Neuroanatomical and Functional Correlates in Bipolar Disorder (BD): A Narrative Review
Abstract
1. Introduction
2. Study Objectives
3. Descriptive, Clinical and Diagnostic Elements of BD

4. The Main Neuroanatomical and Functional Correlates of BD
4.1. The Role of Neuroimaging Research in Bipolar Disorder
4.2. Specific Areas of Interest
5. Clinical Implications
6. Study Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mullins, N.; Forstner, A.J.; O’Connell, K.S.; Coombes, B.; Coleman, J.R.I.; Qiao, Z.; Als, T.D.; Bigdeli, T.B.; Børte, S.; Bryois, J.; et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 2021, 53, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Craddock, N.; Sklar, P. Genetics of bipolar disorder. Lancet 2013, 381, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Muneer, A. The Neurobiology of Bipolar Disorder: An Integrated Approach. Chonnam Med. J. 2016, 52, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Alloy, L.B.; Nusslock, R.; Boland, E.M. The development and course of bipolar spectrum disorders: An integrated reward and circardian rhythm dysregualtion model. Annu. Rev. Clin. Psychol. 2015, 11, 213–250. [Google Scholar] [CrossRef]
- Ashock, A.; Marques, T.R.; Jauhar, S.; Nour, M.M.; Goodwin, G.M.; Young, A.H.; Howes, O.D. The dopamine hypothesis of bipolar affective disorder: The state of the art and implications for treatement. Mol. Psychiatry 2017, 22, 666–679. [Google Scholar] [CrossRef]
- Benedetti, F.; Aggio, V.; Pratesi, M.L.; Greco, G.; Furlan, R. Neuroinflammation in Bipolar Depression. Front. Psychiatry 2020, 26, 71. [Google Scholar] [CrossRef]
- Haarman, B.C.B.; Riemersma-Van der Lek, R.F.; de Groot, J.C.; Ruhé, H.G.E.; Klein, H.C.; Zandstra, T.E.; Burger, H.; Schoevers, R.A.; de Vries, E.F.; Drexhage, H.A.; et al. Neuroinflammation in bipolar disorder—A [11C]-(R)-PK11195 positron emission tomography study. Brain Behav. Immun. 2014, 40, 219–225. [Google Scholar] [CrossRef]
- Dong, D.; Wang, Y.; Chang, X.; Chen, X.; Chang, X.; Luo, C.; Yao, D. Common and diagnosis-specific fractional anisotropy of white matter in schizophrenia, bipolar disorder, and major depressive disorder: Evidence from comparative voxel-based meta-analysis. Schizophr. Res. 2018, 193, 456–458. [Google Scholar] [CrossRef]
- Chepenik, L.G.; Wang, F.; Spencer, L.; Spann, M.; Kalmar, J.H.; Womer, F.; Edmiston, E.K.; Pittman, B.; Blumberg, H.P. Structure-function associations in hippocampus in bipolar disorder. Biol. Psychol. 2012, 90, 18–22. [Google Scholar] [CrossRef]
- O’Connell, K.S.; Koromina, M.; van der Veen, T.; Boltz, T.; David, F.S.; Yang, J.M.K.; Lin, K.-H.; Wang, X.; Coleman, J.R.I.; Mitchell, B.L.; et al. Genomics yields biological and phenotypic insights into bipolar disorder. Nature 2025, 639, 968–975. [Google Scholar] [CrossRef]
- Cotrena, C.; Branco, L.D.; Kochhann, R.; Shansis, F.M.; Fonseca, R.P. Quality of life, functioning and cognition in bipolar disorder and major depression: A latent profile analysis. Psychiatry Res. 2016, 241, 289–296. [Google Scholar] [CrossRef]
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Castaldelli-Maia, J.M. Analysis of global prevalence of mental and substance use disorders within countries: Focus on sociodemographic characteristics and income levels. Int. Rev. Psychiatry 2022, 34, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Plans, L.; Barrot, C.; Nieto, E.; Rios, J.; Schulze, T.G.; Papiol, S.; Mitjans, M.; Vieta, E.; Benabarre, A. Association between completed suicide and bipolar disorder: A systematic review of the literature. J. Affect. Disord. 2019, 1, 111–122. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Trad. It.: Washington, DC, USA, 2022. [Google Scholar]
