Sexual Dysfunction in Melanoma Survivors: A Cross-Sectional Study on Prevalence and Associated Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Variables
- (a)
- International Index of Erectile Function (IIEF-5) [18] and Female Sexual Function Index (FSFI-6) [19] questionnaires: The IIEF-5 encompasses five key domains of male sexual health, with scores ≤ 21 indicating the presence of dysfunction. The FSFI-6 assesses six core dimensions of female sexual function, with a threshold score of ≤19 suggestive of SD.
- (b)
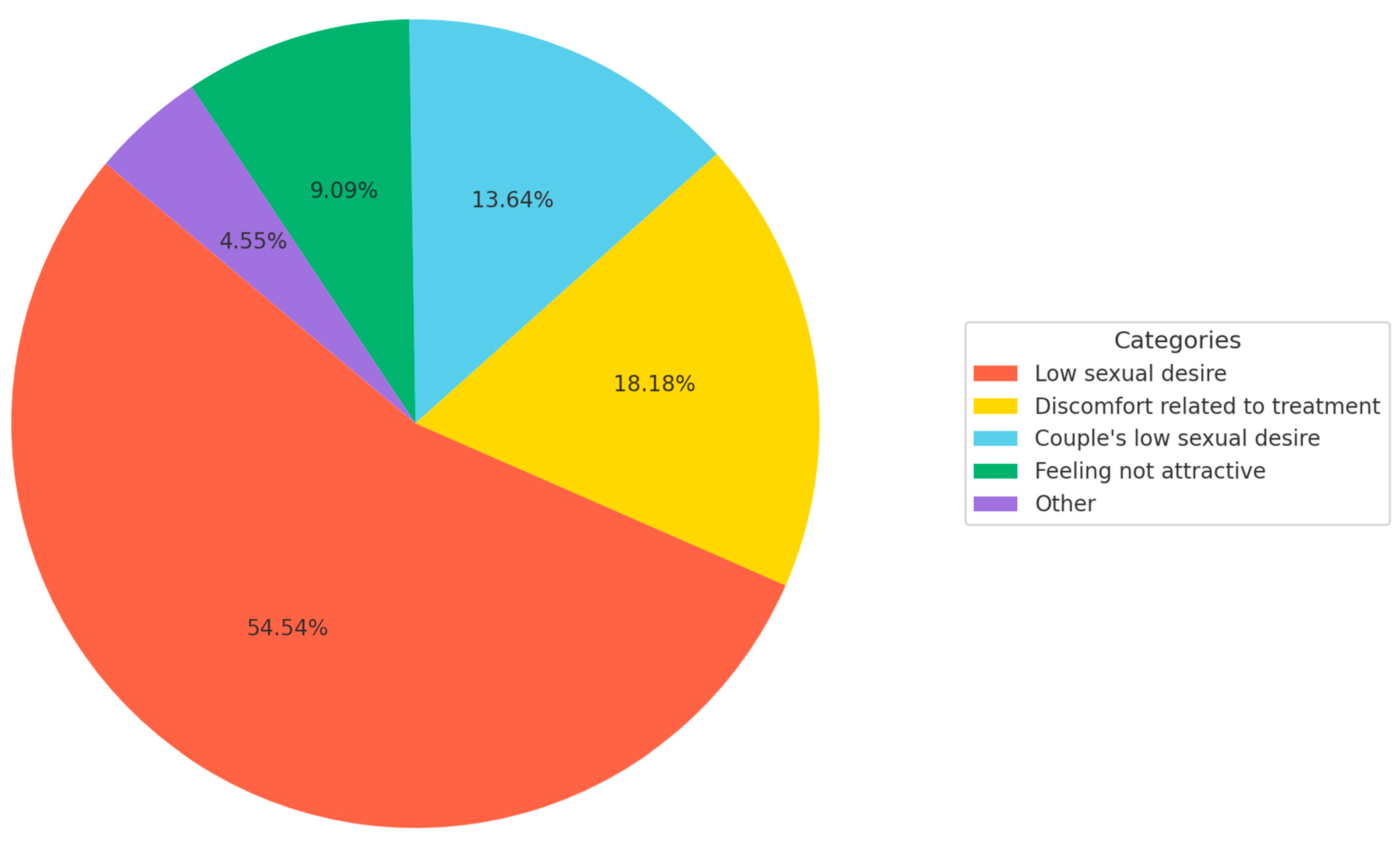
- Patients were asked about the impact of the disease in terms of sexuality. Those patients who answered that melanoma had a negative impact were asked to describe the causes of the negative impact.
