Cutaneous Cancer Trends in Spain: An Emerging Epidemic with Shifting Tumor Types
Abstract
1. Introduction
1.1. Basal Cell Carcinoma
1.2. Squamous Cell Carcinoma
1.3. Melanoma
2. Materials and Methods
2.1. Quality Control
2.2. Statistical Analysis
2.3. Incidence Rates and Trend Analysis
3. Results
3.1. Skin Tumors
3.2. Melanoma
3.2.1. Clinical Variables
3.2.2. Annual Distribution of Melanoma by Sex
3.2.3. Anatomical Location and Sex
3.2.4. Histological Subtypes and Sex
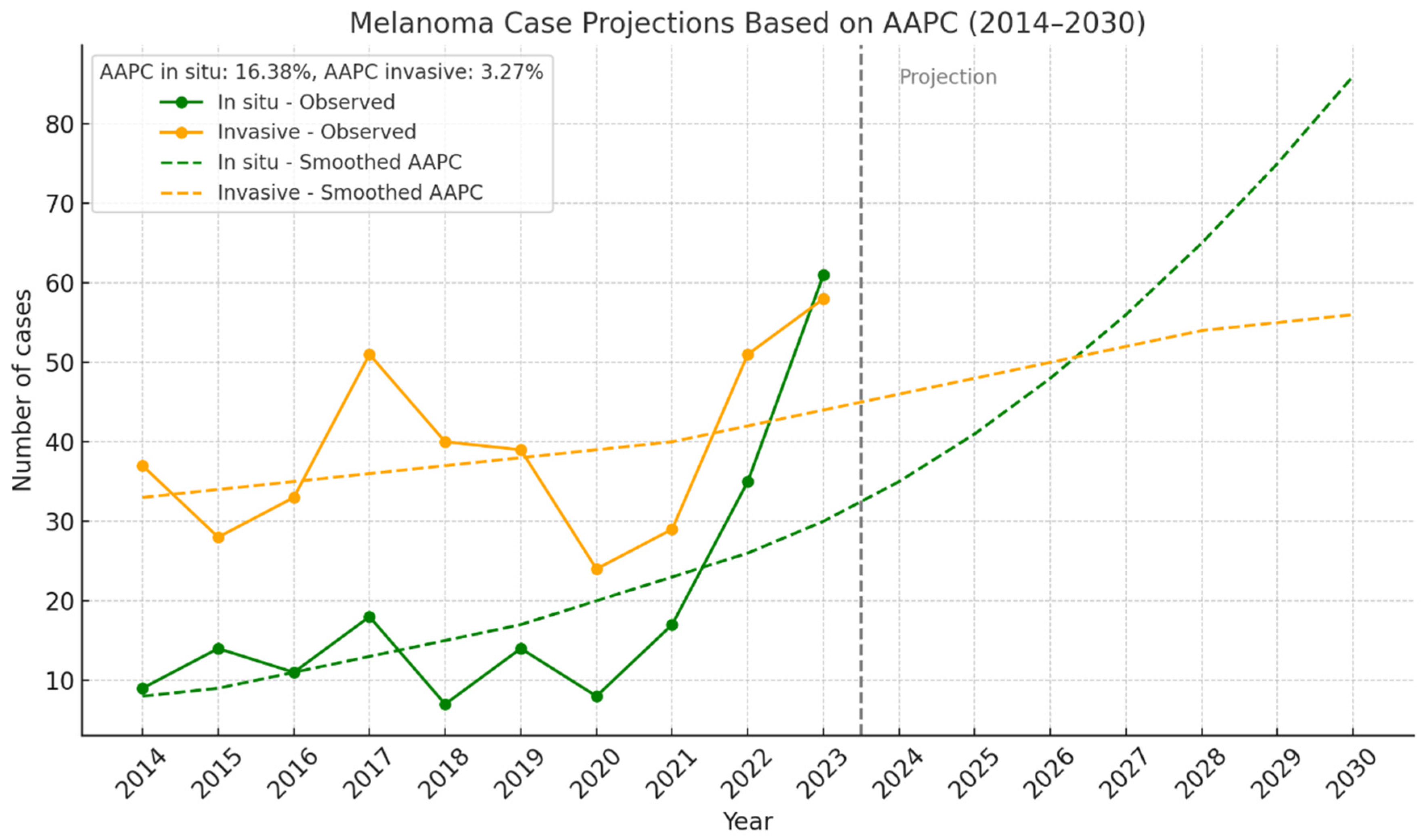
3.2.5. In Situ and Invasive Melanoma by Year
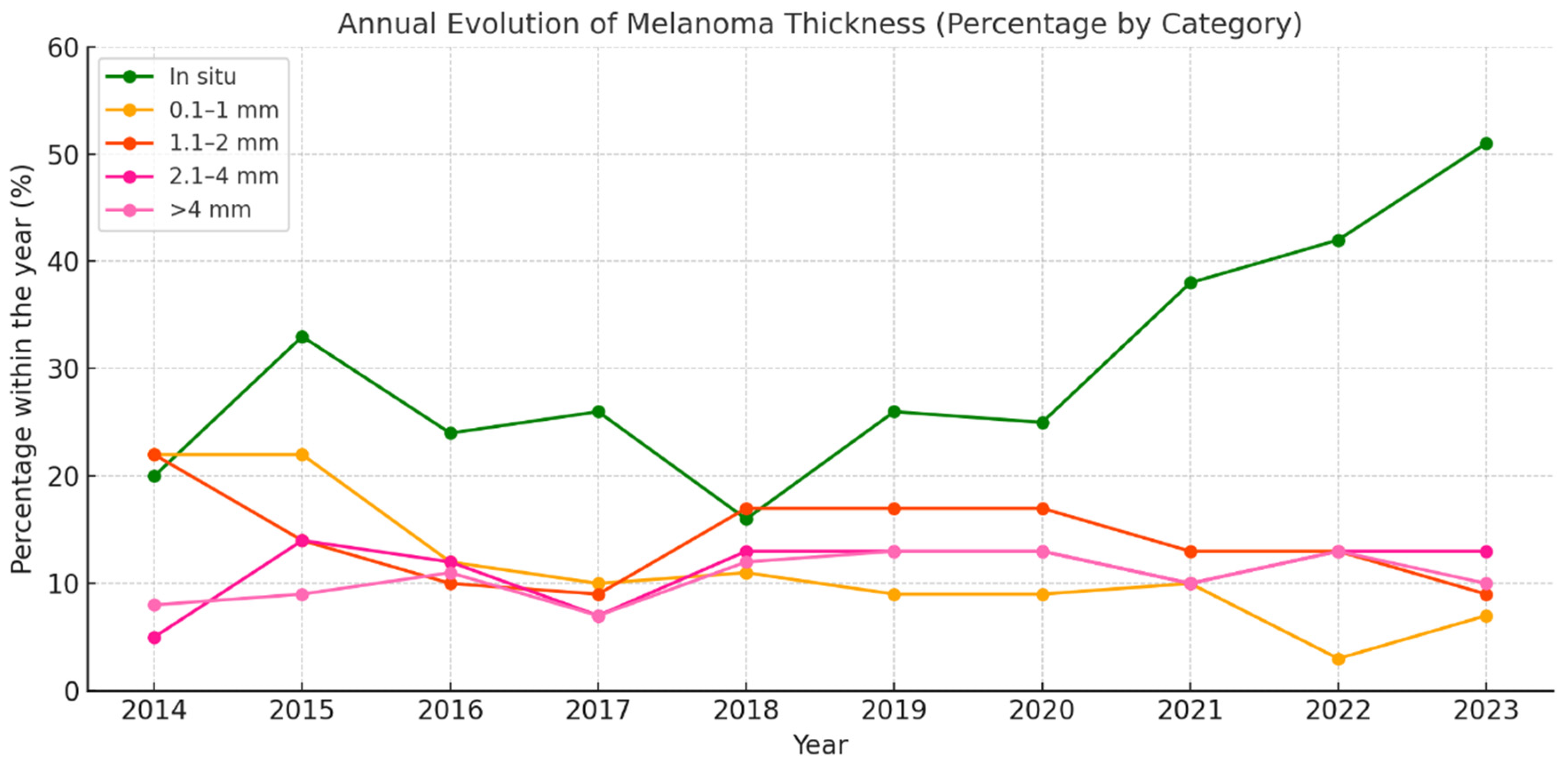
3.2.6. Melanoma by Breslow Thickness Categories
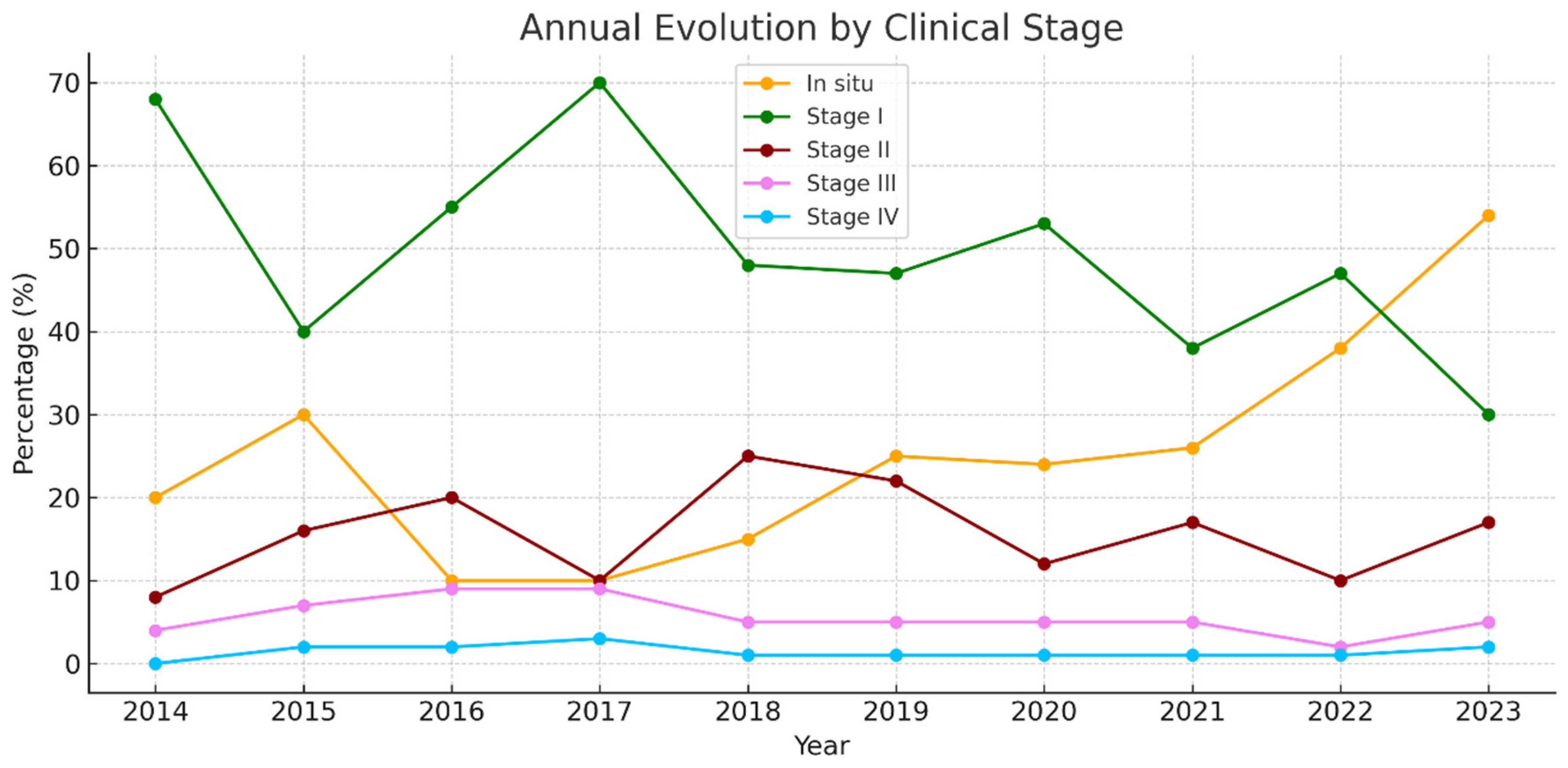
3.2.7. Melanoma by Clinical Stage
3.2.8. Specialist Responsible for the Diagnosis
3.2.9. Crude and Age-Standardized Incidence Rates
4. Discussion
4.1. Basal Cell Carcinoma
4.2. Squamous Cell Carcinoma
4.3. Cutaneous Melanoma