- Machado-Vieira, R.; Luckenbaugh, D.A.; Ballard, E.D.; Henter, I.D.; Tohen, M.; Suppes, T.; Zarate, C.A., Jr. Increased Activity or Energy as a Primary Criterion for the Diagnosis of Bipolar Mania in DSM-5: Findings From the STEP-BD Study. Am. J. Psychiatry 2017, 174, 70–76. [Google Scholar] [CrossRef]
- Baldassano, C.F.; Marangell, L.B.; Gyulai, L.; Ghaemi, S.N.; Joffe, H.; Kim, D.R.; Sagduyu, K.; Truman, C.J.; Wisniewski, S.R.; Sachs, G.S.; et al. Gender differences in bipolar disorder: Retrospective data from the first 500 STEP-BD participants. Bipolar Disord. 2005, 7, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S. Bipolar disorder in the International Classification of Diseases-Eleventh version: A review of the changes, their basis, and usefulness. World J. Psychiatry 2022, 12, 1335–1355. [Google Scholar] [CrossRef]
- Revadigar, N.; Gupta, V. Substance-Induced Mood Disorders. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555887/ (accessed on 7 June 2025). [PubMed]
- Strakowski, S.M.; Adler, C.M.; Almeida, J.; Altshuler, L.L.; Blumberg, H.P.; Chang, K.D.; DelBello, M.P.; Frangou, S.; McIntosh, A.; Phillips, M.L.; et al. The functional neuroanatomy of bipolar disorder: A consensus model. Bipolar Disord. 2012, 14, 313–325. [Google Scholar] [CrossRef]
- Hafeman, D.M.; Chang, K.D.; Garrett, A.S.; Sanders, E.M.; Phillips, M.L. Effects of medication on neuroimaging findings in bipolar disorder: An updated review. Bipolar Disord. 2012, 14, 375–410. [Google Scholar] [CrossRef]
- Maletic, V.; Raison, C. Integrated neurobiology of bipolar disorder. Front. Psychiatry 2014, 5, 98. [Google Scholar] [CrossRef]
- Wollenhaupt-Aguiar, B.; Kapczinski, F.; Pfaffenseller, B. Biological Pathways Associated with Neuroprogression in Bipolar Disorder. Brain Sci. 2021, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; DelBello, M.P.; McNamara, R.K.; Strakowski, S.M.; Adler, C.M. Neuroprogression in bipolar disorder. Bipolar Disord. 2012, 14, 356–374. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Passos, I.C.; Mwangi, B.; Amaral-Silva, H.; Tannous, J.; Wu, M.J.; Zunta-Soares, G.B.; Soares, J.C. Hippocampal subfield volumes in mood disorders. Mol. Psychiatry 2017, 22, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, B.; Wu, M.J.; Cao, B.; Passos, I.C.; Lavagnino, L.; Keser, Z.; Zunta-Soares, G.B.; Hasan, K.M.; Kapczinski, F.; Soares, J.C. Individualized Prediction and Clinical Staging of Bipolar Disorders using Neuroanatomical Biomarkers. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2016, 1, 186–194. [Google Scholar] [CrossRef]
- Bearden, C.E.; Hoffman, K.M.; Cannon, T.D. The neuropsychology and neuroanatomy of bipolar affective disorder: A critical review. Bipolar Disord. 2001, 3, 106–150. [Google Scholar] [CrossRef]
- Di Vincenzo, M.; Sampogna, G.; Della Rocca, B.; Brandi, C.; Mancuso, E.; Landolfi, L.; Volpicelli, A.; Di Cerbo, A.; Fiorillo, A.; Luciano, M. What influences psychological functioning in patients with mood disorders? The role of clinical, sociodemographic, and temperamental characteristics in a naturalistic study. Ann. Gen. Psychiatry 2022, 21, 51. [Google Scholar] [CrossRef]
- Nakamura, S. Integrated pathophysiology of schizophrenia, major depression, and bipolar disorder as monoamine axon disorder. Front. Biosci. (Sch. Ed.) 2022, 14, 4. [Google Scholar] [CrossRef]
