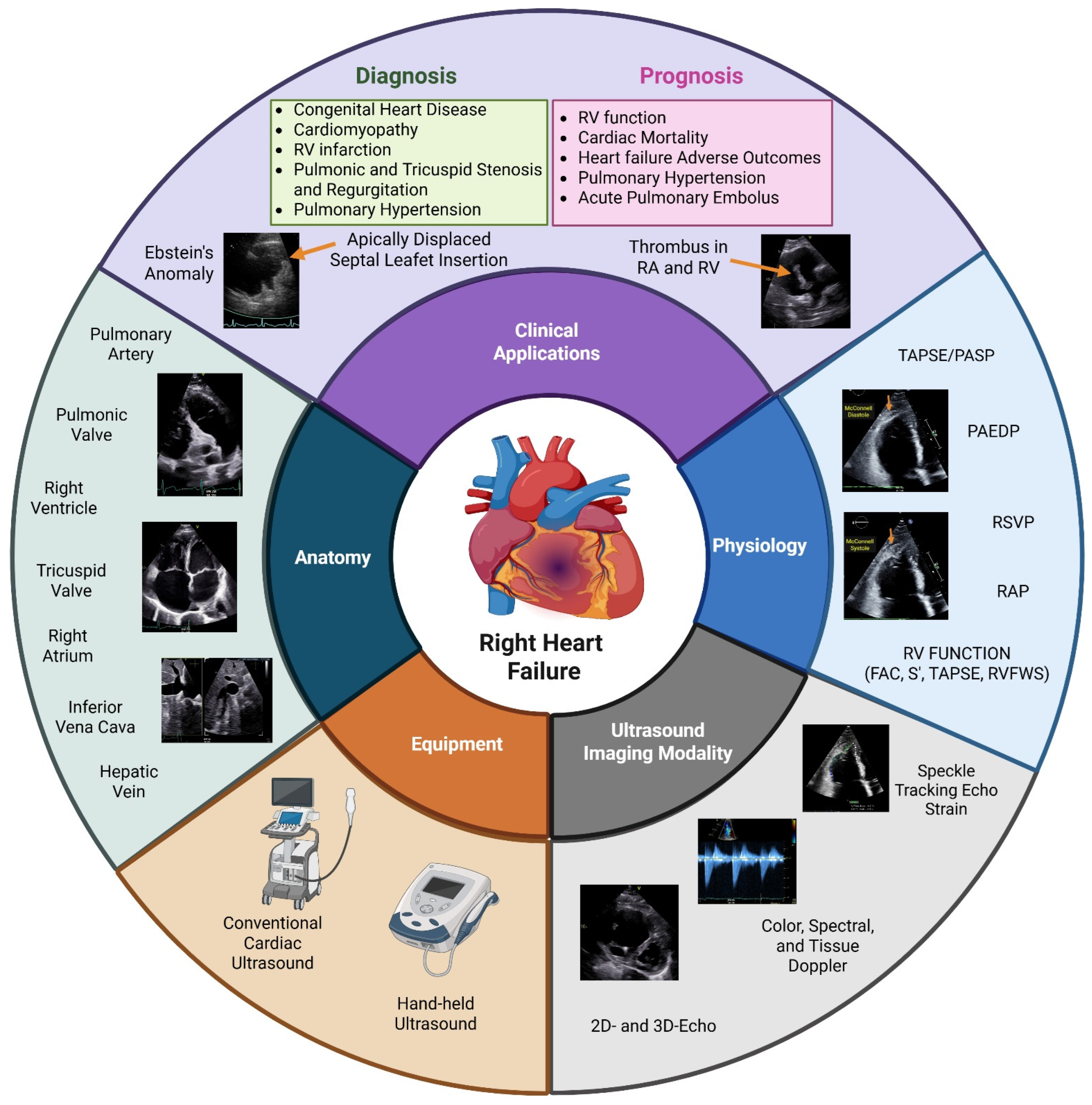
A Narrative Review of the Clinical Applications of Echocardiography in Right Heart Failure
Abstract
1. Introduction
2. Methods
3. Right Ventricular Anatomy
4. Echocardiographic Assessment of the Right Heart
4.1. Right Atrial and Ventricular Size
4.2. Right Ventricular Thickness
4.3. Right Atrial Function
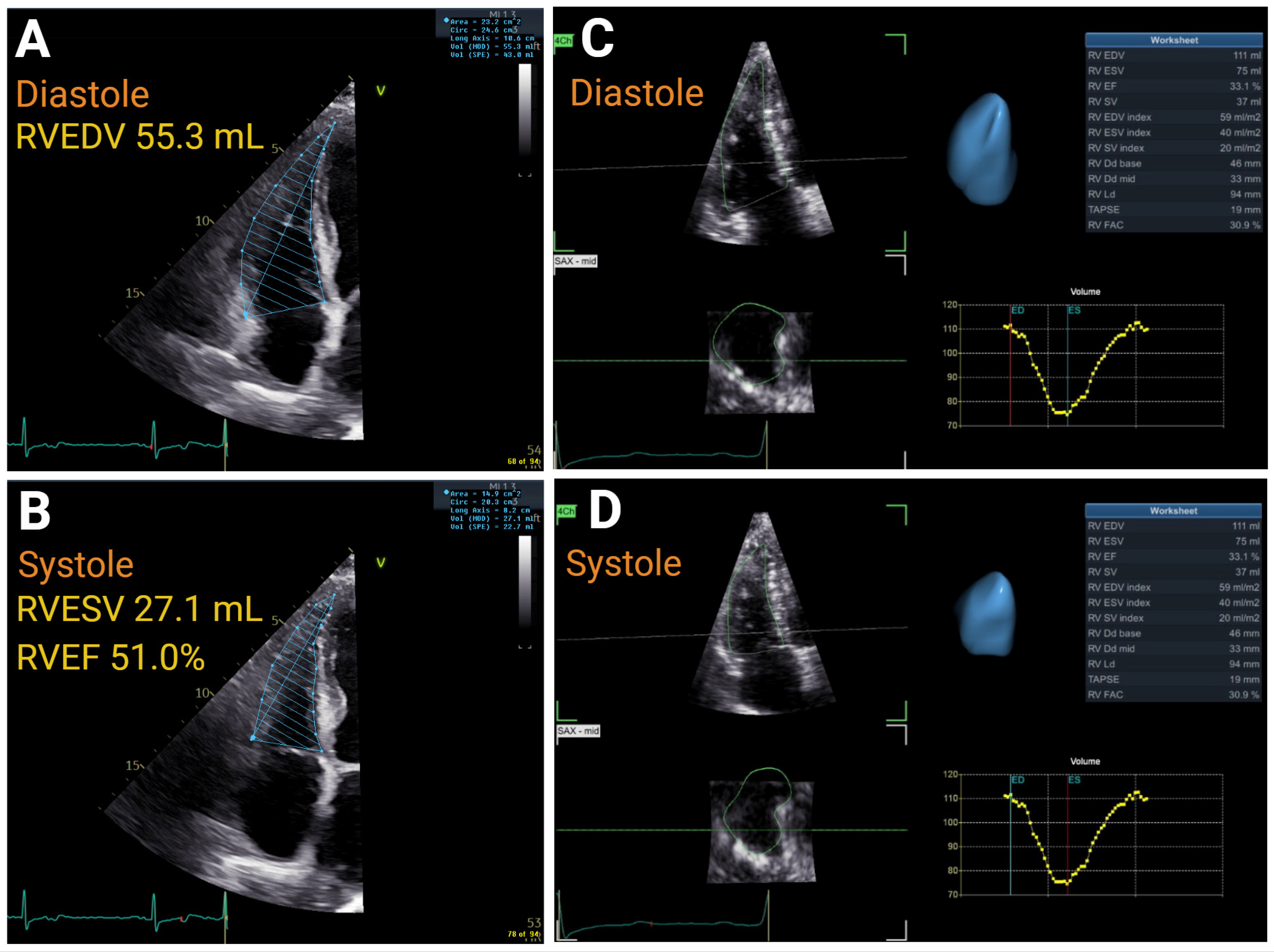
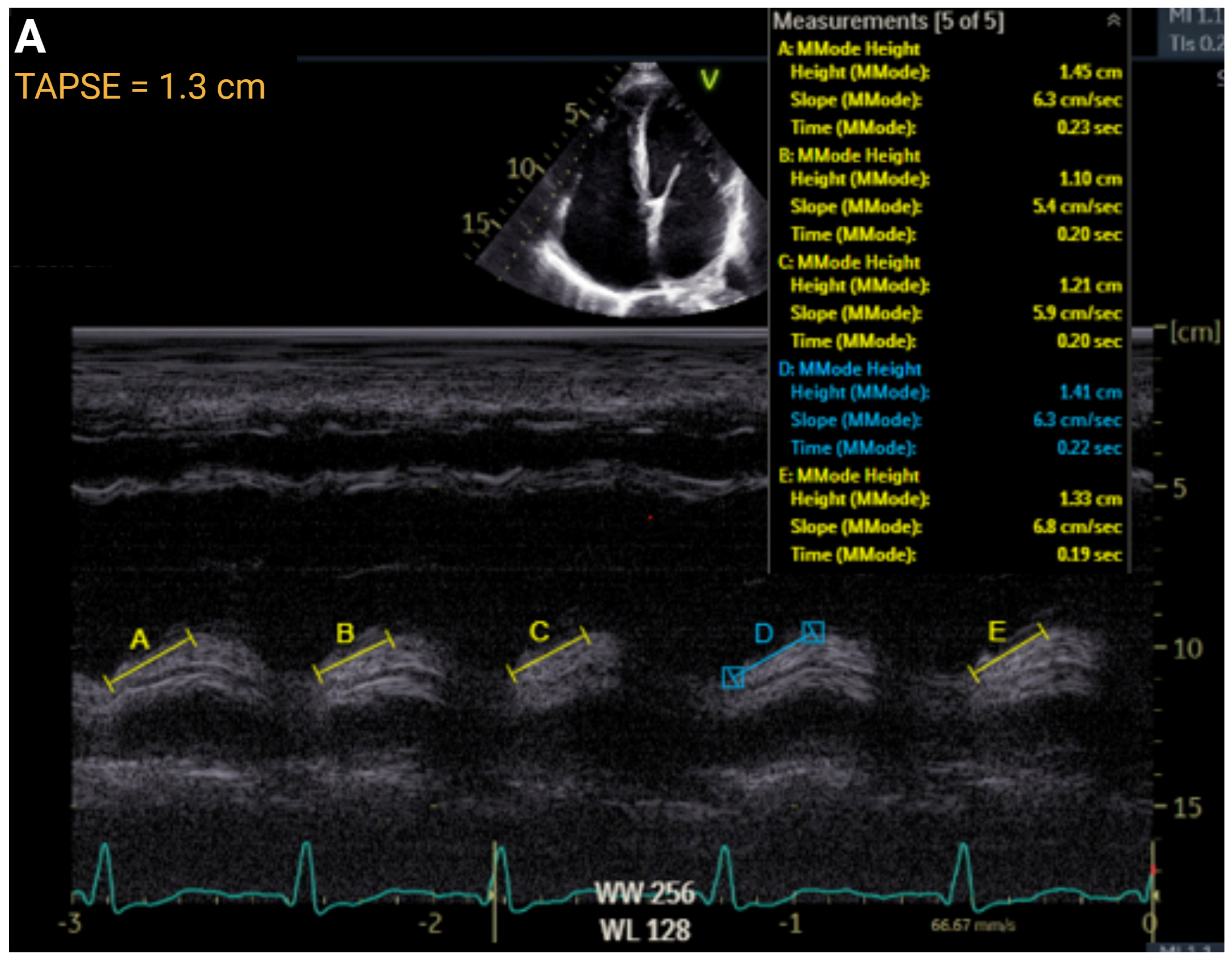
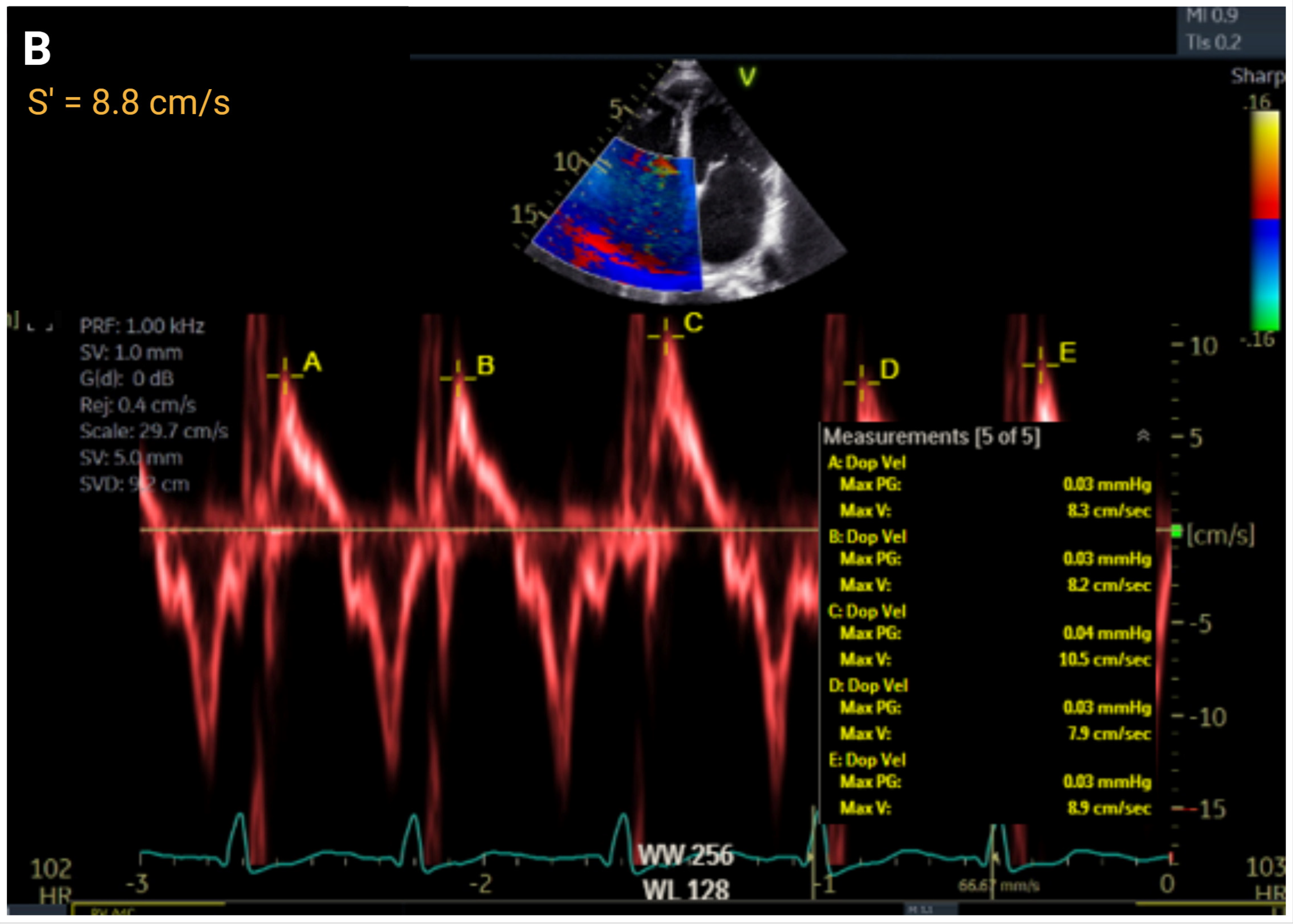
4.4. Right Ventricular Function
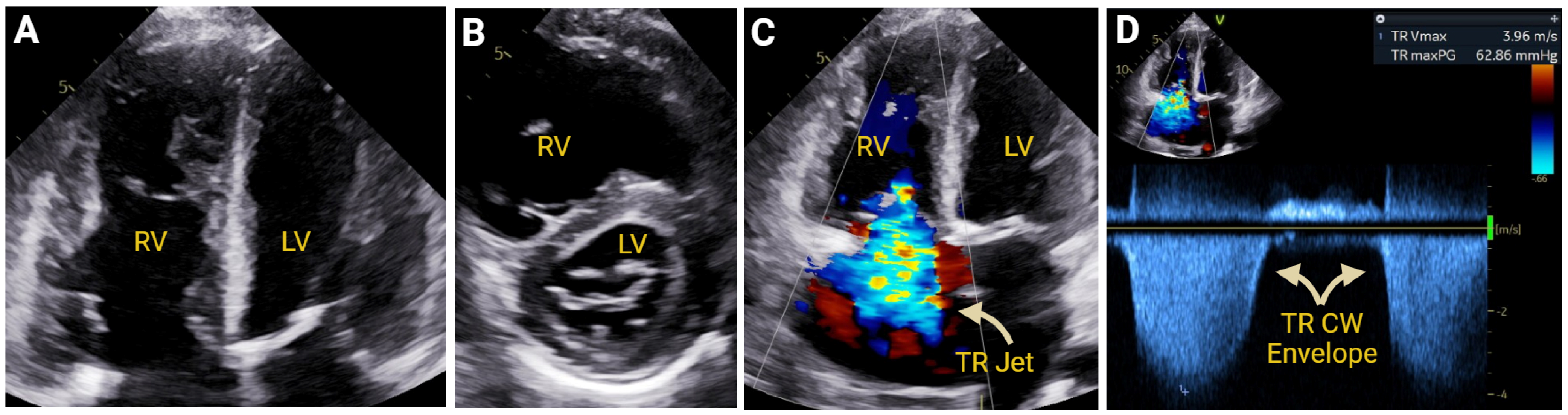
Hemodynamic Assessment
| Cut-Point | Invasive Pressure, mmHg | Sensitivity | Specificity | NPV | PPV | ROC Curve | Correlation Coefficient | N | |
