A Scoping Review of Clinical Features and Mechanisms of Orofacial Pain and Headache in Patients with Head and Neck Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Identifying the Research Question
- What are the typical frequency, intensity, quality, location, and duration of these pain symptoms?
- What mechanisms of pain are proposed (e.g., nociceptive, neuropathic, nociplastic)?
- What assessment tools are used to evaluate these types of pain in HNC populations?
2.2. Identifying Relevant Studies
2.2.1. Eligibility Criteria
2.2.2. Information Sources
2.2.3. Search Strategy
2.3. Study Selection
2.4. Data Charting
2.4.1. Data Extraction
2.4.2. Extracted Variables
- Study characteristics: author, year, and study design.
- Participant characteristics: cancer type, treatment status (pre-/post-therapy, during therapy), sample size, and sex.
- Pain characteristics: location, intensity, and frequency.
- Proposed pain mechanisms: nociceptive, neuropathic, nociplastic, or mixed.
- Affected activities and others.
2.5. Collating, Summarizing, and Reporting the Results
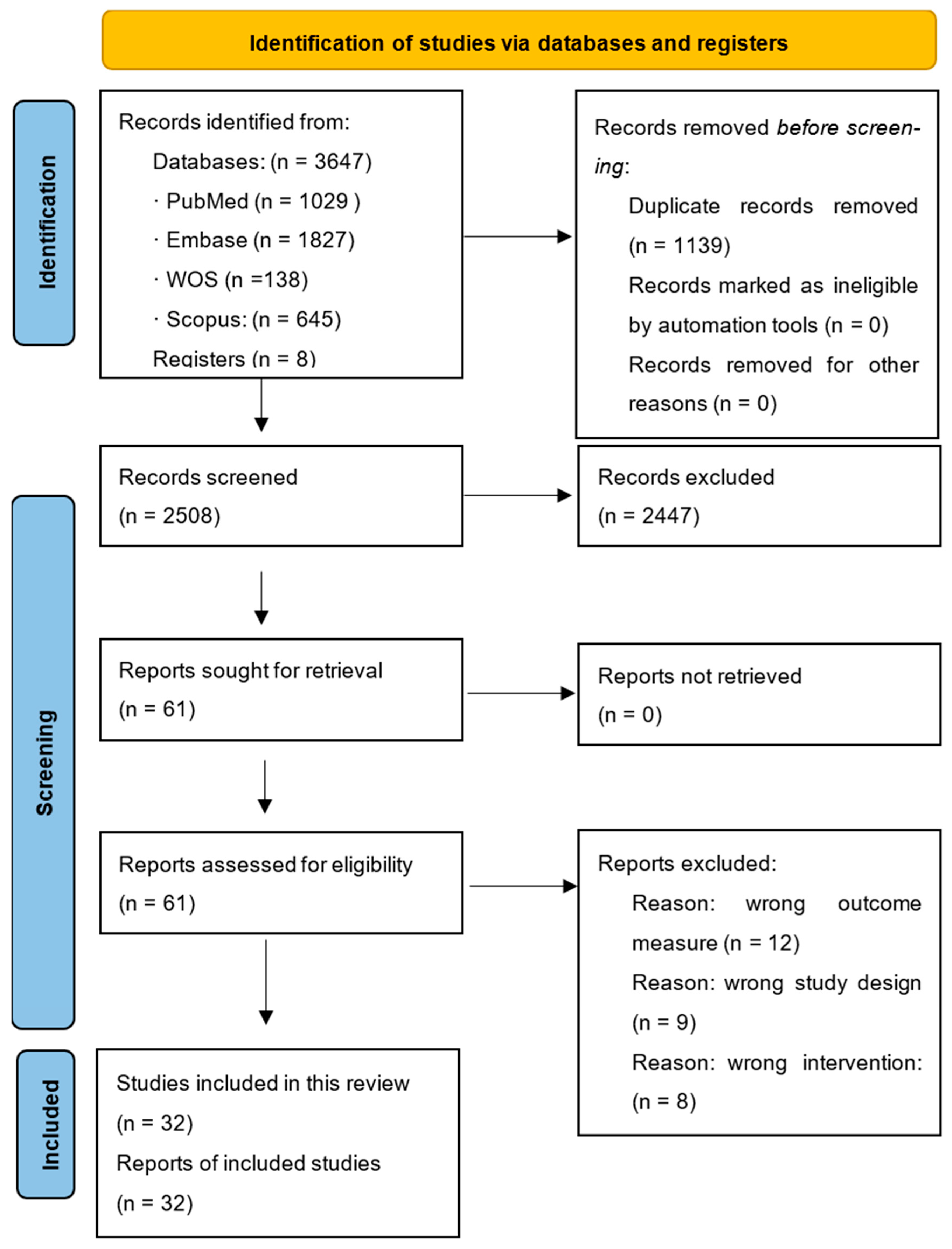
3. Results
- Type of study, sample size, and sex distribution.
- Specific diagnosis and oncologic treatment status.
- Outcome measures.
