Evaluation of Different Initial Doses of Envarsus in De Novo Kidney Transplant Recipients
Abstract
1. Introduction
2. Methods
Outcome Variables: The Analysis Considered Three Outcome Domains
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hart, A.; Smith, J.M.; Skeans, M.A.; Gustafson, S.K.; Stewart, D.E.; Cherikh, W.S.; Wainright, J.L.; Boyle, G.; Snyder, J.J.; Kasiske, B.L.; et al. Kidney. Am. J. Transplant. 2016, 16, 11–46. [Google Scholar]
- Alloway, R.; Steinberg, S.; Khalil, K.; Gourishankar, S.; Miller, J.; Norman, D.; Hariharan, S.; Pirsch, J.; Matas, A.; Zaltzman, J.; et al. Conversion of stable kidney transplant recipients from a twice daily Prograf-based regimen to a once daily modified release tacrolimus-based regimen. Transplant. Proc. 2005, 37, 867–870. [Google Scholar] [CrossRef]
- Florman, S.; Alloway, R.; Kalayoglu, M.; Lake, K.; Bak, T.; Klein, A.; Klintmalm, G.; Busque, S.; Brandenhagen, D.; Lake, J.; et al. Conversion of stable liver transplant recipients from a twice-daily Prograf-based regimen to a once-daily modified release tacrolimus-based regimen. Transplant. Proc. 2005, 37, 1211–1213. [Google Scholar] [CrossRef]
- Gaber, A.O.; Alloway, R.R.; Bodziak, K.; Kaplan, B.; Bunnapradist, S. Conversion from Twice-Daily Tacrolimus Capsules to Once-Daily Extended-Release Tacrolimus (LCPT). Transplant. J. 2013, 96, 191–197. [Google Scholar] [CrossRef]
- Webster, A.C.; Taylor, R.R.; Chapman, J.R.; Craig, J.C. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst. Rev. 2005, CD003961. [Google Scholar] [CrossRef]
- Astellas Pharma Canada, Inc. Prograf® (Tacrolimus) Product Monograph Including Patient Medication Information; Astellas Pharma Canada, Inc.: Markham, ON, Canada, 2022; 95p, Available online: https://www.astellas.com/ca/system/files/pdf/2022-12-01_prograf_pm_-_english_0.pdf (accessed on 7 August 2025).