- The lack of data on relevant confounding factors such as skin phototype, immunosuppression status, rural versus urban residence, socioeconomic level, and access to healthcare services. These variables are known to influence skin cancer risk and incidence trends, but they were not available in the pathology registry used for this analysis. Future studies incorporating these determinants are necessary to gain a more comprehensive understanding of the epidemiological patterns observed.
- This was a cross-sectional study with no clinical follow-up, preventing assessment of outcomes or prognosis.
- Melanomas diagnosed in the private healthcare sector were not included. The literature indicates that this exclusion may result in an underestimation of true incidence due to incomplete reporting of cases diagnosed in outpatient settings not captured by official epidemiological surveillance systems [13]. Underreporting of melanoma has also been documented in certain periods within the Surveillance, Epidemiology, and End Results (SEER) program [13].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HUCA | Central University Hospital of Asturias HUCA |
| BCC | Basal cell carcinoma |
| SCC | Squamous cell carcinoma |
| NMS | Non-melanoma skin cancer |
| SSM | Superficial spreading melanoma |
| LMM | Lentigo maligna melanoma |
| LM | Lentigo maligna |
| NM | Nodular melanoma |
| ALM | Acral lentiginous melanoma |
| UV | Ultraviolet |
| TNM | Tumor, node, metastasis classification |
| AJCC | American Joint Committee on Cancer |
References
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of skin cancer: Update 2019. Adv. Exp. Med. Biol. 2020, 1268, 123–139. [Google Scholar] [PubMed]
- Tejera-Vaquerizo, A.; Descalzo-Gallego, M.; Otero-Rivas, M.; Posada-García, C.; Rodríguez-Pazos, L.; Pastushenko, I.; Marcos-Gragera, R.; García-Doval, I. Skin cancer incidence and mortality in Spain: A systematic review and meta-analysis. Actas. Dermosifiliogr. 2016, 107, 318–328. [Google Scholar] [CrossRef]
- Armstrong, B.K.; Kricker, A. How much melanoma is caused by sun exposure? Melanoma. Res. 1993, 3, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kricker, A.; Armstrong, B.K.; English, D.R.; Heenan, P.J. Does intermittent sun exposure cause basal cell carcinoma? A case-control study in Western Australia. Int. J. Cancer. 1995, 60, 489–494. [Google Scholar] [CrossRef]
- Kricker, A.; Armstrong, B.K.; English, D.R. Sun exposure and non-melanocytic skin cancer. Cancer Causes Control 1994, 5, 367–392. [Google Scholar] [CrossRef]
- El Ghissassi, F.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part D: Radiation. Lancet Oncol. 2009, 10, 751–752. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Savarese, I.; Gori, A.; Scarfi, F.; Topa, A.; Trane, L.; Portelli, F.; Innocenti, A.; Covarelli, P. Advanced basal cell carcinoma: When a good drug is not enough. J. Dermatol. Treat 2020, 31, 552–553. [Google Scholar] [CrossRef]
- McDaniel, B.; Badri, T.; Steele, R.B. Basal Cell Carcinoma; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK482439/ (accessed on 11 March 2025).