- Pain characteristics: phenotype, location, intensity, and frequency.
- Affected activities and other reported outcomes.
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimatesof incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- National Institute of Dental and Craniofacial Research. Oral Health in America. Reviewed May 2022. Available online: https://www.nidcr.nih.gov/research/oralhealthinamerica (accessed on 18 May 2022).
- Hammerlid, E.; Silander, E.; Hörnestam, L.; Sullivan, M. Health-related quality of life three years after diagnosis of head and neck cancer—A longitudinal study. Head Neck 2001, 23, 113–125. [Google Scholar] [CrossRef]
- Dal Fabbro, C.; Harris, P.; Dufresne, E.; Herrero Babiloni, A.; Mayer, P.; Bahig, H.; Filion, E.; Nguyen, F.; Ghannoum, J.; Schmittbuhl, M.; et al. Orofacial Pain and Snoring/Obstructive Sleep Apnea in Individuals with Head and Neck Cancer: A Critical Review. J. Oral Facial Pain Headache 2022, 36, 85–102. [Google Scholar] [CrossRef]
- Mirabile, A.; Airoldi, M.; Ripamonti, C.; Bolner, A.; Murphy, B.; Russi, E.; Numico, G.; Licitra, L.; Bossi, P. Pain management in head and neck cancer patients undergoing chemo-radiotherapy: Clinical practical recommendations. Crit. Rev. Oncol. Hematol. 2016, 99, 100–106. [Google Scholar] [CrossRef]
- Pazdrowski, J.; Gornowicz-Porowska, J.; Kaźmierska, J.; Krajka-Kuźniak, V.; Polanska, A.; Masternak, M.; Szewczyk, M.; Golusiński, W.; Danczak-Pazdrowska, A. Radiation-induced skin injury in the head and neck region: Pathogenesis, clinics, prevention, treatment considerations and proposal for management algorithm. Rep. Pract. Oncol. Radiother. 2024, 29, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Abboud, W.A.; Hassin-Baer, S.; Alon, E.E.; Gluck, I.; Dobriyan, A.; Amit, U.; Yahalom, R.; Yarom, N. Restricted Mouth Opening in Head and Neck Cancer: Etiology, Prevention, and Treatment. JCO Oncol. Pract. 2020, 16, 643–653. [Google Scholar] [CrossRef]
- Daud, M.L.; Simone, G.G. Management of pain in cancer patients—An update. Ecancermedicalscience 2024, 18, 1821. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, T.; Emodi-Perlman, A. Headache and orofacial pain: A traffic-light prognosis-based management approach for the musculoskeletal practice. Front. Neurol. 2023, 14, 1146427. [Google Scholar] [CrossRef]
- Cuffari, L.; Tesseroli de Siqueira, J.T.; Nemr, K.; Rapaport, A. Pain complaint as the first symptom of oral cancer: A descriptive study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 56–61. [Google Scholar] [CrossRef]
- Binczak, M.; Navez, M.; Perrichon, C.; Blanchard, D.; Bollet, M.; Calmels, P.; Couturaud, C.; Dreyer, C.; Espitalier, F.; Testelin, S.; et al. Management of somatic pain induced by head-and-neck cancer treatment: Definition and assessment. Guidelines of the French Oto-Rhino-Laryngology- Head and Neck Surgery Society (SFORL). Eur Ann. Otorhinolaryngol. Head Neck Dis. 2014, 131, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Reyes-Gibby, C.C.; Anderson, K.O.; Merriman, K.W.; Todd, K.H.; Shete, S.S.; Hanna, E.Y. Survival patterns in squamous cell carcinoma of the head and neck: Pain as an independent prognostic factor for survival. J. Pain 2014, 15, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Aupérin, A. Epidemiology of head and neck cancers: An update. Curr. Opin. Oncol. 2020, 32, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Scheff, N.N.; Bhattacharya, A.; Dowse, E.; Dang, R.X.; Dolan, J.C.; Wang, S.; Kim, H.; Albertson, D.G.; Schmidt, B.L. Neutrophil-Mediated Endogenous Analgesia Contributes to Sex Differences in Oral Cancer Pain. Front. Integr. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef]
- Terkawi, A.S.; Tsang, S.; Alshehri, A.S.; Mulafikh, D.S.; Alghulikah, A.A.; AlDhahri, S.F. The burden of chronic pain after major head and neck tumor therapy. Saudi J. Anaesth. 2017, 11 (Suppl. S1), S71–S79. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, P.; Li, W. Comparison of orofacial pain of patients with different stages of precancer and oral cancer. Sci. Rep. 2017, 7, 203. [Google Scholar] [CrossRef]