- Sellarés, J.; de Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the Causes of Kidney Transplant Failure: The Dominant Role of Antibody-Mediated Rejection and Nonadherence. Am. J. Transplant. 2012, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Eisen, S.A.; Miller, D.K.; Woodward, R.S.; Spitznagel, E.; Przybeck, T.R. The effect of prescribed daily dose frequency on patient medication compliance. Arch. Intern. Med. 1990, 150, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Claxton, A.J.; Cramer, J.; Pierce, C. A systematic review of the associations between dose regimens and medication compliance. Clin. Ther. 2001, 23, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.D.; Schoenfeld, P.; Kaulback, K.; Dubinsky, M.C. Effect of medication dosing frequency on adherence in chronic diseases. Am. J. Manag. Care 2009, 15, e22–e33. [Google Scholar] [PubMed]
- Kamar, N.; Cassuto, E.; Piotti, G.; Govoni, M.; Ciurlia, G.; Geraci, S.; Poli, G.; Nicolini, G.; Mariat, C.; Essig, M.; et al. Pharmacokinetics of Prolonged-Release Once-Daily Formulations of Tacrolimus in De Novo Kidney Transplant Recipients: A Randomized, Parallel-Group, Open-Label, Multicenter Study. Adv. Ther. 2019, 36, 462–477. [Google Scholar] [CrossRef]
- Laetitia, A.; Bernhard, B.; Juergen, L.K.; Maciej, G.; Ondrej, V.; Nassim, K. OSAKA Trial. A Randomized, Controlled Trial Comparing Tacrolimus QD and BD in Kidney Transplantation. Transplant. J. 2013, 96, 897–903. [Google Scholar] [CrossRef]
- Baraldo, M. Meltdose Tacrolimus Pharmacokinetics. Transplant. Proc. 2016, 48, 420–423. [Google Scholar] [CrossRef]
- Franco, A.; Más-Serrano, P.; Balibrea, N.; Rodriguez, D.; Javaloyes, A.; Díaz, M.; Gascón, I.; Ramon-Lopez, A.; Perez-Contreras, J.; Selva, J.; et al. Envarsus, a novelty for transplant nephrologists: Observational retrospective study. Nefrología 2019, 39, 506–512, (In English & Spanish). [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Nigro, V.; Weinberg, J.; Woodle, E.S.; Alloway, R.R. A Steady-State Head-to-Head Pharmacokinetic Comparison of All FK-506 (Tacrolimus) Formulations (ASTCOFF): An Open-Label, Prospective, Randomized, Two-Arm, Three-Period Crossover Study. Am. J. Transplant. 