- Zeng, S.; Fu, L.; Zhou, P.; Ling, H. Identifying risk factors for the prognosis of head and neck cutaneous squamous cell carcinoma: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0239586. [Google Scholar] [CrossRef]
- Hadian, Y.; Howell, J.Y.; Ramsey, M.L.; Buckley, C. Cutaneous Squamous Cell Carcinoma; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK441939/ (accessed on 11 March 2025).
- Heistein, J.B.; Acharya, U.; Mukkamalla, S.K.R. Malignant Melanoma; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK470409/ (accessed on 11 March 2025).
- Zaba, L.C.; Wang, J.Y.; Swetter, S.M. Melanoma. In Dermatology, 5th ed.; Bolognia, J.L., Schaffer, J.V., Cerroni, L., Eds.; Elsevier: Dermatology, PA, USA, 2024; pp. 2009–2044. [Google Scholar]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef]
- Dixon, A.J.; Sladden, M.; Zouboulis, C.C.; Popescu, C.M.; Nirenberg, A.; Steinman, H.K.; Longo, C.; Dixon, Z.L.; Thomas, J.M. Primary cutaneous melanoma—Management in 2024. J. Clin. Med. 2024, 13, 1607. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A. Melanoma staging: Evidence-based changes in the AJCC 8th edition cancer staging manual. CA Cancer. J. Clin. 2017, 67, 472–492. [Google Scholar]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef]
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, P.H.; Ardanaz, E. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE-5. Lancet Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef]
- Arnold, M.; Holterhues, C.; Hollestein, L.; Coebergh, J.; Nijsten, T.; Pukkala, E.; Holleczek, B.; Tryggvadóttir, L.; Comber, H.; Bento, M.; et al. Trends in incidence and predictions of cutaneous melanoma across Europe up to 2015. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1170–1178. [Google Scholar] [CrossRef]
- Miller, A.J.; Mihm, M.C. Melanoma. N. Engl. J. Med. 2006, 355, 51–65. [Google Scholar] [CrossRef]
- Sociedad Española de Oncología Médica (SEOM). Se incrementa la Incidencia Interanual de Melanoma en España Con 7.881 Casos Nuevos en 2024. 2024. Available online: https://seom.org/otros-servicios/noticias/210543 (accessed on 12 March 2025).
- Fidler, M.M.; Gupta, S.; Soerjomataram, I.; Ferlay, J.; Steliarova-Foucher, E.; Bray, F. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012. Lancet Oncol. 2017, 18, 1579–1589. [Google Scholar] [CrossRef]
- Allied Market Research. Skin Cancer Treatment Market: Global Opportunity Analysis and Industry Forecast, 2021–2031. Available online: https://www.alliedmarketresearch.com/skin-cancer-treatment-market-A17526 (accessed on 25 March 2025).
