 (95% CI: 0.48–5.30, Figure 3). In the pathological stage II, one-year OS did not significantly differ between the WG and AG (80% vs. 77%, p = 0.40). Three-year OS rates were 28% (WG) and 67% (AG).
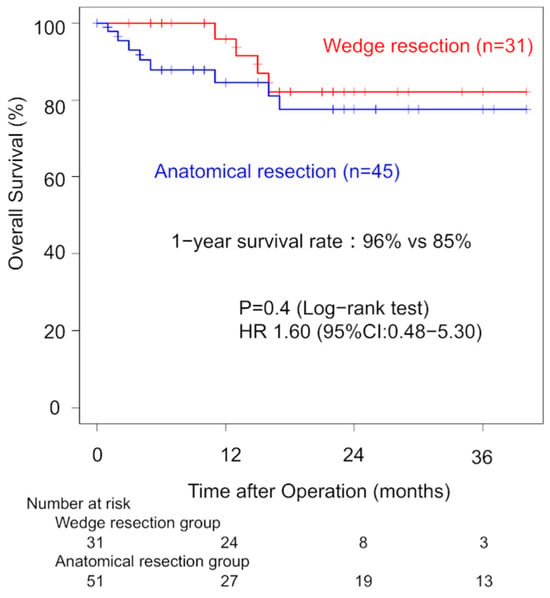
Figure 3.
Comparison of overall survival in pathological stage I patients between the wedge resection group and the anatomical resection group.
Multivariate analysis identified older age (HR: 1.05; p < 0.01), male sex (HR: 2.66; p = 0.01), elevated serum CEA (HR: 1.83; p = 0.01), advanced pathological stage (HR: 2.19; p < 0.01), and non-adenocarcinoma histology (HR: 2.19; p < 0.01) as significant unfavorable prognostic factors (Table 4). Although ILD was associated with poorer survival (HR: 1.58), the result did not reach statistical significance (p = 0.11).

Table 4.
Multivariate analysis for overall survival.
4. Discussion
Balancing perioperative safety with oncological efficacy is critical when determining surgical strategies for lung cancer patients with ILD. In our study, wedge resection was more frequently performed in older patients with smaller tumors. Wedge resection was performed in a minimally invasive manner with shorter operative time (p < 0.01) and less blood loss (p = 0.02). However, there was no significant difference in the frequency of AE-ILD (8% vs. 4%, p = 0.68). Despite being less invasive, wedge resection showed comparable postoperative complication rates to anatomical resection, and notably, no perioperative mortality was observed in the wedge group.
AE-ILD remains one of the most serious postoperative complications following lung resection in ILD patients [7]. Despite efforts to develop prophylactic treatments, such as perioperative use of pirfenidone, AE-ILD continues to pose significant risks [6,14]. In our cohort, AE-ILD occurred in 6% of ILD patients, with a 29% mortality rate among affected individuals. This highlights the importance of cautious treatment planning in this population. Previous studies have also demonstrated that AE can be triggered by other lung cancer treatments, such as chemotherapy and radiotherapy [15,16,17]. Immune checkpoint inhibitors and carbon-ion radiotherapy are among the latest treatment modalities being explored to establish safe therapeutic strategies for patients with lung cancer complicated by interstitial pneumonia [18,19,20,21]. Even among these various treatment modalities, given the unpredictable nature of AE-ILD, surgical decision-making must weigh curability against risk.
Recently, treatment for NSCLC has been improved in stereotactic body radiotherapy (SBRT) and molecular target drugs. SBRT was administered to 55 patients with stage I NSCLC who were deemed operable but declined surgery. The 3-year OS rate was 82% [22]. However, another report demonstrated that the fetal AE-ILD rate was 16.7%, significantly higher than that observed in the non-ILD group (0.8%) [23]. Osimertinib and Alectinib have demonstrated excellent outcomes as adjuvant therapies and have transformed the standard treatment of NSCLC [24,25,26]. However, according to real-world clinical data, 6.5–6.8% of 3578 patients treated with Osimertinib developed ILD, and 29 of them experienced fatal outcomes. In patients with pre-existing ILD, the risk of ILD onset increases by approximately 3.5-fold [27]. As a result, these populations have been excluded from clinical trials of these agents. Patients with ILD are not eligible to benefit from these novel treatments. The development of safe treatment strategies for lung cancer patients with comorbid ILD is an urgent need.