- Haumann, J.; van Kuijk, S.M.J.; Geurts, J.W.; Hoebers, F.J.P.; Kremer, B.; Joosten, E.A.; van den Beuken-van Everdingen, M.H.J. Methadone versus Fentanyl in Patients with Radiation-Induced Nociceptive Pain with Head and Neck Cancer: A Randomized Controlled Noninferiority Trial. Pain Pract. 2018, 18, 331–340. [Google Scholar] [CrossRef]
- Ouadghiri, F.; Salles, C.; Passemard, L.; Lapeyre, M.; Mulliez, A.; Devoize, L.; Pham Dang, N. After 4 years of survival, patients treated for an oral or oropharyngeal cancer have more neurosensorial disorders than chronic pain and a better quality of life. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101924. [Google Scholar] [CrossRef]
- Sato, J.; Yamazaki, Y.; Satoh, A.; Notani, K.; Kitagawa, Y. Pain is associated with an endophytic cancer growth pattern in patients with oral squamous cell carcinoma before treatment. Odontology 2010, 98, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Cardoso, D.M.; Kayahara, G.M.; Bernabé, D.G. A pilot study to improve pain phenotyping in head and neck cancer patients. Front. Pain Res. 2023, 4, 1146667. [Google Scholar] [CrossRef]
- Shinde, S.; Kadam, I.; Dalvi, S.; Jain, P.; Patil, S.; Gudur, A. Effect of Multicomponent Exercise Program on Temporomandibular Joint Dysfunction in Oral Cancer Survivors. J. Head Neck Physicians Surg. 2023, 11, 107–113. [Google Scholar] [CrossRef]
- Schaller, A.K.C.S.; Peterson, A.; Bäckryd, E. Pain management in patients undergoing radiation therapy for head and neck cancer—A descriptive study. Scand. J. Pain 2020, 21, 256–265. [Google Scholar] [CrossRef]
- Saghafi, E.; Tuomi, L.; Kjeller, G. The prevalence and symptoms of temporomandibular disorders in head and neck cancer patients. Acta Odontol. Scand. 2022, 80, 252–257. [Google Scholar] [CrossRef]
- Ortiz-Comino, L.; Fernández-Lao, C.; Castro-Martín, E.; Lozano-Lozano, M.; Cantarero-Villanueva, I.; Arroyo-Morales, M.; Martín-Martín, L. Myofascial pain, widespread pressure hypersensitivity, and hyperalgesia in the face, neck, and shoulder regions, in survivors of head and neck cancer. Support. Care Cancer 2020, 28, 2891–2898. [Google Scholar] [CrossRef]
- Arantes, D.; Costa, N.; Resende, T.; Mikulas, K.; da Silva Júnior, P.; Brito, R.; Noronha, V.; Pedras, R.; Corrêa, L. Dental approach of orofacial pain in head and neck cancer patients. J. Clin. Exp. Dent. 2018, 10, e1082–e1090. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, Y.; Shen, Q.; Rong, X.; Huang, X.; Li, H.; Zhou, L.; Mai, H.Q.; Zheng, D.; Chen, M.Y.; et al. Effect of Pregabalin on Radiotherapy-Related Neuropathic Pain in Patients With Head and Neck Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Wilkie, D.J.; Fischer, D.J.; Kim, Y.O.; Villines, D. Neuropathic and nociceptive pain in head and neck cancer patients receiving radiation therapy. Head Neck Oncol. 2009, 1, 26. [Google Scholar] [CrossRef]
- Chua, K.S.; Reddy, S.K.; Lee, M.C.; Patt, R.B. Pain and loss of function in head and neck cancer survivors. J. Pain. Symptom Manag. 1999, 18, 193–202. [Google Scholar] [CrossRef]
- Sarumpaet, V.T.; Dewi, Y.A.; Mahdiani, S. Head and neck cancer pain. Pharmacia 2024, 71, 1–6. [Google Scholar] [CrossRef]
- Aghajanzadeh, S.; Karlsson, T.; Tuomi, L.; Engström, M.; Finizia, C. Facial pain, health-related quality of life and trismus-related symptoms up to 5 years post-radiotherapy for head and neck cancer. Support. Care Cancer 2023, 31, 699. [Google Scholar] [CrossRef]
- Khawaja, S.; Bavarian, R.; Abdul Rehman, S.; Hafeez, H. Head and Neck Cancer-Related Pain: A Descriptive Analysis of the Pain Phenotypes. J. Pain. Res. 2023, 16, 2919–2927. [Google Scholar] [CrossRef]
- Rojo, R.D.; Ren, J.L.; Lipe, D.N.; Badr, H.; Shete, S.; Hanna, E.Y.; Reyes-Gibby, C.C. Neuropathic pain prevalence and risk factors in head and neck cancer survivors. Head Neck 2022, 44, 2820–2833. [Google Scholar] [CrossRef] [PubMed]