- Nanz, L.; Keim, U.; Katalinic, A.; Meyer, T.; Garbe, C.; Leiter, U. Epidemiology of keratinocyte skin cancer with a focus on cutaneous squamous cell carcinoma. Cancers 2024, 16, 606. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer in the U.S. population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- García Ruiz, R.; Mateu Puchades, A.; Alegre de Miquel, V. [Translated article] Basal cell carcinoma: Incidence and trends in Valencia, Spain. Actas. Dermosifiliogr. 2024, 115, 943–947. [Google Scholar] [CrossRef]
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A.C. Skin cancer and new treatment perspectives: A review. Cancer. Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef]
- Hu, W.; Fang, L.; Ni, R.; Zhang, H.; Pan, G. Changing trends in the disease burden of non-melanoma skin cancer globally from 1990 to 2019 and its predicted level in 25 years. BMC Cancer. 2022, 22, 836. [Google Scholar] [CrossRef]
- Cayuela, L.; Pereyra-Rodríguez, J.J.; Hernández-Rodríguez, J.C.; Sendín-Martín, M.; Cayuela, A. Non-melanoma skin cancer burden in Spain: A nationwide analysis of incidence trends (1990–2019). Eur. J. Dermatol. 2024, 34, 392–397. [Google Scholar] [CrossRef]
- Wall, J.; Gadsby-Davis, K.; Mistry, K.; Levell, N.J.; Venables, Z.C. Impact of the COVID-19 pandemic on international cutaneous squamous cell carcinoma incidence: A systematic review and meta-analysis. Skin Health Dis. 2024, 4, e405. [Google Scholar] [CrossRef]
- Olsen, C.M.; Thompson, J.F.; Pandeya, N.; Whiteman, D.C. Evaluation of sex-specific incidence of melanoma. JAMA Dermatol. 2020, 156, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gómez, B.; Aragonés, N.; Gustavsson, P.; Lope, V.; López-Abente, G.; Pollán, M. Do sex and site matter? Different age distribution in melanoma of the trunk among Swedish men and women. Br. J. Dermatol. 2008, 158, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Buylla-Puente, M.C.; Adsuar Mas, J.; Terrasa Sagristá, F.; Nadal Nadal, A.; Nadal Lladó, C.; Llambrich Mañés, A. Epidemiología del melanoma cutáneo primario en el sector Migjorn en la isla de Mallorca entre los años 2003–2021. Actas. Dermosifiliogr. 2024, 115, 814–818. [Google Scholar] [CrossRef]
- Tejera-Vaquerizo, A.; Paradela, S.; Toll, A.; Santos-Juanes, J.; Jaka, A.; López, A.; Cañueto, J.; Villegas-Romero, I.; Fernández-Pulido, C.; Perandones, H.; et al. Effects of COVID-19 lockdown on tumour burden of melanoma and cutaneous squamous cell carcinoma. Acta. Derm. Venereol. 2021, 101, adv00525. [Google Scholar] [CrossRef]
- Rosés-Gibert, P.; Podlipnik, S.; de la Torre Gomar, F.J.; Saenz Aguirre, A.; Gimeno Castillo, J.; González Pérez, R.; Puig, S. Incidence of melanoma in the Basque province of Álava, Spain, from 2015 to 2018: A descriptive study. Actas. Dermosifiliogr. 2022, 113, T178–T182. [Google Scholar] [CrossRef]
- Cayuela, L.; Pereyra-Rodríguez, J.J.; Hernández-Rodríguez, J.C.; Cayuela, A. Spain’s rising melanoma threat: A comprehensive 30-year analysis (1990–2019). Cancers 2024, 16, 1167. [Google Scholar] [CrossRef]