2017, 17, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Fructuoso, A.; Ruiz, J.C.; Franco, A.; Diekmann, F.; Redondo, D.; Calviño, J.; Serra, N.; Aladrén, M.J.; Cigarrán, S.; Manonelles, A.; et al. Effectiveness and safety of the conversion to MeltDose® extended-release tacrolimus from other formulations of tacrolimus in stable kidney transplant patients: A retrospective study. Clin. Transplant. 2019, 34, e13767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Budde, K.; Bunnapradist, S.; Grinyo, J.M.; Ciechanowski, K.; Denny, J.E.; Silva, H.T.; Rostaing, L. Novel once-daily extended-release tacrolimus (LCPT) versus twice-daily tacrolimus in de novo kidney transplants: One-year results of Phase III, double-blind, randomized trial. Am. J. Transplant. 2014, 14, 2796–2806. [Google Scholar] [CrossRef]
- Fernandez Rivera, C.; Rodríguez, M.C.; Poveda, J.L.; Pascual, J.; Crespo, M.; Gomez, G.; Cabello Pelegrin, S.; Paul, J.; Lauzurica, R.; Perez Mir, M.; et al. Bioavailability of once-daily tacrolimus formulations used in clinical practice in the management of De Novo kidney transplant recipients: The better study. Clin. Transplant. 2021, 36, e14550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Breslin, N.T.; Hedvat, J.; Salerno, D.M.; Jandovitz, N.; Patel, C.; Lee, S.; Lange, N.W. Comparing weight-based dosing of tacrolimus XR in obese and non-obese renal transplant recipients. Clin. Transplant. 2021, 36, e14529. [Google Scholar] [CrossRef]
- Pascual, J.; Berger, S.P.; Witzke, O.; Tedesco, H.; Mulgaonkar, S.; Qazi, Y.; Chadban, S.; Oppenheimer, F.; Sommerer, C.; Oberbauer, R.; et al. Everolimus with Reduced Calcineurin Inhibitor Exposure in Renal Transplantation. J. Am. Soc. Nephrol. 