- Siegel, S.; Schenk, T.; Brabant, G.; Scholl, R.C.; Buchfelder, M.; Kreitschmann-Andermahr, I. Not Simply a Structural Problem: Psychological Determinants of Headache in Patients with Tumors of the Sellar Region. Exp. Clin. Endocrinol. Diabetes 2022, 130, 693–700. [Google Scholar] [CrossRef]
- Vilarim, R.C.B.; Tavares, M.R.; de Siqueira, S.R.D.T.; Jales, S.M.D.C.P.; Formigoni, G.G.S.; Teixeira, M.J.; de Siqueira, J.T.T. Characteristics and prevalence of orofacial pain as an initial symptom of oral and oropharyngeal cancer and its impact on the patient’s functionality and quality of life. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 457–464. [Google Scholar] [CrossRef]
- Arranz-Martín, B.; Del-Castillo-Pardo-de-Vera, J.L.; Cebrián-Carretero, J.L.; Rouco-García, D.; Fernández-Oliva, C.; Gil-Martínez, A. Quality of life, craniomandibular function, and psychosocial factors related to pain and movement in patients with head and neck cancer. Support. Care Cancer 2024, 32, 334. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Chen, Y.; Zhu, Y.; Pan, D.; Rong, X.; Shen, Q.; Li, H.; Xu, Y.; Tang, Y. Radiation-induced Chronic Pain Plagues Head and Neck Cancer Survivors: A Cross-sectional Analysis From the Cohort in Radiotherapy-related Nervous System Complications. J. Pain 2024, 25, 104612. [Google Scholar] [CrossRef] [PubMed]
- Lou, D.I.; Dietrich, M.S.; Deng, J.; Murphy, B.A. Mechanisms of pain and their manifestations in head and neck cancer: Importance of classifying pain subtypes. Head Neck 2021, 43, 3720–3729. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, S.N.; Jamshed, A.; Hussain, R.T. Prevalence of pain in oral cancer: A retrospective study. Oral Dis. 2021, 27, 1806–1812. [Google Scholar] [CrossRef]
- Kouri, M.; Nicolatou Galitis, O.; Vadalouca, A.; Kouloulias, V.; Papadopoulou, E.; Vardas, E.; Kyrodimos, E.; Trichas, M.; Zygogianni, A.; Liakouli, Z.; et al. Oral mucositis-related neuropathic pain in head and neck cancer patients receiving radiotherapy or chemo-radiotherapy. A prospective study. J. BUON. 2021, 26, 2010–2018. [Google Scholar] [PubMed]
- Salwey, L.; L’Huillier, V.; Zaid, M.; Vené, Y.; Tavernier, L.; Mauvais, O. Neuropathic pain at diagnosis of head and neck squamous cell carcinoma. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Kallurkar, A.; Kulkarni, S.; Delfino, K.; Ferraro, D.; Rao, K. Characteristics of Chronic Pain among Head and Neck Cancer Patients Treated with Radiation Therapy: A Retrospective Study. Pain. Res. Manag. 2019, 2019, 9675654. [Google Scholar] [CrossRef] [PubMed]
- Buchakjian, M.R.; Davis, A.B.; Sciegienka, S.J.; Pagedar, N.A.; Sperry, S.M. Longitudinal Perioperative Pain Assessment in Head and Neck Cancer Surgery. Ann. Otol. Rhinol. Laryngol. 2017, 126, 646–653. [Google Scholar] [CrossRef]
- Van Abel, K.M.; Starkman, S.; O’Reilly, A.G.; Price, D.L. Craniofacial pain secondary to occult head and neck tumors. Otolaryngol. Head Neck Surg. 2014, 150, 813–817. [Google Scholar] [CrossRef]
- Lee, Y.L.; Ho, C.Y. Headache as the sole symptom of nasopharyngeal carcinoma and its clinical implications. Sci. World J. 2012, 2012, 143829. [Google Scholar] [CrossRef]
- Lam, D.K.; Schmidt, B.L. Orofacial pain onset predicts transition to head and neck cancer. Pain 2011, 152, 1206–1209. [Google Scholar] [CrossRef]
- Potter, J.; Higginson, I.J.; Scadding, J.W.; Quigley, C. Identifying neuropathic pain in patients with head and neck cancer: Use of the Leeds Assessment of Neuropathic Symptoms and Signs Scale. J. R. Soc. Med. 2003, 96, 379–383. [Google Scholar] [CrossRef]
- Vecht, C.J.; Hoff, A.M.; Kansen, P.J.; de Boer, M.F.; Bosch, D.A. Types and causes of pain in cancer of the head and neck. Cancer 1992, 70, 178–184. [Google Scholar] [CrossRef][Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Doo, A.R.; Shin, Y.S.; Yoo, S.; Park, J.K. Radiation-induced neuropathic pain successfully treated with systemic lidocaine administration. J. Pain. Res. 2018, 11, 545–548. [Google Scholar] [CrossRef]