- (c)
- Numeric Rating Scale (NRS) for sexual impairment: To assess perceived sexual impairment related to melanoma, patients were asked to rate the impact ranging from 1 to 10 [20].
- (d)
- Dermatology Life Quality Index (DLQI): This instrument serves as a general indicator of dermatology-related quality of life for individuals aged 16 years and older. It comprises 10 items, each rated on a 4-point Likert scale ranging from 0 (no impact) to 3 (maximum impact), yielding a total score between 0 and 30. The items assess the impact of skin disease over the preceding seven days [21].
2.2. Secondary Variables: Other Variables of Interest Included
- (a)
- The Hospital Anxiety and Depression Scale (HADS) was used to assess the presence of anxiety and depression. It consists of two subscales, one for anxiety and another for depression. A score ≥ 8 on any of the subscales was considered indicative of anxiety or depression, respectively [22].
- (b)
- Variables related to the severity and characteristics of the disease:
- TNM stage for melanoma as recommended by the 8th American Joint Committee on Cancer (AJCC) [23].
- Age of onset, evolution time of the disease, date of diagnosis, location of the melanoma, and current and past treatments were collected.
2.3. Statistical Analysis
3. Results
3.1. Sociodemographic and Clinical Characteristics of the Sample
3.2. Impact of Melanoma on Perceived Sexual Function
3.3. Objective Assessment of Sexual Dysfunction
3.4. Factors Associated with Sexual Dysfunction
3.4.1. Female Patients
3.4.2. Male Patients
3.4.3. Global Perceived Impairment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SD | Sexual Dysfunction |
| HRQOL | Health-related quality of life |
| IIEF-5 | International Index of Erectile Function |
| FSFI-6 | Female Sexual Function Index |
| NRS | Numeric Rating Scale |
| DLQI | Dermatology Life Quality Index |
| HADS | Hospital Anxiety and Depression Scale |
| AJCC | American Joint Committee on Cancer |
References
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur. J. Cancer 2022, 170, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Hines, A.S.; Demer, A.M.; Brewer, J.D. The impact of surgical delay in primary cutaneous melanoma: A systematic review. Dermatol. Surg. 2024, 50, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Kunonga, T.P.; Kenny, R.P.W.; Astin, M.; Bryant, A.; Kontogiannis, V.; Coughlan, D.; Richmond, C.; Eastaugh, C.H.; Beyer, F.R.; Pearson, F.; et al. Predictive accuracy of risk prediction models for recurrence, metastasis and survival for early-stage cutaneous melanoma: A systematic review. BMJ Open 2023, 13, e073306. [Google Scholar] [CrossRef] [PubMed]
- Molina-Leyva, A.; Jiménez-Moleón, J.J.; Naranjo-Sintes, R.; Ruiz-Carrascosa, J.C. Sexual dysfunction in psoriasis: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 649–655. [Google Scholar] [CrossRef]
- Misery, L.; Seneschal, J.; Reguiai, Z.; Merhand, S.; Héas, S.; Huet, F.; Taieb, C.; Ezzedine, K. The impact of atopic dermatitis on sexual health. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 428–432. [Google Scholar] [CrossRef]
- Sánchez-Díaz, M.; Salazar-Nievas, M.C.; Molina-Leyva, A.; Arias-Santiago, S. Risk factors of quality-of-life and sexual function impairment in chronic spontaneous urticaria patients: Cross-sectional study. Dermatology 2023, 239, 601–608. [Google Scholar] [CrossRef]
- Weitman, E.S.; Perez, M.; Thompson, J.F.; Andtbacka, R.H.I.; Dalton, J.; Martin, M.L.; Miller, T.; Gwaltney, C.; Sarson, D.; Wachter, E.; et al. Quality of life patient-reported outcomes for locally advanced cutaneous melanoma. Melanoma Res. 2018, 28, 134–142. [Google Scholar] [CrossRef]