|---|---|---|---|---|---|---|---|---|---|
| IVC Diameter, cm | |||||||||
| Brennan JM et al. 2007 [30] | >2.0 | RAP ≥ 10 mmHg | 0.73 | 0.85 | 0.62 | 0.9 | AUC = 0.76 | r = 0.5 | 102 |
| Egbe AC et al. 2002 [31] | >2.0 | RAP > 10 mmHg | 0.63 | 0.89 | 0.57 | 0.74 | AUC = 0.67 | r = 0.56 | 918 |
| Moreno FL et al. 1984 [32] | >2.3 | RAP > 7 mmHg | 0.4 | 0.97 | 0.58 | 0.93 | NR | NR | 65 |
| Prekker ME et al. 2013 [33] | <2.0 | CVP < 10 mmHg | 0.85 | 0.81 | 0.87 | 0.78 | AUC = 0.91 | r = 0.76 | 65 |
| Taniguchi T et al. 2015 [34] | >2.0 | RAP ≥ 10 mmHg | NR | NR | NR | NR | AUC = 0.83 | r = 0.67 | 90 |
| Utsunomiya H et al. 2009 [35] | >2.3 | RAP > 10 mmHg | 0.87 | 0.83 | NR | NR | AUC = 0.85 | NR | 50 |