- Glare, P.A.; Davies, P.S.; Finlay, E.; Gulati, A.; Lemanne, D.; Moryl, N.; Oeffinger, K.C.; Paice, J.A.; Stubblefield, M.D.; Syrjala, K.L. Pain in cancer survivors. J. Clin. Oncol. 2014, 32, 1739–1747. [Google Scholar] [CrossRef]
- Byrd, H.F.; Kohutek, Z.A. Painful Realities: Navigating the Complexities of Head and Neck Cancer Pain. Oral Dis. 2024; Epub ahead of print. [Google Scholar]
- Azzam, P.; Mroueh, M.; Francis, M.; Daher, A.A.; Zeidan, Y.H. Radiation-induced neuropathies in head and neck cancer: Prevention and treatment modalities. Ecancermedicalscience 2020, 14, 1133. [Google Scholar] [CrossRef]
- Fernández-Gualda, M.Á.; Postigo-Martin, P.; Fernandez-Gonzalez, M.; Martin-Martin, L.; Vargas-Arrabal, M.P.; Lozano-Lozano, M.; Fernández-Lao, C. Prevalence and characteristics of persistent pain among head and neck cancer survivors: A systematic review and meta-analysis. Pain Med. 2025, 28, pnaf051. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Calderon, J.; Flores-Cortes, M.; Morales-Asencio, J.M.; Luque-Suarez, A. Which Psychological Factors Are Involved in the Onset and/or Persistence of Musculoskeletal Pain? An Umbrella Review of Systematic Reviews and Meta-Analyses of Prospective Cohort Studies. Clin. J. Pain 2020, 36, 626–637. [Google Scholar] [CrossRef]
- Doan, L.V.; Yoon, J.; Chun, J.; Perez, R.; Wang, J. Pain associated with breast cancer: Etiologies and therapies. Front. Pain Res. 2023, 4, 1182488. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018, 38, 1–211. [CrossRef]
- Cuesta-Vargas, A.I.; Neblett, R.; Chiarotto, A.; Kregel, J.; Nijs, J.; van Wilgen, C.P.; Pitance, L.; Knezevic, A.; Gatchel, R.J.; Mayer, T.G.; et al. Dimensionality and Reliability of the Central Sensitization Inventory in a Pooled Multicountry Sample. J. Pain. 2018, 19, 317–329. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Wodehouse, T. Conditioned pain modulation-A comprehensive review. Neurophysiol. Clin. 2021, 51, 197–208. [Google Scholar] [CrossRef]
- Istenič, S.; Jerman, A.; Pušnik, L.; Stopar Pintarič, T.; Umek, N. Transnasal spread of bupivacaine into the pterygopalatine fossa following endoscopically assisted cotton swab placement: A cadaveric study. Reg. Anesth. Pain Med. 2025, 26, rapm-2025-106553. [Google Scholar] [CrossRef]
| Study | Type of Study | Specific HNC Diagnosis | Sample Size | Sex (F/M) | Oncologic Treatment Status |
|---|---|---|---|---|---|
| Yang et al., 2017 [19] | Non-RCT | OSCC | 124 | 21/103 | During treatment |
| Haumann et al., 2018 [20] | RCT | HNC with nociceptive pain | 82 | 32/50 | During treatment |
| Ouadghiri et al., 2024 [21] | Prospective observational study | Oral or oropharyngeal cancer | 17 | 6/11 | Post-treatment (4 years) |
| Sato et al., 2010 [22] | Cross-sectional study | OSCC | 113 | 31/82 | Pre-treatment |
| Ye et al., 2023 [23] | Prospective study | Oral cavity, oropharynx, or larynx | 25 | 6/19 | During treatment |
| Shinde et al., 2023 [24] | RCT | Oral cancer survivor with TMJD | 100 | 26/74 | Post-treatment |
| Schaller et al., 2021 [25] | Cross-sectional study | HNC with oral mucositis | 63 | 24/39 | During treatment |
| Saghafi et al., 2021 [26] | Retrospective | HNC in the tonsil and the base of the tongue | 217 | 57/160 | Post-treatment |
| Ortiz-Comino et al., 2019 [27] | Cross-sectional study | HNC with oral cavity, pharyngeal, laryngeal, salivary gland, or nasal cavity cancer | 30 | 10/20 | During treatment |
| Arantes et al., 2018 [28] | Case series | HNC and OFP | 22 | 4/18 | Post-treatment |
| Jiang et al., 2019 [29] | RCT | HNC with radiotherapy-related neuropathic pain | 128 | 51/77 | Post-treatment |
| Epstein et al., 2009 [30] | Prospective observational study | HNC with pain | 124 | 29/95 | During treatment |
| Chua et al., 1999 [31] | Retrospective study | HNC with pain | 40 | - | Post-treatment |
| Sarumpaet et al., 2023 [32] | Cross-sectional study | HNC detected through histopathology | 127 | 46/81 | During treatment |