- Mondragón-Maya, A.; Flores-Medina, Y.; Silva-Pereyra, J.; Ramos-Mastache, D.; Yáñez-Téllez, G.; Escamilla-Orozco, R.; Saracco-Álvarez, R. Neurocognition in Bipolar and Depressive Schizoaffective Disorder: A Comparison with Schizophrenia. Neuropsychobiology 2021, 80, 45–51. [Google Scholar] [CrossRef]
- Weathers, J.; Lippard, E.T.C.; Spencer, L.; Pittman, B.; Wang, F.; Blumberg, H.P. Longitudinal Diffusion Tensor Imaging Study of Adolescents and Young Adults With Bipolar Disorder. J. Am. Acad. Child. Adolesc. Psychiatry 2018, 57, 111–117. [Google Scholar] [CrossRef]
- Li, W.; Lei, D.; Tallman, M.J.; Welge, J.A.; Blom, T.J.; Fleck, D.E.; Klein, C.C.; Adler, C.M.; Patino, L.R.; Strawn, J.R.; et al. Morphological abnormalities in youth with bipolar disorder and their relationship to clinical characteristics. J. Affect. Disord. 2023, 338, 312–320. [Google Scholar] [CrossRef]
- Phillips, M.L.; Ladouceur, C.D.; Drevets, W.C. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry 2008, 13, 829, 833–857. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Nguyen, L.; Hu, R.; Stavish, C.M.; Leibenluft, E.; Linke, J.O. The uncinate fasciculus in individuals with and at risk for bipolar disorder: A meta-analysis. J. Affect. Disord. 2022, 297, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.F.; Bracher-Smith, M.; Tansey, K.E.; Harrison, J.R.; Parker, G.D.; Caseras, X. Fractional anisotropy of the uncinate fasciculus and cingulum in bipolar disorder type I, type II, unaffected siblings and healthy controls. Br. J. Psychiatry 2018, 213, 548–554. [Google Scholar] [CrossRef]
- Villa, L.M.; Colic, L.; Kim, J.A.; Sankar, A.; Goldman, D.A.; Lessing, B.; Pittman, B.; Alexopoulos, G.S.; van Dyck, C.H.; Blumberg, H.P. Aging of the brain in bipolar disorder: Illness- and onset-related effects in cortical thickness and subcortical gray matter volume. J. Affect. Disord. 2023, 323, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Roman Meller, M.; Patel, S.; Duarte, D.; Kapczinski, F.; de Azevedo Cardoso, T. Bipolar disorder and frontotemporal dementia: A systematic review. Acta Psychiatr. Scand. 2021, 144, 433–447. [Google Scholar] [CrossRef]
- Perrotta, G. Bipolar disorder: Definition, differential diagnosis, clinical contexts and therapeutic approaches. J. Neurosci. Neurol. Surg. 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Drevets, W.C.; Savitz, J.; Trimble, M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008, 13, 663–681. [Google Scholar] [CrossRef]
- Ongür, D.; Drevets, W.C.; Price, J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. USA 1998, 95, 13290–13295. [Google Scholar] [CrossRef]
- Rajashekar, N.; Blumberg, H.P.; Villa, L.M. Neuroimaging Studies of Brain Structure in Older Adults with Bipolar Disorder: A Review. J. Psychiatry Brain Sci. 2022, 7, e220006. [Google Scholar] [CrossRef]
- Tabak, B.A.; Young, K.S.; Torre, J.B.; Way, B.M.; Burklund, L.J.; Eisenberger, N.I.; Lieberman, M.D.; Craske, M.G. Preliminary Evidence That CD38 Moderates the Association of Neuroticism on Amygdala-Subgenual Cingulate Connectivity. Front. Neurosci. 2020, 14, 11. [Google Scholar] [CrossRef]
- Abé, C.; Ching, C.R.; Liberg, B.; Lebedev, A.V.; Agartz, I.; Akudjedu, T.N.; Alda, M.; Alnæs, D.; Alonso-Lana, S.; Benedetti, F.; et al. Longitudinal Structural Brain Changes in Bipolar Disorder: A Multicenter Neuroimaging Study of 1232 Individuals by the ENIGMA Bipolar Disorder Working Group. Biol. Psychiatry 2022, 91, 582–592. [Google Scholar] [CrossRef]
- Velosa, J.; Delgado, A.; Finger, E.; Berk, M.; Kapczinski, F.; de Azevedo Cardoso, T. Risk of dementia in bipolar disorder and the interplay of lithium: A systematic review and meta-analyses. Acta Psychiatr. Scand. 2020, 141, 510–521. [Google Scholar] [CrossRef]