2018, 29, 1979–1991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rummo, O.O.; Carmellini, M.; Rostaing, L.; Oberbauer, R.; Christiaans, M.H.L.; Mousson, C.; Langer, R.M.; Citterio, F.; Charpentier, B.; Brown, M.; et al. ADHERE: Randomized controlled trial comparing renal function in de novo kidney transplant recipients receiving prolonged-release tacrolimus plus mycophenolate mofetil or sirolimus. Transpl. Int. 2016, 30, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Bulatova, N.; Yousef, A.-M.; Al-Khayyat, G.; Qosa, H. Adverse effects of tacrolimus in renal transplant patients from living donors. Curr. Drug Saf. 2011, 6, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Brunet, M.; van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Nigro, V.; Weinberg, J.; Woodle, E.S. Conversion from twice daily tacrolimus capsules to once daily extended-release tacrolimus (LCPT): A phase 3 study of stable renal transplant recipients. Am. J. Transplant. 2017, 17, 814–822. [Google Scholar]
- Woillard, J.-B.; Monchaud, C.; Saint-Marcoux, F.; Labriffe, M.; Marquet, P. Can the area under the curve/trough level ratio be used to optimize tacrolimus individual dose adjustment? Transplantation 2023, 107, e27–e35. [Google Scholar] [CrossRef]
- Trofe-Clark, J.; Brennan, D.C.; West-Thielke, P.; Milone, M.C.; Lim, M.A.; Neubauer, R.; Nigro, V.; Bloom, R.D. Results of ASERTAA, a Randomized Prospective Crossover Pharmacogenetic Study of Immediate-Release Versus Extended-Release Tacrolimus in African American Kidney Transplant Recipients. Am. J. Kidney Dis. 2018, 71, 315–326. [Google Scholar] [CrossRef]
- Rostaing, L.; Bunnapradist, S.; Grinyó, J.M.; Ciechanowski, K.; Denny, J.E.; Silva, H.T.; Budde, K.; Kulkarni, S.; Hricik, D.; Bresnahan, B.A.; et al. Novel Once-Daily Extended-Release Tacrolimus Versus Twice-Daily Tacrolimus in De Novo Kidney Transplant Recipients: Two-Year Results of Phase 3, Double-Blind, Randomized Trial. Am. J. Kidney Dis. 2016, 67, 648–659. [Google Scholar] [CrossRef]