| Aghajanzadeh et al., 2023 [33] | Prospective observational study | HNC | 194 | 38/146 | Pre-treatment |
| Khawaja et al., 2023 [34] | Cross-sectional study | HNC-related pain according to the International Headache Society | 100 | 32/68 | - |
| Rojo et al., 2022 [35] | Cross-sectional study | HNC survivors | 505 | 113/392 | Post-treatment |
| Siegel et al., 2022 [36] | Cross-sectional study | HNC with tumor in the sellar region | 112 | 59/53 | During treatment |
| Vilarim et al., 2022 [37] | Cross-sectional study | HNC | 74 | 15/59 | Pre-treatment |
| Arranz-Martin et al., 2024 [38] | Cross-sectional study | HNC | 78 | 26/52 | Pre-treatment, during treatment, and post-treatment |
| Zuo et al., 2024 [39] | Cross-sectional study | HNC with radiotherapy-related neuropathic pain | 1002 | 288/714 | Post-treatment |
| Lou et al., 2021 [40] | Prospective longitudinal study | HNC | 77 | 24/53 | Pre- and post-treatment |
| Khawaja et al., 2021 [41] | Retrospective study | Oral cancer (90.2% OSCC) | 1067 | 385/682 | Pre-treatment |
| Kouri et al., 2021 [42] | Prospective observational study | HNC | 26 | 6/20 | Post-treatment |
| Salwey et al., 2020 [43] | Prospective observational study | OSCC | 60 | 17/43 | Pre-treatment |
| Kallurcar et al., 2019 [44] | Retrospective study | HNC | 53 | 14/39 | Post-treatment |
| Buchakjian et al., 2017 [45] | Cross-sectional study | HNC | 27 | 7/20 | Pre-treatment |
| Van Abel et al., 2014 [46] | Retrospective study | HNC | 38 | 14/24 | Pre-treatment |
| Lee and Ho, 2012 [47] | Retrospective study | Nasopharyngeal carcinoma | 14 | 4/10 | Pre-treatment |
| Lam and Schmidt, 2011 [48] | Cross-sectional study | Oral cancer | 44 | 19/25 | Pre-treatment |
| Potter et al., 2003 [49] | Cross-sectional study | HNC | 25 | 11/14 | Post-treatment |
| Vecht et al., 1993 [50] | Cross-sectional study | HNC | 25 | 11/14 | Post-treatment |
| Study | Outcome Measures | Pain Characteristics/Phenotype | Location | Intensity | Frequency | Affected Activities in Relation to Pain | Others |
|---|---|---|---|---|---|---|---|
| Yang et al., 2017 [19] | UCSF-OCPQ (0–100 mm) | Spontaneous sharpness: 15.6 (12.85) Functional sharpness: 20.87 (13.03) Spontaneous aching: 33.33 (13.54) Sensitivity to touch: 44.87 (21.13) Functional restriction: 46.2 (16.2) | - | Spontaneous intensity: 29.53 (14.80) Functional intensity: 32.73 (16.34) | - | - | - |
| Haumann et al., 2018 [20] | NRS (0–10); HADS | - | - | 5.4 (1.9) | - | - | Anxiety: 52% Depression: 21% |
| Ouadghiri et al., 2024 [21] | VAS (0–100) | Two with neuropathic and spontaneous pain Only one with paresthesia | - | With pain: 2/17 Mean intensity: 4.5 | - | - | - |
| Sato et al., 2010 [22] | Location of pain | Spontaneous pain: 37% Function-related pain: 68% | Tongue: 34%; lower gingiva: 11%; buccal mucosa: 8%; floor of the mouth: 2%; upper gingiva: 4% | - | - | - | - |
| Ye et al., 2023 [23] | UCSF-OCPQ (0–10) | Pain in 88% All at least one NP descriptor, 54% two Burning: 21/22 Pins and needles: 17/22 Tingling: 2/22 Numbness, itching pinching, and shooting: 1/22 | With pain at the site of tumor: 88% With pain in other orofacial region: 36% | 5.4 | - | Chewing, (15), mouth/jaw opening (12), talking (13), eating (12), drinking (9) | Advanced clinical stage of tumor is related to pain severity |
| Shinde et al., 2023 [24] | VAS (0–100) | - | - | 4.34 | - | - | - |
| Schaller et al., 2021 [25] | NRS (0–10) | - | - | 3.0 | - | - | - |
| Saghafi et al., 2021 [26] | - | Symptom from the TMJ + pain upon palpation: 2/217 Symptom from the muscles + pain upon palpation: 12/217 Pain upon chewing: 4/217 Pain upon mouth opening: 2/217 | TMJ (23/217) | - | - | - | - |
| Ortiz-Comino et al., 2019 [27] | VAS (0–10) | - | Cervical | Cervical: 3.5 (3.3) TMJ: 2.4 (3.4) | - | - | - |
| Arantes et al., 2018 [28] | VAS (0–10); LANSS | Nociceptive: 45.4% Neuropathic: 18.1% Mixed: 36.3% Pain sensations: stinging (36.3%), throbbing (27.2%), pressure (18.1%), burning (18.1%), needle prick (9%), tingling (4.5%), painful cold sensation (4.5%) | - | Severe pain: 45.5% Moderate pain: 22.7% Mild pain: 31.8% | Continuous pain in 36.3% | - | - |