- Hummer, T.A.; Hulvershorn, L.A.; Karne, H.S.; Gunn, A.D.; Wang, Y.; Anand, A. Emotional response inhibition in bipolar disorder: A functional magnetic resonance imaging study of trait- and state-related abnormalities. Biol. Psychiatry 2013, 73, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Guo, H.; Liu, S.; Xue, W.; Fan, F.; Li, H.; Fan, H.; An, H.; Wang, Z.; Tan, S.; et al. Subcortical Brain Volumes Relate to Neurocognition in First-Episode Schizophrenia, Bipolar Disorder, Major Depression Disorder, and Healthy Controls. Front. Psychiatry 2022, 12, 747386. [Google Scholar] [CrossRef] [PubMed]
- Vizueta, N.; Rudie, J.D.; Townsend, J.D.; Torrisi, S.; Moody, T.D.; Bookheimer, S.Y.; Altshuler, L.L. Regional fMRI hypoactivation and altered functional connectivity during emotion processing in nonmedicated depressed patients with bipolar II disorder. Am. J. Psychiatry 2012, 169, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, L.; Bookheimer, S.; Proenza, M.A.; Townsend, J.; Sabb, F.; Firestine, A.; Bartzokis, G.; Mintz, J.; Mazziotta, J.; Cohen, M.S. Increased amygdala activation during mania: A functional magnetic resonance imaging study. Am. J. Psychiatry 2005, 162, 1211–1213. [Google Scholar] [CrossRef]
- Usher, J.; Leucht, S.; Falkai, P.; Scherk, H. Correlation between amygdala volume and age in bipolar disorder—A systematic review and meta-analysis of structural MRI studies. Psychiatry Res. 2010, 182, 1–8. [Google Scholar] [CrossRef]
- Librenza-Garcia, D.; Suh, J.S.; Watts, D.P.; Ballester, P.L.; Minuzzi, L.; Kapczinski, F.; Frey, B.N. Structural and Functional Brain Correlates of Neuroprogression in Bipolar Disorder. Curr. Top. Behav. Neurosci. 2021, 48, 197–213. [Google Scholar] [CrossRef]
- Damme, K.S.F.; Alloy, L.B.; Young, C.B.; Kelley, N.J.; Chein, J.; Ng, T.H.; Titone, M.K.; Black, C.L.; Nusslock, R. Amygdala subnuclei volume in bipolar spectrum disorders: Insights from diffusion-based subsegmentation and a high-risk design. Hum. Brain Mapp. 2020, 41, 3358–3369. [Google Scholar] [CrossRef]
- Blond, B.N.; Fredericks, C.A.; Blumberg, H.P. Functional neuroanatomy of bipolar disorder: Structure, function, and connectivity in an amygdala-anterior paralimbic neural system. Bipolar Disord. 2012, 14, 340–355. [Google Scholar] [CrossRef]
- McDonald, C.; Zanelli, J.; Rabe-Hesketh, S.; Ellison-Wright, I.; Sham, P.; Kalidindi, S.; Murray, R.M.; Kennedy, N. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol. Psychiatry 2004, 56, 411–417. [Google Scholar] [CrossRef]
- Hozer, F.; Sarrazin, S.; Laidi, C.; Favre, P.; Pauling, M.; Cannon, D.; McDonald, C.; Emsell, L.; Mangin, J.-F.; Duchesnay, E.; et al. Lithium prevents grey matter atrophy in patients with bipolar disorder: An international multicenter study. Psychol. Med. 2021, 51, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Bauer, I.E.; Sharma, A.N.; Mwangi, B.; Frazier, T.; Lavagnino, L.; Zunta-Soares, G.B.; Walss-Bass, C.; Glahn, D.C.; Kapczinski, F.; et al. Reduced hippocampus volume and memory performance in bipolar disorder patients carrying the BDNF val66met met allele. J. Affect. Disord. 2016, 198, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Baldessarini, R.J.; Vieta, E.; Yucel, M.; Bora, E.; Sim, K. Longitudinal neuroimaging and neuropsychological changes in bipolar disorder patients: Review of the evidence. Neurosci. Biobehav. Rev. 2013, 37, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Koziol, L.F.; Budding, D.; Andreasen, N.; D’Arrigo, S.; Bulgheroni, S.; Imamizu, H.; Ito, M.; Manto, M.; Marvel, C.; Parker, K.; et al. Consensus paper: The cerebellum’s role in movement and cognition. Cerebellum 2014, 13, 151–177. [Google Scholar] [CrossRef]