- Wlodarczyk, Z.; Squifflet, J.-P.; Ostrowski, M.; Rigotti, P.; Stefoni, S.; Citterio, F.; Vanrenterghem, Y.; Krämer, B.K.; Abramowicz, D.; Oppenheimer, F.; et al. Pharmacokinetics for once- versus twice-daily tacrolimus formulations in de novo kidney transplantation: A randomized, open-label trial. Am. J. Transplant. 2009, 9, 2505–2513. [Google Scholar] [CrossRef]
- Bunnapradist, S.; Ciechanowski, K.; West-Thielke, P.; Mulgaonkar, S.; Rostaing, L.; Vasudev, B.; Budde, K. Conversion from twice-daily tacrolimus to once-daily extended release tacrolimus (LCPT): The phase III randomized MELT trial. Am. J. Transplant. 2012, 13, 760–769. [Google Scholar] [CrossRef]
- Dirks, N.L.; Huth, B.; Yates, C.R.; Meibohm, B. Pharmacokinetics of immunosuppressants: A perspective on ethnic differences. Int. J. Clin. Pharmacol. Ther. 2004, 42, 701–718. [Google Scholar] [CrossRef]
- Vidal-Alabró, A.; Colom, H.; Fontova, P.; Cerezo, G.; Melilli, E.; Montero, N.; Coloma, A.; Manonelles, A.; Favà, A.; Cruzado, J.M.; et al. Tools for a personalized tacrolimus dose adjustment in the follow-up of renal transplant recipients. Metabolizing phenotype according to CYP3A genetic polymorphisms versus concentration-dose ratio. Nefrología 2024, 44, 204–216. [Google Scholar] [CrossRef] [PubMed]
| Variable | Group 1 (n = 39) [0.15 mg/kg] | Group 2 (n = 43) [0.12 mg/kg] | Group 3 (n = 42) [0.10 mg/kg] | Group 4 (n = 43) [0.08 mg/kg] | p |
|---|---|---|---|---|---|
| Sex (men) (n (%)) | 19 (48.7) | 29 (67.4) | 32 (76.2) | 28 (65.1) | 0.074 |
| Body weight (kg) | 66.0 (58.0–80.0) | 68.0 (57.0–82.0) | 72.5 (65.0–79.5) | 73.0 (65.0–80.0) | 0.302 |
| Age (yrs) | 62.0 (54.0–74.0) | 67.0 (58.0–74.0) | 66.0 (58.0–71.0) | 59.0 (50.0–65.0) | 0.016 |
| Donor age (yrs) | 53.2 (50.4–56.1) | 54.6 (51.6–57.1) | 52.6 (50.1–53.8) | 53.7 (50.7–56.8) | 0.687 |
| Cold ischemia (h) | 15.8 (12.4–18.3) | 15.4 (11.7–17.3) | 16.4 (12.4–17.8) | 16.1 (11.9–19.7) | 0.636 |
| PRAs > 50 (n (%)) | 3 (7.7) | 4 (9.3) | 3 (7.1) | 3 (7.0) | 0.980 |