| Jiang et al., 2019 [29] | NRS; BPI; POMS | - | - | 6.4 | - | - | Pain interference and psychological distress associated with pain intensity |
| Epstein et al., 2009 [30] | McGill Pain Questionnaire Pain intensity (0–5) | Aching: 20.2%; boring: 5.6%; burning: 26.6%; drilling: 2.4%; flashing: 4.0%; freezing: 0.8%; hot: 9.3%; lancinating: 0.8%; pricking: 1.6%; beating: 3.2%; dull: 21.8%; hurting: 12.9%; pressing: 10.5%; sharp: 15.3%; rasping: 8.1%; pulsing: 7.3% | Head: 98/124 Arm: 3/124 Abdomen: 1 Back: 3 Missing/unknown: 19 | 1.51 (1.01) | - | - | Other affective and evaluative descriptors were included, such as tiring (25%) or exhausting (8.9%) |
| Chua et al., 1999 [31] | BPI; NRS | Somatic: 13 Neuropathic: 3 Somatic + neuropathic: 15 Myofascial: 6 Others: 3 | Frontal region: 17 Maxillofacial region: 50 Anterior neck: 13 Posterior neck: 10 Occipito-parietal region: 9 | 5.7 (2.5) | Continuous: 60% | - | Trigeminal nerve was the most frequently affected nerve (16) |
| Sarumpaet et al., 2023 [32] | VAS; BPI; HADS; LANSS | Neuropathic pain: 65.4% | - | Moderate pain: 53.5%; severe pain: 26%; mild pain: 20.5% | - | Overall, 91.3% experienced interference while eating, speaking, chewing and/or opening the mouth | Overall, 64.6% experienced depression |
| Aghajanzadeh et al., 2023 [33] | Likert Scale for pain intensity; EORTC-QLQ 30 | - | A total of 97 presented with facial pain | Mild pain: 55%; moderate: 33%; severe: 12% | - | - | Pain intensity was correlated with less mouth opening |
| Khawaja et al., 2023 [34] | NRS | Sharp: 29; pressing: 1; electric: 22; burning: 57; throbbing: 24; stinging: 19; dull ache: 86; stabbing: 22 Myofascial pain: 81; jaw bone pain disorder: 53; burning pain disorder: 40; neuropathic: 20; unknown: 16 | Unilateral: 65% | 5.9 (2.5) | Constant: 93% | - | - |
| Rojo et al., 2022 [35] | NRS; S-LANSS | Neuropathic pain: 13.7% | Head and oral cavity: 46.2% Neck and throat: 41.5% Shoulders: 24.6% Upper extremities: 26.2% Anterior chest: 1.5% Posterior chest: 10.8% Abdomen: 1.5% Lower back and pelvis: 24.6% Lower extremities: 24.6% | Mild: 31% Moderate: 52% Severe: 17% | - | - | Severe pain was more frequent in those with neuropathic pain |
| Siegel et al., 2022 [36] | PCS; EHI | Migraine without aura: 47%; migraine with aura: 27%; chronic daily headache: 29%; tension-type headache: 27%; cluster headache: 11%; medication induced headache: 4%; not classifiable: 18% | Frontal: 53%; holocranial: 14%; occipital: 6%; right side: 4%; various locations: 22% | - | . | - | Headache occurrence was associated with catastrophism |
| Vilarim et al., 2022 [37] | Verbal scale of pain; Standardized Evaluation of orofacial pain | Orofacial pain: 91.9% Jumping: 51.4%; burning: 45.9%; throbbing: 40.5% Toothache-like: 10.8%. | Mouth: 28.4%; tongue: 40.5%; throat: 16.2%; ear: 10.8%; palate: 8.1%; face: 8.1%; tooth: 4.1%; gingiva: 4.1%; neck: 2.7% | Mild: 16.2%; moderate: 29.7%; severe: 45.9% | Morning: 1.4%; afternoon: 6.8%; night: 6.8%; variable: 77% | Triggering factors: chewing: 16.2%; speaking: 1.4%; swallowing: 10.8% | Overall, 55.4% reported that pain woke up them during the night |
| Arranz-Martin et al., 2024 [38] | EORTC QLQ; PCS; NRS; TSK-TMD; CF-PDI-11 | - | - | Mild: 21.8%; moderate: 15,4%; severe: 3.8% | - | - | Kinesiofhobia was correlated with low ranges of mouth opening |
| Zuo et al., 2024 [39] | NRS; SAS; SDS; WHOQOL-BREF | Radiotherapy-induced chronic pain: 67.1% | - | Mild: 41.6%; moderate: 18.9%; severe: 11.3% | - | Insomnia: 22.5% | Radiation-induced chronic pain increased risk of anxiety, depression, sleep, and QOL |