The choice of surgical procedure is important. The large-scale prospective study of the postoperative AE-ILD by the JACS demonstrated seven risk factors: (i) male sex, (ii) preoperative steroid use for ILD, (iii) previous history of AE, (iv) radiological UIP pattern, (v) %VC less than 80%, (vi) elevation of the serum KL-6 level, and (vii) surgical procedure besides segmentectomy [7]. Consequently, wedge resection is often considered a safer surgical option in this context. In our study, the wedge resection rate was 31%, nearly double that of previous reports [1]. This patient selection strategy contributed to a lower observed incidence of AE-ILD and achieved zero perioperative mortality in the WG. However, the incidence of AE-ILD was not significantly different between the WG and AG (8% vs. 4%, p = 0.68), indicating that wedge resection cannot entirely eliminate the risk.
Previous studies of surgical biopsy for ILD diagnosis have similarly reported the safety of wedge resection, with extremely low morbidity and mortality [28,29]. However, therapeutic resections for malignancy involve additional risks, especially in patients with high-risk profiles. The high proportion (61%) of patients with a radiological UIP pattern in our study may explain the persistence of AE-ILD despite conservative surgical approaches.
Multivariate analysis showed that male sex (HR 2.66), advanced pathological stage (HR 2.19), and non-adenocarcinoma histology (HR 2.19) have a strong influence among the significant independent prognostic factors. The ILD group had high frequencies of these unfavorable characteristics—92% were male, 34% had advanced-stage cancer, and 50% had squamous cell carcinoma. Consequently, ILD was not independently associated with poor overall survival in multivariate analysis. In the short term, wedge resection showed a slight advantage in 1-year OS, likely reflecting its lower perioperative risk. Although wedge resection may be acceptable from a safety standpoint in patients with ILD, it has not been adopted as the standard surgical procedure due to lower survival rates caused by a high incidence of local recurrence, including recurrence at the surgical margins [30]. In our study, 11% of patients in the WG developed local recurrence at the surgical margin. This may explain the convergence of long-term survival curves between the WG and AG. Similar trends have been reported previously, with lobectomy demonstrating superior survival in some cohorts [14]. While long-term follow-up is necessary to draw definitive conclusions, recent evidence supports the efficacy of sublobar resection for small, peripheral lung cancers [31]. The median tumor size in our study (17 mm) aligns with these indications, suggesting that wedge resection may offer comparable long-term outcomes if appropriately selected.
This study has several limitations. First, it is a retrospective analysis across multiple institutions, and the follow-up period was relatively short (median: 14 months). And surgical protocols across institutions could introduce variability. Second, the total number of objective patients was small, with no mention of propensity score matching or adjusted multivariate models comparing the WG vs. AG. We consider any adjustments or statistical controls important. We will continue accumulating cases and address them as a subject for analysis in the future. Third, the classification of ILD patterns was based on radiological findings, which are subject to interobserver variability. Although thoracic surgeons reviewed imaging in consultation with radiologists and pulmonologists, some diagnostic inconsistency may have occurred. Fourth, while recent guidelines (2022) offer updated criteria, our study relied on the 2018 ATS guideline, which was applicable to the majority of our cohort.
In conclusion, wedge resection can reduce perioperative mortality in lung cancer patients with ILD but does not eliminate the risk of AE-ILD. Moreover, it may not offer superior long-term survival. Careful patient selection and long-term monitoring remain essential.
5. Conclusions
Wedge resection appears to be a safer surgical option for lung cancer patients with underlying interstitial lung disease, significantly reducing perioperative mortality compared to anatomical resection. However, it does not eliminate the risk of postoperative acute exacerbation of ILD or show significant difference in mortality. Additionally, wedge resection may offer limited benefit in terms of long-term survival. Therefore, surgical strategies for this population must be individualized, taking into account tumor characteristics, patient comorbidities, and the risks of postoperative complications. Continued prospective studies and long-term follow-up are essential to better defining the role of wedge resection in this high-risk patient group.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by K.S. and K.F. The first draft of the manuscript was written by K.S., and all authors commented on previous versions of the manuscript and provided overall direction and guidance of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Hamamatsu University School of Medicine (approval number 24-303, dated on 28 February 2025).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to express their deep appreciation to Yusuke Takanashi for valuable advice and constructive comments throughout this study. We also gratefully acknowledge the following institutions for their participation in this multicenter retrospective study: Seirei Hamamatsu General Hospital, Hamamatsu Medical Center, Iwata city Hospital, Yaizu City Hospital, Fujieda Municipal General Hospital, Japanese Red Cross Shizuoka Hospital, and Fujinomiya City General Hospital.