- Lupo, M.; Olivito, G.; Siciliano, L.; Masciullo, M.; Bozzali, M.; Molinari, M.; Leggio, M. Development of a Psychiatric Disorder Linked to Cerebellar Lesions. Cerebellum 2018, 17, 438–446. [Google Scholar] [CrossRef]
- Saleem, A.; Harmata, G.; Jain, S.; Voss, M.W.; Fiedorowicz, J.G.; Williams, A.J.; Shaffer, J.J.; Richards, J.G.; Barsotti, E.J.; Sathyaputri, L.; et al. Functional connectivity of the cerebellar vermis in bipolar disorder and associations with mood. Front. Psychiatry 2023, 14, 1147540. [Google Scholar] [CrossRef]
- Mills, N.P.; Delbello, M.P.; Adler, C.M.; Strakowski, S.M. MRI analysis of cerebellar vermal abnormalities in bipolar disorder. Am. J. Psychiatry 2005, 162, 1530–1532. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Li, F.; Zhao, L.; Bo, Q.; Zhou, Y.; Wang, C. Decreased dynamic variability of the cerebellum in the euthymic patients with bipolar disorder. BMC Psychiatry 2024, 24, 137. [Google Scholar] [CrossRef]
- Olivito, G.; Lupo, M.; Gragnani, A.; Saettoni, M.; Siciliano, L.; Pancheri, C.; Panfili, M.; Cercignani, M.; Bozzali, M.; Chiaie, R.D.; et al. Aberrant Cerebello-Cerebral Connectivity in Remitted Bipolar Patients 1 and 2: New Insight into Understanding the Cerebellar Role in Mania and Hypomania. Cerebellum 2022, 21, 647–656. [Google Scholar] [CrossRef]
- Olivito, G.; Lupo, M.; Siciliano, L.; Gragnani, A.; Saettoni, M.; Pancheri, C.; Panfili, M.; Pignatelli, F.; Chiaie, R.D.; Leggio, M. Theory of mind profile and cerebellar alterations in remitted bipolar disorder 1 and 2: A comparison study. Front. Behav. Neurosci. 2022, 16, 971244. [Google Scholar] [CrossRef]
- Thiel, K.; Lemke, H.; Winter, A.; Flinkenflügel, K.; Waltemate, L.; Bonnekoh, L.; Grotegerd, D.; Dohm, K.; Hahn, T.; Förster, K.; et al. White and gray matter alterations in bipolar I and bipolar II disorder subtypes compared with healthy controls—Exploring associations with disease course and polygenic risk. Neuropsychopharmacology 2024, 49, 814–823. [Google Scholar] [CrossRef]
- Baldassarini, R.J.; Tondo, L.; Vazquez, G.H.; Undurraga, J.; Bolzani, L.; Yildiz, A.; Khalsa, H.K.; Lai, M.; Lepri, B.; Lolich, M.; et al. Age at onset versus family history and clinical outcomes in 1665 international bipolar-I disorder patients. World Psychiatry 2012, 11, 40–46. [Google Scholar] [CrossRef]
- Vieta, E.; Berk, M.; Schulze, T.G.; Carvalho, A.F.; Suppes, T.; Calabrese, J.R.; Gao, K.; Miskowiak, K.W.; Grande, I. Bipolar disorders. Nat. Rev. Dis. Primers 2018, 8, 18008. [Google Scholar] [CrossRef]
| Neuroanatomical Areas | Healthy Subject (Average Adult) | Person with BD |
|---|---|---|
| Prefrontal Cortex (PFC) | Located anteriorly to the frontal lobe, the PFC—one of the last regions of the cortex (neocortex) to develop—plays a pivotal role in executive function, orchestrating, together with other areas with which it is reciprocally connected, a complex symphony of motor, cognitive and emotional functions. Specifically, vmPFC and vlPFC are involved in emotional and behavioral regulation, while dlPFC is associated with cognitive functions such as working memory and sustained attention. | In adolescents of both sexes, a reduction in cortical thickness has been documented particularly in the vmPFC and vlPFC. These abnormalities seem particularly evident in subjects with BD-I, comorbid ADHD