- Schadendorf, D.; Amonkar, M.M.; Stroyakovskiy, D.; Levchenko, E.; Gogas, H.; de Braud, F.; Grob, J.-J.; Bondarenko, I.; Garbe, C.; Lebbe, C.; et al. Health-related quality of life impact in a randomized phase III study of the combination of dabrafenib and trametinib versus dabrafenib monotherapy in patients with BRAF V600 metastatic melanoma. Eur. J. Cancer 2015, 51, 833–840. [Google Scholar] [CrossRef]
- Suzuki, K.; Morishita, S.; Nakano, J.; Okayama, T.; Inoue, J.; Tanaka, T.; Fukushima, T. Association between quality of life and mortality risk in patients with breast cancer: A systematic review and meta-analysis. Breast Cancer 2024, 31, 552–561. [Google Scholar] [CrossRef]
- Blidari, A.R.; Muntean, C. Sexual functioning and impact on quality of life in patients with early-onset colorectal cancer: A systematic review. Diseases 2024, 12, 66. [Google Scholar] [CrossRef]
- Seguin, L.; Touzani, R.; Bouhnik, A.-D.; Ben Charif, A.; Marino, P.; Bendiane, M.-K.; Gonçalves, A.; Gravis, G.; Mancini, J. Deterioration of Sexual Health in Cancer Survivors Five Years after Diagnosis: Data from the French National Prospective VICAN Survey. Cancers 2020, 12, 3453. [Google Scholar] [CrossRef] [PubMed]
- Mols, F.; Holterhues, C.; Nijsten, T.; van de Poll-Franse, L.V. Personality is associated with health status and impact of cancer among melanoma survivors. Eur. J. Cancer 2010, 46, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Young, J.N.; Griffith-Bauer, K.; Hill, E.; Latour, E.; Samatham, R.; Leachman, S. The benefit of early-stage diagnosis: A registry-based survey evaluating the quality of life in patients with melanoma. Skin Health Dis. 2023, 3, e237. [Google Scholar] [CrossRef] [PubMed]
- Kungwengwe, G.; Gowthorpe, C.; Ali, S.R.; Warren, H.; Drury, D.J.; Ang, K.L.; Gibson, J.A.G.; Dobbs, T.D.; Whitaker, I.S. Prevalence & odds of anxiety & depression in cutaneous malignant melanoma: A proportional meta-analysis & regression. Br. J. Dermatol. 2024, 191, 24–35. [Google Scholar]
- Özdemir, B.C. Immune checkpoint inhibitor-related hypogonadism and infertility: A neglected issue in immuno-oncology. J. Immunother. Cancer 2021, 9, e002220. [Google Scholar] [CrossRef]
- Garutti, M.; Lambertini, M.; Puglisi, F. Checkpoint inhibitors, fertility, pregnancy, and sexual life: A systematic review. ESMO Open 2021, 6, 100276. [Google Scholar] [CrossRef]
- de Melo, A.C.; Barbosa, E.; de Andrade Vieira, M.; de Carvalho, L.M.; de Souza Fonseca, L.; Lopes Carvalho, A.; de Almeida, C.E. Genital mucosal melanoma: Challenges in diagnosis and treatment. Cancer Treat. Rev. 2020, 88, 102062. [Google Scholar]
- Rosen, R.C.; Cappelleri, J.C.; Smith, M.D.; Lipsky, J.; Peña, B.M. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int. J. Impot. Res. 1999, 11, 319–326. [Google Scholar] [CrossRef]
- Isidori, A.M.; Pozza, C.; Esposito, K.; Giugliano, D.; Morano, S.; Vignozzi, L.; Corona, G.; Lenzi, A.; Jannini, E.A. Development and validation of a 6-item version of the female sexual function index (FSFI) as a diagnostic tool for female sexual dysfunction. J. Sex. Med. 2010, 7, 1139–1146. [Google Scholar] [CrossRef]
- Cuenca-Barrales, C.; Molina-Leyva, A. Risk Factors of Sexual Dysfunction in Patients with Hidradenitis Suppurativa: A Cross-Sectional Study. Dermatology 2020, 236, 37–45. [Google Scholar] [CrossRef]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI): A simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Clinical Guidelines for the Staging, Diagnosis, and Management of Cutaneous Malignant Melanoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Linares-Gonzalez, L.; Lozano-Lozano, I.; Gutierrez-Rojas, L.; Lozano-Lozano, M.; Rodenas-Herranz, T.; Ruiz-Villaverde, R. Sexual Dysfunction and Atopic Dermatitis: A Systematic Review. Life 2021, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, V.; Laan, E.T.M.; den Oudsten, B.L. Sexual health-related care needs among young adult cancer patients and survivors: A systematic literature review. J. Cancer Surviv. 2022, 16, 913–924. [Google Scholar] [CrossRef]