| HLA mismatches (n (%)) | 2 (5.1) | 3 (7.0) | 2 (4.8) | 2 (4.7) | 0.960 |
| Delayed graft function (n (%)) | 5 (12.8) | 5 (11.8) | 8 (19.0) | 5 (11.6) | 0.720 |
| Delayed tacrolimus start (n (%)) | 23 (59.0) | 19 (44.2) | 24 (57.1) | 18 (41.9) | 0.281 |
| Delayed tacrolimus start (days) | 4 (3–6) | 3 (2–9) | 4 (2–11) | 3 (2–7) | 0.431 |
| Dose (mg) | 10.0 (9.0–12.0) | 8.0 (6.8–10.0) | 7.00 (6.0–8.0) | 6.0 (5.0–6.5) | <0.001 |
| Dose (mg/kg) | 0.15 (0.14–0.15) | 0.12 (0.12–0.12) | 0.09 (0.10–0.10) | 0.08 (0.08–0.08) | <0.001 |
| Variable | Group 1 (n = 39) [0.15 mg/kg] | Group 2 (n = 43) [0.12 mg/kg] | Group 3 (n = 42) [0.10 mg/kg] | Group 4 (n = 43) [0.08 mg/kg] | p |
|---|---|---|---|---|---|
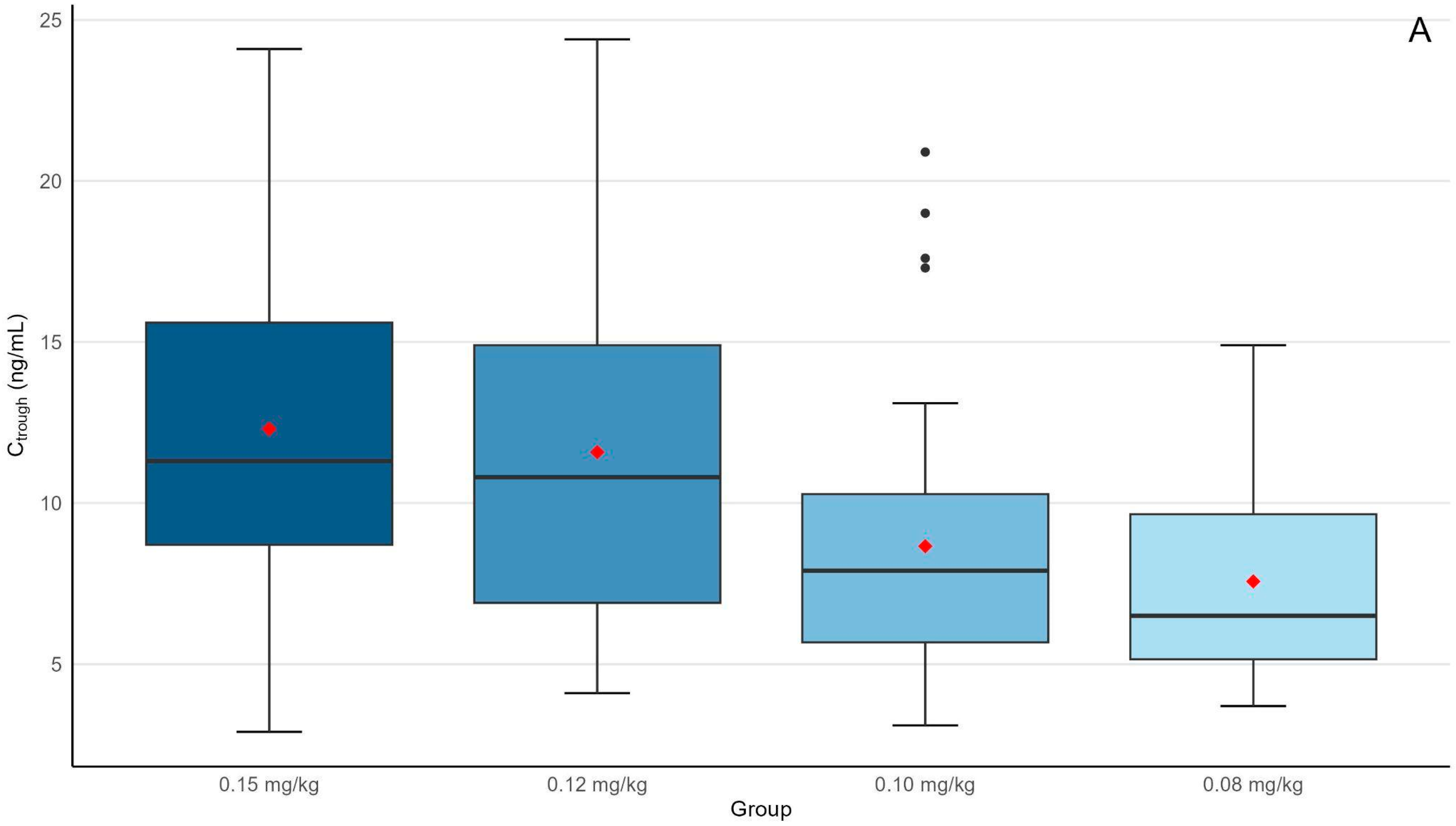
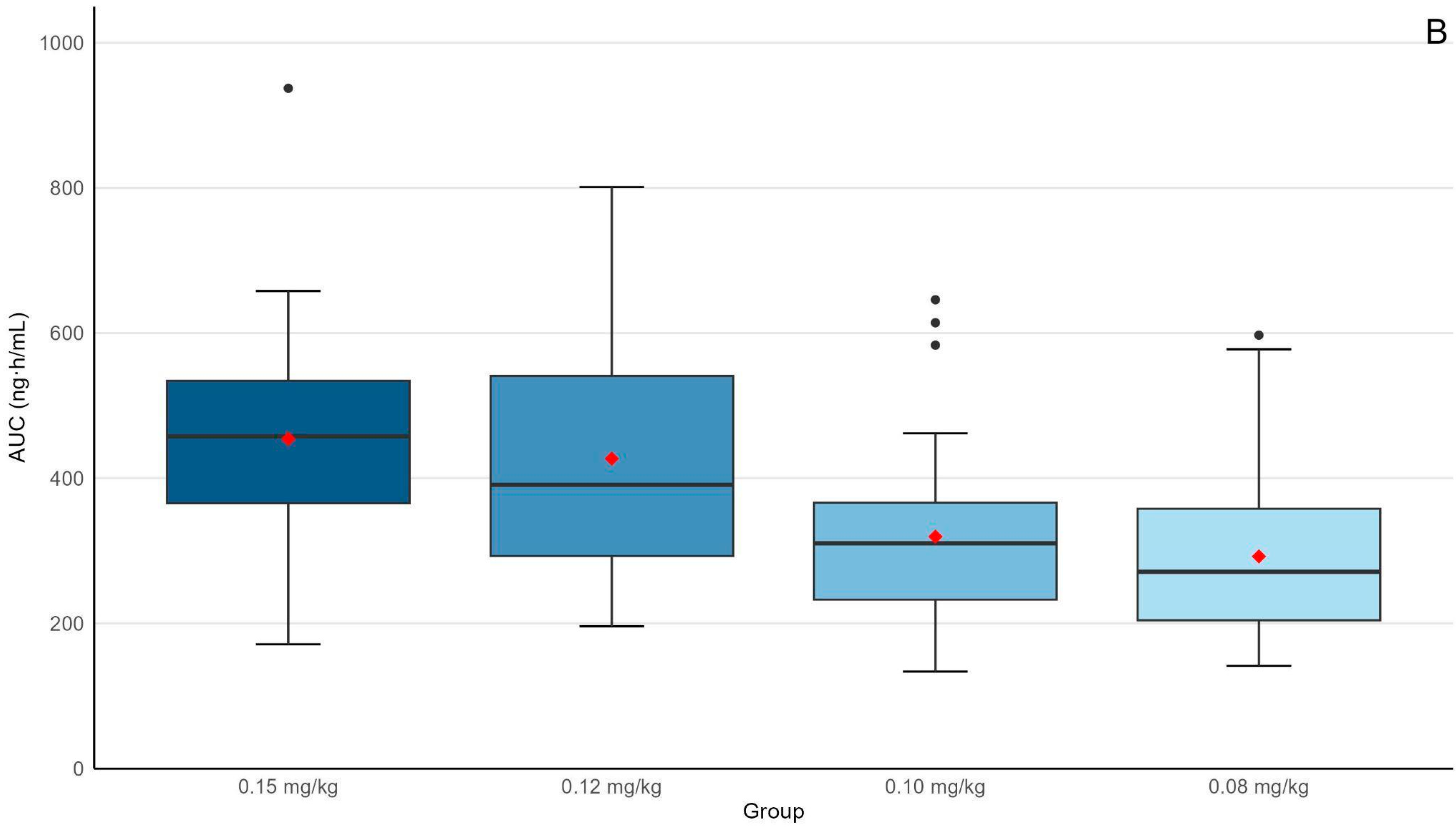
| Ctrough (ng/mL) | 11.3 (8.7–15.6) | 10.8 (6.9–14.9) | 7.9 (5.6–10.3) * | 6.5 (5.2–9.7) **,‡ | <0.001 |
| C/D ratio (ng/mL/mg) | 1.14 (0.85–1.45) | 1.27 (1.06–1.74) | 1.07 (0.88–1.49) | 1.11 (0.95–1.60) | 0.277 |
| AUC0–24h (ng × h/mL) | 458 (366–534) | 3901 (293–541) | 310 (223–366) * | 271 (204–358) **,‡ | <0.001 |
| AUC0–24h/D ratio (ng × h/mL/mg) | 42.4 (36.0–55.2) | 48.9 (40.0–63.8) | 43.0 (34.0–57.3) | 47.2 (35.5–58.6) | 0.169 |
| Cr (mg/dL, 3 m) | 1.38 (1.14–1.78) | 1.67 (1.40–2.24) | 1.72 (1.20–2.15) | 1.48 (1.25–1.77) | 0.115 |
| GFR CKD-EPI (ml/min, 3 m) | 48.6 (35.2–59.1) | 43.2 (35.1–50.7) | 44.0 (36.4–51.9) | 44.4 (38.9–50.7) | 0.329 |
| Group 1 (n = 39) [0.15 mg/kg] | Group 2 (n = 43) [0.12 mg/kg] | Group 3 (n = 42) [0.10 mg/kg] | Group 4 (n = 43) [0.08 mg/kg] | p * | |
|---|---|---|---|---|---|
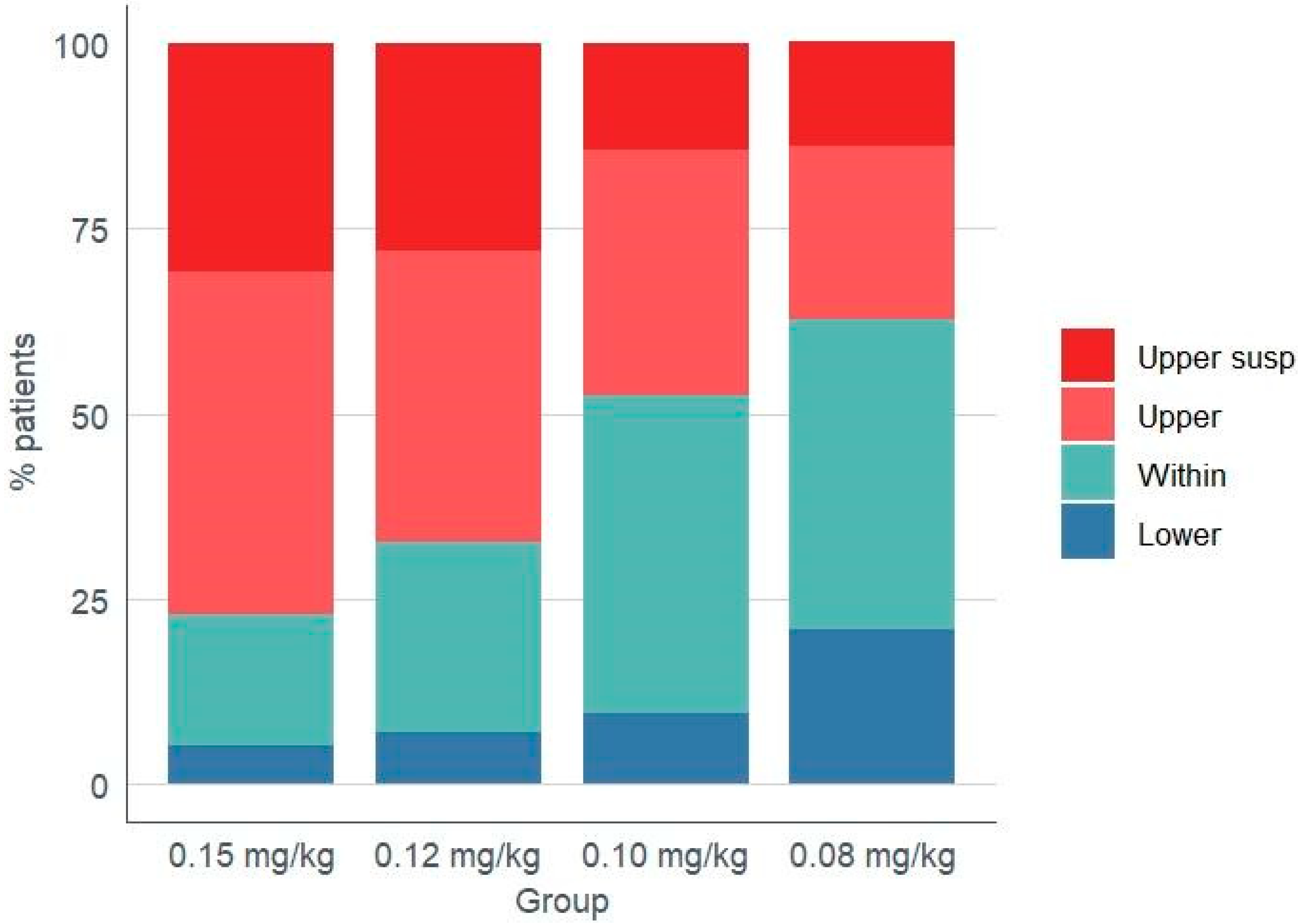
| Lower | 5.1 (2) | 7.0 (3) | 9.5 (4) | 20.9 (9) | 0.085 |
| Within | 17.9 (7) | 25.6 (11) | 42.9 (18) | 41.9 (18) | 0.037 |
| Upper (Total) | 76.9 (30) | 67.4 (29) | 47.6 (20) | 37.2 (16) † | 0.001 |
| Upper (Skipped) ** | 30.8 (12) | 27.9 (12) | 14.3 (6) | 14.0 (6) | 0.124 |