Conflicts of Interest
All authors have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AE-ILD | Acute Exacerbation of Interstitial Lung Disease |
| ILD | Interstitial Lung Disease |
| UIP | Usual Interstitial Pneumonia |
| HRCT | High-Resolution Computed Tomography |
| CEA | Carcinoembryonic Antigen |
| KL-6 | Krebs von den Lungen-6 |
| WG | Wedge Resection Group |
| AG | Anatomical Resection Group |
| OS | Overall Survival |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| %VC | Percent Vital Capacity |
| %DLco | Percent Diffusing Capacity for Carbon Monoxide |
| JACS | Japanese Association for Chest Surgery |
| LCNEC | Large Cell Neuroendocrine Carcinoma |
| R0 | Complete Macroscopic and Microscopic Tumor Removal |
| SBRT | Stereotactic Body Radiotherapy |
References
- Sato, T.; Watanabe, A.; Kondo, H.; Kanzaki, M.; Okubo, K.; Yokoi, K.; Matsumoto, K.; Marutsuka, T.; Shinohara, H.; Teramukai, S.; et al. Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J. Thorac. Cardiovasc. Surg. 2015, 149, 64–69, 70.e1–70.e2. [Google Scholar] [CrossRef] [PubMed]
- Sekihara, K.; Aokage, K.; Oki, T.; Omori, T.; Katsumata, S.; Ueda, T.; Miyoshi, T.; Goto, M.; Nakasone, S.; Ichikawa, T.; et al. Long-term survival after complete resection of non-small-cell lung cancer in patients with interstitial lung disease. Interact. Cardiovasc. Thorac. Surg. 2017, 26, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Natsuizaka, M.; Chiba, H.; Kuronuma, K.; Otsuka, M.; Kudo, K.; Mori, M.; Bando, M.; Sugiyama, Y.; Takahashi, H. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am. J. Respir. Crit. Care Med. 2014, 190, 773–779. [Google Scholar] [CrossRef]
- Rudd, R.M.; Prescott, R.J.; Chalmers, J.C.; Johnston, I.D.; Fibrosing Alveolitis Subcommittee of the Research Committee of the British Thoracic Society. British Thoracic Society Study on cryptogenic fibrosing alveolitis: Response to treatment and survival. Thorax 2007, 62, 62–66. [Google Scholar] [CrossRef]
- Hubbard, R.; Venn, A.; Lewis, S.; Britton, J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am. J. Respir. Crit. Care Med. 2000, 161, 5–8. [Google Scholar] [CrossRef]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery; Shimizu, H.; Okada, M.; Tangoku, A.; Doki, Y.; Endo, S.; Fukuda, H.; Hirata, Y.; Iwata, H.; Kobayashi, J.; et al. Thoracic and cardiovascular surgeries in Japan during 2017: Annual report by the Japanese Association for Thoracic Surgery. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 414–449. [Google Scholar] [CrossRef]
- Sato, T.; Teramukai, S.; Kondo, H.; Watanabe, A.; Ebina, M.; Kishi, K.; Fujii, Y.; Mitsudomi, T.; Yoshimura, M.; Maniwa, T.; et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J. Thorac. Cardiovasc. Surg. 2014, 147, 1604–1611.e3. [Google Scholar] [CrossRef]
- Kobayashi, S.; Karube, Y.; Nishihira, M.; Inoue, T.; Araki, O.; Maeda, S.; Sado, T.; Matsumura, Y.; Chida, M. Postoperative pyothorax a risk factor for acute exacerbation of idiopathic interstitial pneumonia following lung cancer resection. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 476–480. [Google Scholar] [CrossRef]
- Fukui, M.; Takamochi, K.; Suzuki, K.; Ando, K.; Matsunaga, T.; Hattori, A.; Oh, S.; Suzuki, K. Advantages and disadvantages of corticosteroid use for acute exacerbation of interstitial pneumonia after pulmonary resection. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 472–477. [Google Scholar] [CrossRef]
- Iwata, T.; Yoshino, I.; Yoshida, S.; Ikeda, N.; Tsuboi, M.; Asato, Y.; Katakami, N.; Sakamoto, K.; Yamashita, Y.; Okami, J.; et al. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan Oncology Group 6711 L (PEOPLE Study). Respir. Res. 2016, 17, 90. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Iwata, T.; Yoshida, S.; Nagato, K.; Nakajima, T.; Suzuki, H.; Tagawa, T.; Mizobuchi, T.; Ota, S.; Nakatani, Y.; Yoshino, I. Experience with perioperative pirfenidone for lung cancer surgery in patients with idiopathic pulmonary fibrosis. Surg. Today 2015, 45, 1263–1270. [Google Scholar] [CrossRef]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery; Shimizu, H.; Matsumiya, G.; Sato, Y.; Takeuchi, H.; Abe, T.; Endo, S.; Hirata, Y.; Ishida, M.; Iwata, H.; et al. Thoracic and cardiovascular surgeries in Japan during 2020: Annual report by the Japanese Association for Thoracic Surgery. Gen. Thorac. Cardiovasc. Surg. 2024, 72, 61–94. [Google Scholar]