and those with predominantly manic symptoms. In peers with BD-II and those with a prevalence of depressive states, an impairment of the volume and functionality of the dlPFC has been documented. In adults there are also anomalies at the level of the uncinate fasciculus (UF), more serious in patients with BD-I. In seniors abnormalities in PFC are widespread, thus leading to an higher risk of neurodegenerative disorders. Functional MRI studies have shown increased activity in the ventral striatum and left prefrontal cortex during reward processing tasks in patients with bipolar disorder. |
| Amygdala | Placed bilaterally in the anterior portion of each of the medial temporal lobes. Reaches an average vol. of ≈2.30 ± 10 cm3 (larger on the right), wider in males. It consists of 13 distinct nuclei, each one with its own functions and connections to other brain structures. Overall, it participates in emotional and olfactory memories, sensory input processing, emotion managing—particularly anger and fear- and their behavioral, neurovegetative and hormonal responses. | Although the results are not always homogeneous, a marked hypoactivity of this structure has been observed especially in patients with BD-II and in those with comorbid depression. In contrast, in patients with BD-I and those with a predominance of manic states the amygdala is generally hyperactive. Also, while the volume of the amygdala appears generally smaller than normal in younger patients, in adults it tends to increase. |
| Hippocampus | Bilateral medial temporal lobe fold of mean length ≈ 8 cm. Diffusely innervated by afferent and efferent fibers to other CNS structures. Principal Center for Memory and Learning, also handles functions of spatial orientation, intra- and extractor-portal sensory and perceptual processing, object recognition, socioemotional info processing and subsequent behavioral responses, and stress management. | Although scientific findings are quite inhomogeneous, a large amount of data show a larger hippocampal volumes in younger BD patients compared to adults. On the contrary, in adult patients a progressive decrease in hippocampal and parahippocampal density has been reported, probably associated with the presence of a genetic polymorphism involved in the regulation of BDNF functionality (val66met). |
| Cerebral Ventricles | There are four cerebral ventricles: two lateral ones are situated within each hemisphere of the cerebrum. The third ventricle is located in the diencephalon, between the right and left thalamus, while the fourth is situated at the back of the pons and upper half of the medulla oblongata. The ventricles produce and store cerebrospinal fluid (CSF, approximately 20–25 mL in total), which surrounds the brain and spinal cord, providing protection from trauma. CSF also removes waste and delivers nutrients to the brain. The choroid plexuses in each ventricle are responsible for the synthesis of CSF itself. | Patients with BD often show an abnormal and progressive increase of ventricular volumes, together with a thinning (also progressively worsening) of the prefrontal, fusiform, and parahippocampal cortices, the latter however mostly associated with the presence of frequent manic episodes. No significant differences were documented between BD-I and BD-II subtypes. |
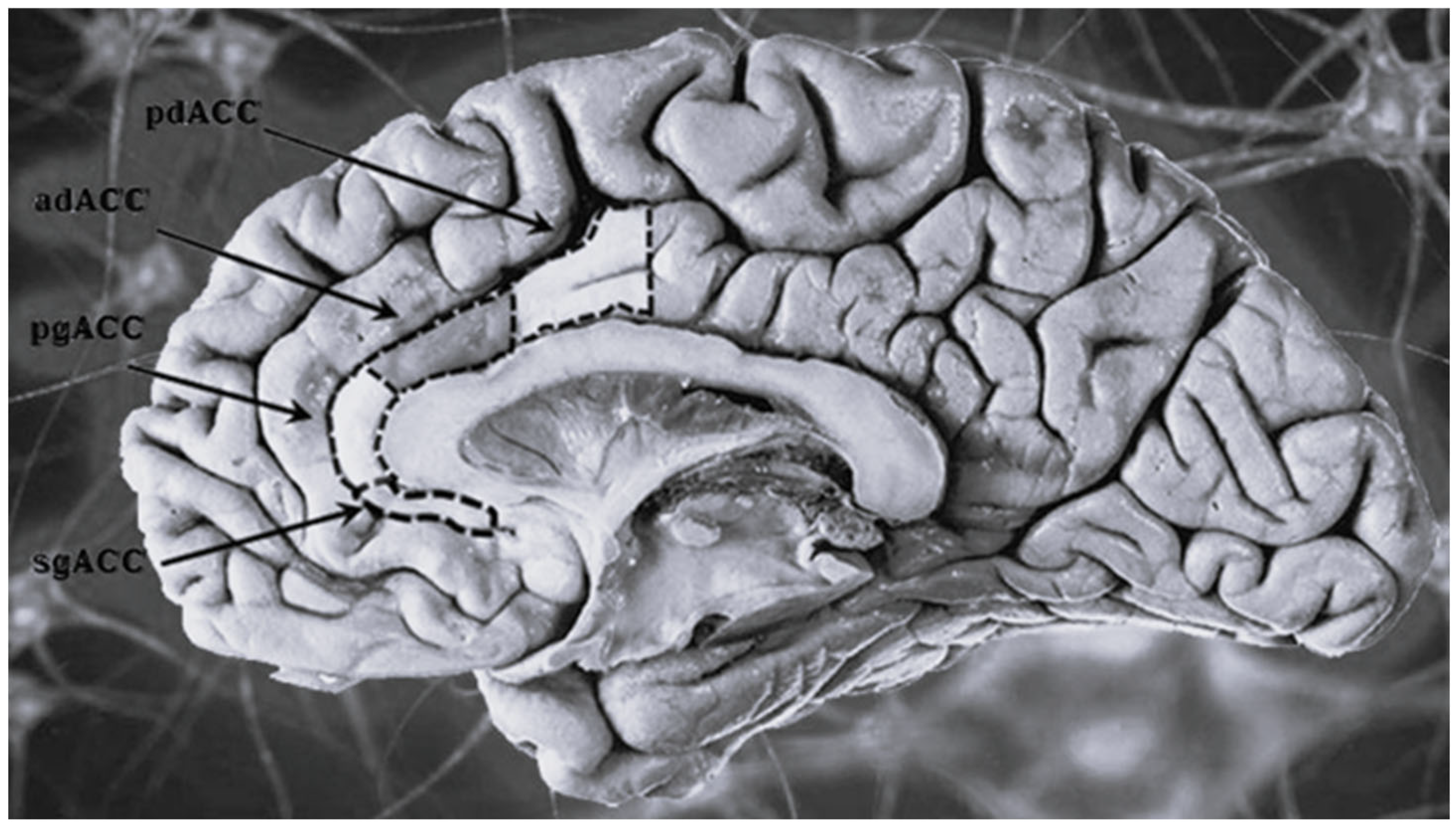