| Lou et al., 2021 [40] | VHNSS; SF-MPQ; 2.4.4|Head and Neck Lymphedema and Fibrosis Symptom Inventory | Chronic pain joint (pain/arthritis, low back pain, neck pain, and facial/jaw pain): 40% Nociceptive pain from mouth and throat sores (largely due to mucositis) was most prevalent at end of treatment and resolved by 3–6 months: 54.5% | Widespread pain outside of the head and neck region: 36%; at 12 months: 40% | Moderate to severe (4–10): 31%; at 12 months: 40% | - | Mucosal and dental sensitivity on dental hygiene and eating: 39.7% at 3 months and 23% at 12 months | Patients with pain at 3–6 months post-treatment were likely to have transitioned to a chronic pain state |
| Khawaja et al., 2021 [41] | NRS | Chronicity of pain Pain <3 months: 228 (33.7%) Pain ≥3 months: 448 (66.3%) | Tongue: 55.3% Buccal mucosa: 22% Maxillary or mandibular alveolar ridges: 16.1% | Pain: 67.5% Moderate to severe (4–10): 42% | Intermittent: 31% | Mouth Opening: 10% Restricted tongue mobility with interference with mastication, speech, and swallowing 12.5% | - |
| Kouri et al., 2021 [42] | NRS; DN-4; EORTC | Neuropathic pain, descriptors: “burning” (34.62%), “electric shocks” (30.77%), and “pins and needles” (30.77%) | - | Overall, 65% developed moderate or severe pain (NRS ≥ 5) | - | - | Strong correlation between DN4 questionnaire scores and the intensity of oral mucositis |
| Salwey et al., 2020 [43] | NRS; DN-4; NPSI | NP+: 24 (40%) NP−: 36 (60%) | - | Mild: 20%; moderate to severe: 53% |
Pain/24 h: Permanent: 8 (33%); 8–12 h: 4 (17%); 4–7 h: 3 (12.5%); 1–3 h: 6 (25%); <1 h: 3 (12.5%). Episodes/24 h: 20: 1 (4%); 1–20: 6 (25%); 6–10: 7 (29%); 1–5: 6 (25%). Absent: 4 (17%). | - | - |
| Kallurcar et al., 2019 [44] | NRS | Chronic NP with descriptors of neuropathic pain, such as stinging or burning pain: 32 (60%) | Oral cavity: 24.5% Pharynx: 26.4% Eyes: 1.9% Nasal cavity/paranasal sinuses: 1.9% Odynophagia: 17% Headache: 5.7% Face: 9.4% Ear: 15.1% Neck: 15.1% | - | - | - | Significant association between chronic pain and number of pain sites |
| Buchakjian et al., 2017 [45] | S-LANSS; HADS; WPI/SS; BPI | NP+: 38% | Jaw: 62% Ears: 52% Tongue: 29% Other: 76% | With pain: 21/27 Mean intensity: 4 | - | - | Anxiety: 52% Depression: 30% + Fibromyalgia: 0% |
| Van Abel et al., 2014 [46] | - | Sharp: 33 (86.8%) Dull: 4 (10.5%) Burning: 1 (2.6%) | - | Mild: 2.6%; moderate: 52.6%; severe: 44.8% | Constant: 6 (15.8%) Intermittent: 32 (84.2%) | Dysphagia: 21% | - |
| Lee and Ho, 2012 [47] | Location and pain characteristics | Headache: 100% Dull: 3 Pulsating: 3 Explosive: 2 Pressing: 2 Tightening: 1 Debilitating: 1 Pressure-like: 1 Nonspecific: 1 | Temporal: 6 Parietal: 3 Frontal: 1 Diffuse: 4 | - | - | - | Overall, 71% had improved headache during or after treatment |
| Lam and Schmidt, 2011 [48] | UCSF-OCPQ | - | - | Moderate to severe (>30 mm): 84% | - | - | - |
| Potter et al., 2003 [49] | LANSS | NP+: 56% | - | - | - | Swallowing warm or cold drinks: 16% | - |
| Vecht et al., 1993 [50] | NRS | Nociceptive pain: 9 (36%) Nociceptive nerve pain: 8 (32%) Referred: 2 (8%) Neuropathic pain: 6 (24%) | - | All patients > 50; 80% patients > 70 | Constant: 19 (76%) Intermittent: 6 (24%) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anarte-Lazo, E.; Bernal-Utrera, C. A Scoping Review of Clinical Features and Mechanisms of Orofacial Pain and Headache in Patients with Head and Neck Cancer. J. Clin. Med. 2025, 14, 5722. https://doi.org/10.3390/jcm14165722
Anarte-Lazo E, Bernal-Utrera C. A Scoping Review of Clinical Features and Mechanisms of Orofacial Pain and Headache in Patients with Head and Neck Cancer. Journal of Clinical Medicine. 2025; 14(16):5722. https://doi.org/10.3390/jcm14165722
Chicago/Turabian StyleAnarte-Lazo, Ernesto, and Carlos Bernal-Utrera. 2025. "A Scoping Review of Clinical Features and Mechanisms of Orofacial Pain and Headache in Patients with Head and Neck Cancer" Journal of Clinical Medicine 14, no. 16: 5722. https://doi.org/10.3390/jcm14165722
APA StyleAnarte-Lazo, E., & Bernal-Utrera, C. (2025). A Scoping Review of Clinical Features and Mechanisms of Orofacial Pain and Headache in Patients with Head and Neck Cancer. Journal of Clinical Medicine, 14(16), 5722. https://doi.org/10.3390/jcm14165722