- Qian, M.; Wang, L.; Xing, J.; Shan, X.; Wu, J.; Liu, X. Prevalence of sexual dysfunction in women with cervical cancer: A systematic review and meta-analysis. Psychol. Health Med. 2023, 28, 494–508. [Google Scholar] [CrossRef]
- McCabe, M.P.; Sharlip, I.D.; Lewis, R.; Atalla, E.; Balon, R.; Fisher, A.D.; Laumann, E.; Lee, S.W.; Segraves, R.T. Incidence and Prevalence of Sexual Dysfunction in Women and Men: A Consensus Statement from the Fourth International Consultation on Sexual Medicine 2015. J. Sex. Med. 2016, 13, 144–152. [Google Scholar] [CrossRef]
- Wojciechowska-Zdrojowy, M.; Reid, A.; Szepietowski, J.C.; Wojciechowski, A. Analysis of Sexual Problems in Men with Psoriasis. J. Sex. Marital. Ther. 2018, 44, 737–745. [Google Scholar] [CrossRef]
- Quinn-Nilas, C.; Milhausen, R.R.; McKay, A.; Holzapfel, S. Prevalence and Predictors of Sexual Problems Among Midlife Canadian Adults: Results from a National Survey. J. Sex. Med. 2018, 15, 873–879. [Google Scholar] [CrossRef]
- Åkeflo, L.; Dunberger, G.; Elmerstig, E.; Skokic, V.; Steineck, G.; Bergmark, K. Sexual health and wellbeing among female pelvic cancer survivors following individualized interventions in a nurse-led clinic. Support. Care Cancer 2022, 30, 8981–8996. [Google Scholar] [CrossRef]
- Ask, S.; Schildmeijer, K.; Kaldo, V.; Hellström, A. The effect of psychosocial interventions for sexual health in patients with pelvic cancer: A systematic review and meta-analysis. Acta Oncol. 2024, 63, 230–239. [Google Scholar] [CrossRef]
- Everaars, K.E.; Welbie, M.; Hummelink, S.; Tjin, E.P.M.; de Laat, E.H.; Ulrich, D.J.O. The impact of scars on health-related quality of life after breast surgery: A qualitative exploration. J. Cancer Surviv. 2021, 15, 224–233. [Google Scholar] [CrossRef]
- Park, E.R.; Norris, R.L.; Bober, S.L. Sexual health communication during cancer care: Barriers and recommendations. Cancer J. 2009, 15, 74–77. [Google Scholar] [CrossRef]
| Distribution of Sociodemographic Characteristics, Melanoma-Related Clinical Variables, and Key Indicators of Quality of Life, Mood, and Sexual Function in Patients with Melanoma (N = 75) | |||||
|---|---|---|---|---|---|
| Socio-demographic features | |||||
| Age (years) | 52.70 ± 14.07 | Marital status | With couple | 77.33% (58/75) | |
| Single | 22.67% (17/75) | ||||
| Sex (%) | Male: | 38.67% (29/75) | Occupation | Employed | 54.67% (41/75) |
| Female: | 61.33% (46/75) | Unemployed | 45.33% (34/75) | ||
| Educational level | Basic education | 26.67% (20/75) | Professional education | 17.33% (13/75) | |
| Secondary education | 21.33% (16/75) | University education | 34.67% (26/75) | ||
| Disease characteristics | |||||
| Disease duration (years) | 3.26 ± 6.77 | Visible location of scar | 41.33% (31/75) | ||
| Melanoma stage | I–II | 81.33% (61/75) | Melanoma location | Trunk | 57.33% (43/75) |
| Upper limbs | 4.00% (3/75) | ||||
| Lower limbs | 30.67% (23/75) | ||||
| III–IV | 18.67% (14/75) | Face | 8.00% (6/75) | ||
| Quality of life indicators | |||||
| DLQI | 3.60 ± 3.95 | Negative impact of the disease on sexuality (%) | 29.33% (22/75) | ||
| HADS Depression (score) | 2.34 ± 2.98 | HADS Anxiety (score) | 3.88 ± 4.16 | ||
| FSFI (% of female sexual dysfunction) | 41.30% (19/46) | IIEF (% of male sexual dysfunction) | 68.96% (20/29) | ||