| Anterior cingulate cortex (ACC) | The most distal portion of the cingulate gyrus (bilateral structure surrounding the corpus callosum). Given the direct connections it establishes with the prefrontal cortex and some limbic structures (amygdala, hypothalamus, and hippocampus) it participates in the encoding of emotions particularly anxiety, anger and fear. Also it regulates some endocrine and vegetative functions and participates in emotional language production. | Regardless of the subtype it has been documented the existence of a general volumetric reduction of the ACC, localized in particular in its subgenual portion (sgACC), more evident in patients with affective and/or depressive comorbidity. No significant age- or subtype- related differences have been reported between patients. |
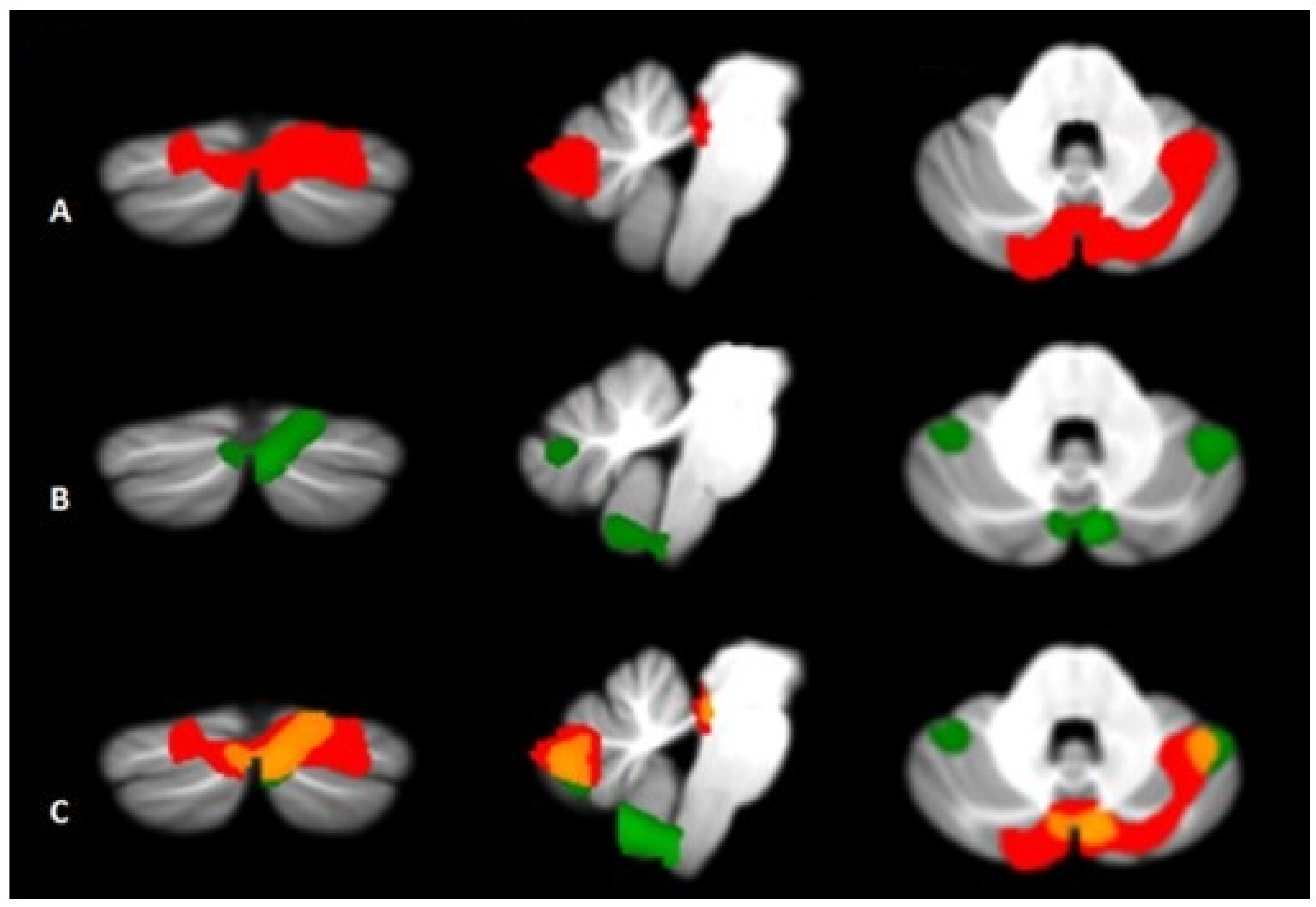
| Cerebellum | Located in the posterior cranial fossa behind the pons and medulla oblongata, separated from them by the fourth ventricle. It is divided into two hemispheres (left and right) and three lobes (anterior, posterior and flocculonodular). It is responsible for dealing with motor learning, coordination and precision of motor functions, but it also plays a role in cognitive, emotional, linguistic and visuospatial functions, thanks to connections [cortico-ponto-cerebellar (CPC) and the circuit cerebello-thalamo-cortical (CTC)] with the frontal, temporal, parietal cortices and paralimbic regions. | Functional imaging studies have highlighted a pattern of significant atrophy affecting various regions of the cerebellum (including the vermis, anterior lobe V, and posterior lobules Crus I and II), with some distinctive characteristics between subtypes: in BD-I it has been observed a reduction of the anterior and posterior cerebellar portions, more evident on the right hemisphere, while BD-II subjects show a pattern of diffuse and bilateral cerebellar atrophy. |
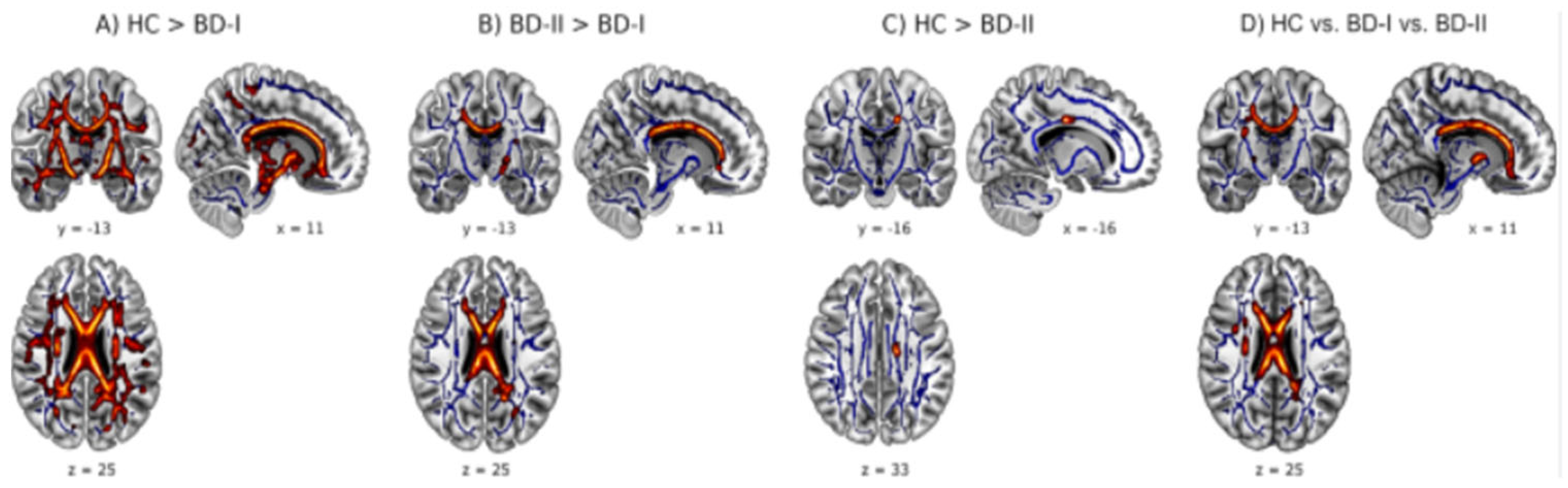
| White matter | White matter is a component of the central nervous system, consisting primarily of myelinated nerve fibers that connect different areas of the brain and spinal cord. Myelin, a fatty substance that coats axons, gives them their characteristic white color and allows for the rapid transmission of nerve impulses. White matter is essential for communication and information processing within the nervous system. | An increase in deep white matter hyperintensity (areas that show abnormal signal intensity on MRI) is also observed. |
| Grey matter | Gray matter consists of the cell bodies of neurons and is primarily found in the cerebral cortex. | Reductions in gray matter volume may be observed in specific brain areas, such as those involved in emotional control and reasoning. These reductions may be associated with difficulties regulating emotions and making decisions. |
| Cortical thickness | Cerebral cortical thickness, which refers to the thickness of the cerebral cortex, is generally between 2 and 4 mm. The cortex, a layer of gray matter, is the outer surface of the brain and is rich in neurons. Its thickness varies slightly depending on the different areas of the brain, but generally remains thin, about 2–4 mm in adults. | Although there are no significant changes in cortical surface area, reduced cortical thickness has been reported in areas such as the operculum and midfrontal cortex. |
| Neuroanatomical Areas | Person with BD-I | Person with BD-II |
|---|---|---|