- Puig, S.; Marcoval, J.; Gallardo Hernández, F. Melanoma incidence increases in the elderly of Catalonia but not in the younger population: Effect of prevention or consequence of immigration? Acta. Derm. Venereol. 2015, 95, 422–426. [Google Scholar] [CrossRef]
- Podlipnik, S.; Carrera, C.; Boada, A.; Richarz, N.; Marcoval, J.; Ferreres, J.R.; Bodet, D.; Martí, R.M.; Segura, S.; Sabat, M.; et al. Incidence of melanoma in Catalonia, Spain, is rapidly increasing in the elderly population: A multicentric cohort study. J. Clin. Med. 2020, 9, 3396. [Google Scholar] [CrossRef] [PubMed]
- Grau-Pérez, M.; Carretero, G.; Almeida, P.; Castro-González, E.; de la Rosa del Rey, M.D.P.; González-Martín, J.M.; Borrego, L. The incidence of skin melanoma in Gran Canaria (Canary Islands, Spain) is lower than expected in Southern Europe despite high-risk environmental conditions: An island-wide cross-sectional study. Cancer Causes Control. 2021, 32, 525–535. [Google Scholar] [PubMed]
- Fernández-Canedo, I.; Rivas-Ruiz, F.; Fúnez-Liébana, R.; Blázquez-Sánchez, N.; de Troya-Martín, M. Epidemiología del melanoma en una población multicultural mediterránea. Piel 2014, 29, 401–405. [Google Scholar] [CrossRef]
- Karampinis, E.; Koumaki, D.; Sgouros, D.; Nechalioti, P.-M.; Toli, O.; Pappa, G.; Papadakis, M.; Georgopoulou, K.-E.; Schulze-Roussaki, A.-V.; Kouretas, D. Non-Melanoma Skin Cancer: Assessing the Systemic Burden of the Disease. Cancers 2025, 17, 703. [Google Scholar] [CrossRef] [PubMed]



| 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | 367,844 | 364,233 | 361,319 | 359,500 | 357,745 | 356,436 | 355,586 | 352,868 | 349,953 | 352,493 | 3,577,977 |
| BCC | 623 | 705 | 723 | 734 | 843 | 786 | 591 | 699 | 857 | 916 | 7477 |
| Crude incidence rate—BCC | 169.37 | 193.56 | 200.10 | 204.17 | 235.64 | 220.52 | 166.20 | 198.10 | 244.89 | 259.86 | 208.97 |
| SCC | 191 | 164 | 202 | 240 | 296 | 248 | 317 | 338 | 349 | 344 | 2689 |
| Crude incidence rate—SCC | 51.92 | 45.03 | 55.91 | 66.76 | 82.74 | 69.58 | 89.15 | 95.79 | 99.73 | 97.59 | 75.15 |
| Melanoma | 46 | 42 | 43 | 69 | 47 | 53 | 32 | 47 | 87 | 119 | 585 |
| Crude incidence rate—melanoma | 12.51 | 11.53 | 11.90 | 19.19 | 13.14 | 14.87 | 9.00 | 13.32 | 24.86 | 33.76 | 16.35 |
| Total tumors | 860 | 911 | 968 | 1043 | 1186 | 1087 | 940 | 1084 | 1293 | 1379 | 10,751 |
| Year | Men n (%) | Women n (%) | Total |
|---|---|---|---|
| 2014 | 21 (45.7%) | 25 (54.3%) | 46 |
| 2015 | 15 (35.7%) | 27 (64.3%) | 42 |
| 2016 | 18 (41.9%) | 25 (58.1%) | 43 |
| 2017 | 29 (42.0%) | 40 (58.0%) | 69 |
| 2018 | 17 (36.2%) | 30 (63.8%) | 47 |
| 2019 | 23 (43.4%) | 30 (56.6%) | 53 |
| 2020 | 10 (31.2%) | 22 (68.8%) | 32 |
| 2021 | 10 (21.3%) | 37 (78.7%) | 47 |
| 2022 | 37 (42.5%) | 50 (57.5%) | 87 |
| 2023 | 54 (45.4%) | 65 (54.6%) | 119 |
| Total | 234 (40.0%) | 351 (60.0%) | 585 |
| Gender | Trunk | Head/Neck | Extremities | Acral | Genital | Total |