| Overall sexual dysfunction | 52.00% (39/75) | NRS for sexual dysfunction | 2.25 ± 2.95 | ||
| Univariate Analyses of Factors Associated with Sexual Dysfunction in Patients with Melanoma. Associations Were Explored for Female Sexual Dysfunction (FSFI Scores), Male Sexual Dysfunction (IIEF Scores), and Global Sexual Dysfunction (Measured by the Numeric Rating Scale, NRS) | ||||||
|---|---|---|---|---|---|---|
| Factors | Female sexual dysfunction (FSFI) | Male sexual dysfunction (IIEF) | Global sexual dysfunction (NRS for SD) | |||
| Mean/%/beta | p value | Mean/%/beta | p value | Mean/Beta | p value | |
| Age (years) | −0.32 ± 0.08 | <0.01 | −0.06 ± 0.10 | 0.53 | 0.06 ± 0.02 | <0.01 |
| Educational level | Basic: 17.05 ± 2.13 | 0.64 | Basic: 18 ± 1.44 | 0.72 | Basic: 2.52 ± 0.49 | 0.44 |
| Superior: 18.38 ± 1.87 | Superior: 17.23 ± 1.59 | Superior: 2 ± 0.47 | ||||
| Marital status | Couple: 20.02 ± 1.46 | <0.01 | Couple: 17.73 ± 1.20 | 0.78 | Couple: 2.48 ± 0.38 | 0.21 |
| Single: 10.72 ± 2.61 | Single: 17.33 ± 2.35 | Single: 1.47 ± 0.71 | ||||
| Age at onset of disease (years) | −0.30 ± 0.08 | <0.01 | −0.02 ± 0.07 | 0.76 | 0.04 ± 0.02 | 0.07 |
| Disease duration (months) | 0.11 ± 0.04 | 0.01 | 0.03 ± 0.02 | 0.23 | −0.01 ± 0.01 | 0.05 |
| Melanoma stage | I–II: 17.71 ± 1.53 | 0.88 | I–II: 18.71 ± 2.17 | 0.57 | I–II: 2.14 ± 0.37 | 0.52 |
| III–IV: 18.28 ± 3.61 | III–IV: 17.31 ± 1.22 | III–IV: 2.71 ± 0.79 | ||||
| Visible scar location | Yes: 15.84 ± 1.86 | 0.05 | Yes: 16 ± 2.33 | 0.43 | Yes: 1.93 ± 0.53 | 0.43 |
| No: 20.14 ± 2.03 | No: 18.08 ± 1.19 | No: 2.47 ± 0.44 | ||||
| Sentinel lymph node biopsy performed | Yes: 73.68% (14/19) | 0.44 | Yes: 65% (13/20) | 0.48 | Yes: 66.67% (24/36) | 0.52 |
| No: 26.32% (5/19) | No: 35% (7/20) | No: 33.33% (12/36) | ||||
| Melanoma location (Face/Lower limbs/Upper Limbs/Trunk) | Yes: 5.26%/36.84%/5.26%/52.63% | 0.45 | Yes: 15%/5%/5%/75% | 1.0 | Yes: 10.26%/20.51%/5.13%/64.10% | 0.23 |
| No: 3.70%/55.56%/0%/40.74% | No: 11.11%/0%/11.11%/77.78% | No: 5.56%/41.67%/2.78%/50% | ||||
| Anxiety (HADS-A ≥ 8) | Yes: 19.25 ± 2.75 | 0.54 | Yes: 9 ± 5.52 | 0.03 | Yes: 4.76 ± 0.75 | <0.01 |
| No: 17.29 ± 1.63 | No: 17.96 ± 1.04 | No: 1.75 ± 0.34 | ||||
| Depression (HADS-D ≥ 8) | Yes: 1 ± 9.21 | 0.03 | Yes: 9 ± 5.52 | 0.04 | Yes: 7 ± 2.02 | 0.02 |
| No: 18.17 ± 1.37 | No: 17.96 ± 1.04 | No: 2.12 ± 0.33 | ||||
| DLQI | −0.22 ± 0.23 | 0.50 | −1.00 ± 0.53 | 0.04 | 0.21 ± 0.08 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Barba, D.; Sánchez-Díaz, M.; Molina-Leyva, A.; Martínez-López, A.; Arias-Santiago, S. Sexual Dysfunction in Melanoma Survivors: A Cross-Sectional Study on Prevalence and Associated Factors. J. Clin. Med. 2025, 14, 4891. https://doi.org/10.3390/jcm14144891
Muñoz-Barba D, Sánchez-Díaz M, Molina-Leyva A, Martínez-López A, Arias-Santiago S. Sexual Dysfunction in Melanoma Survivors: A Cross-Sectional Study on Prevalence and Associated Factors. Journal of Clinical Medicine. 2025; 14(14):4891. https://doi.org/10.3390/jcm14144891
Chicago/Turabian StyleMuñoz-Barba, Daniel, Manuel Sánchez-Díaz, Alejandro Molina-Leyva, Antonio Martínez-López, and Salvador Arias-Santiago. 2025. "Sexual Dysfunction in Melanoma Survivors: A Cross-Sectional Study on Prevalence and Associated Factors" Journal of Clinical Medicine 14, no. 14: 4891. https://doi.org/10.3390/jcm14144891
APA StyleMuñoz-Barba, D., Sánchez-Díaz, M., Molina-Leyva, A., Martínez-López, A., & Arias-Santiago, S. (2025). Sexual Dysfunction in Melanoma Survivors: A Cross-Sectional Study on Prevalence and Associated Factors. Journal of Clinical Medicine, 14(14), 4891. https://doi.org/10.3390/jcm14144891