| In general | The neuroanatomical differences between bipolar disorder type 1 and type 2, although not yet fully understood, primarily concern the extent and severity of mood episodes. Type 1 is characterized by full-blown manic episodes, potentially with psychotic symptoms, while type 2 presents hypomanic episodes (less severe than mania) and major depressive episodes. This is reflected in some neuroanatomical differences, with type 1 showing greater impairment in certain brain areas involved in emotional processing and mood regulation. In DB-1, the presence of manic episodes with possible psychosis and greater functional impairment suggest greater neuroanatomical dysregulation, involving larger brain areas. In DB-2, with the predominance of hypomanic and depressive episodes, it may have a less marked impact on some brain areas, although the specific differences are not yet fully elucidated. | |
| Hippocampus | The volume is markedly reduced, generating a greater impairment of functions such as memory, learning, problem solving and mood regulation. | The volume is reduced but significantly less than the DB-1. |
| Prefrontal cortex | The volume appears significantly reduced, generating a greater impairment of functions such as planning and problem solving, with a greater tendency towards impulsivity. | The volume is reduced but significantly less than the DB-1. |
| Anterior cingulate | The volume appears moderately reduced, generating a greater impairment of functions such as emotional processing and the management of fear and frustration. | The volume is reduced but significantly less than the DB-1. |
| Cerebral ventricles | The volume appears significantly increased, with greater presence of fluid in the ventricular spaces, generating a greater impairment of functions such as memory, attention, executive function and planning, favoring degenerative dementia processes. | Volume increased but significantly less than DB-1. |
| Cerebellum | Reduction of the anterior and posterior cerebellar portions, with greater evidence in the right hemisphere. | Diffuse and bilateral cerebellar atrophy. |
| Grey and White matters | Significant structural and functional reduction | Mild or moderate structural and functional reduction |
| Different Alterations Between the Two Main Forms of Bipolarism (BD-I and BD-II) | ||
|---|---|---|
| BD-I | Elements Shared in Both Forms but with Different Intensity and Frequency | BD-II |
| Anxiety disorders and Emotional reactivity | Cyclic nature of manic and depressive symptoms | Age of BD onset |
| Metabolic syndrome | Residual mood symptoms | Number of episodes |
| Poor patient cooperation and adherence to therapy | Poor Sleep quality | Patient cooperation and adherence to the- rapy |
| Psychiatric drugs such as antidepressants, mood stabilizers, and antipsychotics | Childhood trauma | Psychiatric drugs such as antidepressants and mood stabilizers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liberati, A.S.; Eleuteri, S.; Perrotta, G. Neuroanatomical and Functional Correlates in Bipolar Disorder (BD): A Narrative Review. J. Clin. Med. 2025, 14, 5689. https://doi.org/10.3390/jcm14165689
Liberati AS, Eleuteri S, Perrotta G. Neuroanatomical and Functional Correlates in Bipolar Disorder (BD): A Narrative Review. Journal of Clinical Medicine. 2025; 14(16):5689. https://doi.org/10.3390/jcm14165689
Chicago/Turabian StyleLiberati, Anna Sara, Stefano Eleuteri, and Giulio Perrotta. 2025. "Neuroanatomical and Functional Correlates in Bipolar Disorder (BD): A Narrative Review" Journal of Clinical Medicine 14, no. 16: 5689. https://doi.org/10.3390/jcm14165689
APA StyleLiberati, A. S., Eleuteri, S., & Perrotta, G. (2025). Neuroanatomical and Functional Correlates in Bipolar Disorder (BD): A Narrative Review. Journal of Clinical Medicine, 14(16), 5689. https://doi.org/10.3390/jcm14165689