| Variable | Group 1 (n = 39) [0.15 mg/kg] | Group 2 (n = 43) [0.12 mg/kg] | Group 3 (n = 42) [0.10 mg/kg] | Group 4 (n = 43) [0.08 mg/kg] | p * |
|---|---|---|---|---|---|
| Nephrotoxicity | 4 (10.3) | 8 (18.6) | 6 (14.3) | 8 (18.6) | ns |
| Neurotoxicity | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ns |
| CMV infection | 5 (12.8) | 4 (9.3) | 4 (9.5) | 3 (7.0) | ns |
| BK infection | 3 (7.7) | 4 (9.3) | 4 (9.5) | 4 (9.3) | ns |
| Admission for bacterial infection | 4 (10.3) | 3 (7.0) | 4 (9.5) | 3 (7.0) | ns |
| Tacrolimus withdrawal | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Más-Serrano, P.; Franco, A.; Beltrá-Picó, I.; Díaz-González, M.; Colomer, C.; Gascón, I.; de la Cruz, E.; Pérez-Contreras, J.; Nalda-Molina, R.; Ramón-López, A. Evaluation of Different Initial Doses of Envarsus in De Novo Kidney Transplant Recipients. J. Clin. Med. 2025, 14, 5687. https://doi.org/10.3390/jcm14165687
Más-Serrano P, Franco A, Beltrá-Picó I, Díaz-González M, Colomer C, Gascón I, de la Cruz E, Pérez-Contreras J, Nalda-Molina R, Ramón-López A. Evaluation of Different Initial Doses of Envarsus in De Novo Kidney Transplant Recipients. Journal of Clinical Medicine. 2025; 14(16):5687. https://doi.org/10.3390/jcm14165687
Chicago/Turabian StyleMás-Serrano, Patricio, Antonio Franco, Iván Beltrá-Picó, Marcos Díaz-González, Claudia Colomer, Isabel Gascón, Elena de la Cruz, Javier Pérez-Contreras, Ricardo Nalda-Molina, and Amelia Ramón-López. 2025. "Evaluation of Different Initial Doses of Envarsus in De Novo Kidney Transplant Recipients" Journal of Clinical Medicine 14, no. 16: 5687. https://doi.org/10.3390/jcm14165687
APA StyleMás-Serrano, P., Franco, A., Beltrá-Picó, I., Díaz-González, M., Colomer, C., Gascón, I., de la Cruz, E., Pérez-Contreras, J., Nalda-Molina, R., & Ramón-López, A. (2025). Evaluation of Different Initial Doses of Envarsus in De Novo Kidney Transplant Recipients. Journal of Clinical Medicine, 14(16), 5687. https://doi.org/10.3390/jcm14165687