|---|---|---|---|---|---|---|
| Men | 105 (44.9%) | 60 (25.6%) | 47 (20.1%) | 21 (9.0%) | 1 (0.4%) | 234 |
| Women | 91 (25.9%) | 53 (15.1%) | 177 (50.4%) | 30 (8.5%) | 0 (0.0%) | 351 |
| Total | 196 (33.5%) | 113 (19.3%) | 224 (38.3%) | 51 (8.7%) | 1 (0.2%) | 585 |
| Gender | SSM | LM | NM | ALM | Other | Total |
|---|---|---|---|---|---|---|
| Men | 160 (68.4%) | 29 (12.4%) | 23 (9.8%) | 17 (7.3%) | 5 (2.1%) | 234 (100.0%) |
| Women | 263 (74.9%) | 29 (8.3%) | 31 (8.8%) | 26 (7.4%) | 2 (0.6%) | 351 (100.0%) |
| Total | 423 (72.3%) | 58 (9.9%) | 54 (9.2%) | 43 (7.4%) | 7 (1.2%) | 585 (100.0%) |
| Year | In Situ (n, %) | Invasive (n, %) | Total |
|---|---|---|---|
| 2014 | 9 (19.6%) | 37 (80.4%) | 46 |
| 2015 | 14 (33.3%) | 28 (66.7%) | 42 |
| 2016 | 10 (23.3%) | 33 (76.7%) | 43 |
| 2017 | 18 (26.1%) | 51 (73.9%) | 69 |
| 2018 | 7 (14.9%) | 40 (85.1%) | 47 |
| 2019 | 14 (26.4%) | 39 (73.6%) | 53 |
| 2020 | 8 (25.0%) | 24 (75.0%) | 32 |
| 2021 | 18 (38.3%) | 29 (61.7%) | 47 |
| 2022 | 36 (41.4%) | 51 (58.6%) | 87 |
| 2023 | 61 (51.3%) | 58 (48.7%) | 119 |
| Total | 195 (33.3%) | 390 (66.7%) | 585 |
| Year | Crude | Age-Standardized | CI (95%) |
|---|---|---|---|
| 2014 | 12.5053 | 8.8519 | 6.3781–12.5397 |
| 2015 | 11.5311 | 7.7178 | 5.4370–11.2829 |
| 2016 | 11.9008 | 8.7172 | 6.2704–12.4742 |
| 2017 | 19.1933 | 12.3394 | 9.4036–16.5768 |
| 2018 | 13.1378 | 8.8098 | 6.2883–12.7656 |
| 2019 | 14.8694 | 9.2957 | 6.7200–13.2855 |
| 2020 | 8.9992 | 6.5627 | 4.3572–10.3103 |
| 2021 | 13.3194 | 8.6537 | 6.1995–12.6959 |
| 2022 | 24.8604 | 14.2388 | 11.1150–18.8906 |
| 2023 | 33.7595 | 18.4991 | 15.0767–23.4161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Juanes, J.; Santos-Juanes, R.; López-Pando, M.; García-Puente, M.; Plaza-López, M.; Gómez de Castro, C.; Palacios-García, L.; Galache, C. Cutaneous Cancer Trends in Spain: An Emerging Epidemic with Shifting Tumor Types. J. Clin. Med. 2025, 14, 5654. https://doi.org/10.3390/jcm14165654
Santos-Juanes J, Santos-Juanes R, López-Pando M, García-Puente M, Plaza-López M, Gómez de Castro C, Palacios-García L, Galache C. Cutaneous Cancer Trends in Spain: An Emerging Epidemic with Shifting Tumor Types. Journal of Clinical Medicine. 2025; 14(16):5654. https://doi.org/10.3390/jcm14165654
Chicago/Turabian StyleSantos-Juanes, Jorge, Raquel Santos-Juanes, Marta López-Pando, Marta García-Puente, Miguel Plaza-López, Celia Gómez de Castro, Laura Palacios-García, and Cristina Galache. 2025. "Cutaneous Cancer Trends in Spain: An Emerging Epidemic with Shifting Tumor Types" Journal of Clinical Medicine 14, no. 16: 5654. https://doi.org/10.3390/jcm14165654
APA StyleSantos-Juanes, J., Santos-Juanes, R., López-Pando, M., García-Puente, M., Plaza-López, M., Gómez de Castro, C., Palacios-García, L., & Galache, C. (2025). Cutaneous Cancer Trends in Spain: An Emerging Epidemic with Shifting Tumor Types. Journal of Clinical Medicine, 14(16), 5654. https://doi.org/10.3390/jcm14165654