- Ando, M.; Okamoto, I.; Yamamoto, N.; Takeda, K.; Tamura, K.; Seto, T.; Ariyoshi, Y.; Fukuoka, M. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Sanuki, N.; Ono, A.; Komatsu, E.; Kamei, N.; Akamine, S.; Yamazaki, T.; Mizunoe, S.; Maeda, T. Association of computed tomography-detected pulmonary interstitial changes with severe radiation pneumonitis for patients treated with thoracic radiotherapy. J. Radiat. Res. 2012, 53, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kenmotsu, H.; Naito, T.; Kimura, M.; Ono, A.; Shukuya, T.; Nakamura, Y.; Tsuya, A.; Kaira, K.; Murakami, H.; Takahashi, T.; et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2011, 6, 1242–1246. [Google Scholar] [CrossRef]
- Ikeda, S.; Kato, T.; Kenmotsu, H.; Sekine, A.; Baba, T.; Ogura, T. Current Treatment Strategies for Non-Small-Cell Lung Cancer with Comorbid Interstitial Pneumonia. Cancers 2021, 13, 3979. [Google Scholar] [CrossRef]
- Aoki, S.; Ishikawa, H.; Nakajima, M.; Yamamoto, N.; Mori, S.; Omatsu, T.; Tada, Y.; Mizobuchi, T.; Ikeda, S.; Yoshino, I.; et al. Safety and Efficacy of Single-Fraction Carbon-Ion Radiotherapy for Early-Stage Lung Cancer with Interstitial Pneumonia. Cancers 2024, 16, 562. [Google Scholar] [CrossRef]
- Otoshi, R.; Ikeda, S.; Kaneko, T.; Sagawa, S.; Yamada, C.; Kumagai, K.; Moriuchi, A.; Sekine, A.; Baba, T.; Ogura, T. Treatment Strategies for Non-Small-Cell Lung Cancer with Comorbid Respiratory Disease; Interstitial Pneumonia, Chronic Obstructive Pulmonary Disease, and Tuberculosis. Cancers 2024, 16, 1734. [Google Scholar] [CrossRef]
- Mauclet, C.; Dupont, M.V.; Roelandt, K.; Regnier, M.; Delos, M.; Pirard, L.; Vander Borght, T.; Dahlqvist, C.; Froidure, A.; Rondelet, B.; et al. Treatment and Prognosis of Patients with Lung Cancer and Combined Interstitial Lung Disease. Cancers 2023, 15, 3876. [Google Scholar] [CrossRef]
- Kocak Uzel, E.; Bagci Kilic, M.; Morcali, H.; Uzel, O. Stereotactic body radiation therapy for stage I medically operable non-small cell lung cancer. Sci. Rep. 2023, 13, 10384. [Google Scholar] [CrossRef]
- van den Berg, L.L.; Klinkenberg, T.J.; Groen, H.J.; Widder, J. Patterns of recurrence and survival after surgery or stereotactic radiotherapy for early stage NSCLC. J. Thorac. Oncol. 2015, 10, 826–831. [Google Scholar] [CrossRef]
- Wu, Y.L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.-C.; et al. Overall survival with osimertinib in resected EGFR-mutated NSCLC. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef]
- Wu, Y.L.; Dziadziuszko, R.; Ahn, J.S.; Barlesi, F.; Nishio, M.; Lee, D.H.; Lee, J.-S.; Zhong, W.; Horinouchi, H.; Mao, W.; et al. Alectinib in resected ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2024, 390, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Gemma, A.; Kusumoto, M.; Sakai, F.; Endo, M.; Kato, T.; Saito, Y.; Baba, T.; Sata, M.; Yamaguchi, O.; Yabuki, Y.; et al. Real-world evaluation of factors for interstitial lung disease incidence and radiologic characteristics in patients with EGFR T790M-positive NSCLC treated with osimertinib in Japan. J. Thorac. Oncol. 2020, 15, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Sano, A.; Azuma, Y.; Sakai, T.; Koezuka, S.; Sugino, K.; Sakamoto, S.; Tochigi, N.; Homma, S.; Iyoda, A. Surgical lung biopsy for interstitial lung diseases-a single center study of 129 patients. J. Thorac. Dis. 2022, 14, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Sekihara, K.; Tajiri, T.; Shibata, M.; Fujisawa, T.; Suda, T.; Shiiya, N.; Funai, K. Safe surgical lung biopsy in the diagnosis of interstitial lung disease under strict patient selection. Respir. Investig. 2025, 63, 81–85. [Google Scholar] [CrossRef]
- Ginsberg, R.J.; Rubinstein, L.V.; Lung Cancer Study Group. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann. Thorac. Surg. 1995, 60, 615–622. [Google Scholar] [CrossRef]
- Altoki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).