| Respirophasic Variation—IVC Collapsibility % (sniff) | |||||||||
| Brennan JM et al. 2007 [30] | <40 | RAP ≥ 10 mmHg | 0.73 | 0.84 | 0.62 | 0.9 | AUC = 0.91 | NR | 102 |
| Egbe AC et al. 2002 [31] | <50 | RAP > 10 mmHg | 85 | 72 | 80 | 78 | AUC = 0.76 | r = 0.72 | 918 |
| Kircher BJ et al. 1990 [36] | <50 | RAP ≥ 10 mmHg | 87 | 82 | NR | NR | NR | r = 0.75 | 83 |
| Taniguichi T et al. 2015 [34] | <50 | RAP ≥ 10 mmHg | NR | NR | NR | NR | NR | NR | 90 |
| Respirophasic Variation—IVC Collapsibility % (passive) | |||||||||
| Brennan MJ et al. 2007 [30] | <20 | RAP > 10 mmHg | 0.73 | 0.82 | 0.57 | 0.9 | AUC = 0.93 | NR | 102 |
| Moreno FL et al. 1985 [32] | <40 | RAP > 7 mmHg | 0.91 | 0.9 | 0.9 | 0.91 | NR | NR | 65 |
| Nagueh SF et al. 1996 [28] | <50 | RAP > 8 mmHg | 0.72 | 0.75 | NR | NR | NR | NR | 85 |
| Prekker ME et al. 2013 [33] | >50 | CVP < 10 mmHg | 0.47 | 0.77 | 0.75 | 0.5 | AUC = 0.66 | NR | 65 |
| Taniguichi T et al. 2015 [34] | <25 | RAP ≥ 10 mmHg | NR | NR | NR | NR | AUC = 0.79 | NR | 90 |
| IVC Diameter + Respirophasic Variation | |||||||||
| Arbo JE et al. 2013 [37] | ≥1.9 cm and >50% | CVP ≥ 10 mmHg | 0.46 | 0.9 | 0.75 | 0.72 | NR | NR | 30 |
| Egbe AC et al. 2002 [31] | ≥2.1 cm and <50% (sniff) | RAP > 10 mmHg | 65 | 99 | 55 | 89 | AUC = 0.76 | r = 0.681 | 918 |
| Doppler—E/A Ratio | |||||||||
| Nagueh SF et al. 1996 [28] | ≥1.1 | RAP > 8 mmHg | NR | NR | NR | NR | NR | r = 0.66 | 85 |
| Tissue Doppler Imaging—E/e′ | |||||||||
| Arbo JE et al. 2013 [37] | >4.6 | CVP ≥ 10 mmHg | 0.85 | 0.9 | 0.85 | 0.9 | NR | NR | 30 |
| Nagueh SF et al. 1996 [28] | >6 | RAP > 8 mmHg | 0.79 | 0.73 | NR | NR | NR | NR | 85 |
| Sade LE et al. 2007 [38] | ≥4 | RAP > 10 | 0.88 | 0.85 | NR | NR | AUC = 0.93 | r = 0.83 | 53 |
| Said K et al. 2012 [39] | ≥4.5 | RAP > 10 mmHg | 0.89 | 1 | NR | NR | AUC = 0.95 | r = 0.84 | 50 |
| Sundereswaran L et al. 1998 [40] | >8 | RAP ≥ 10 mmHg | 0.78 | 0.85 | NR | NR | NR | r = 0.63 | 50 |
| Utsunomiya H et al. 2009 [35] | >7.3 | RAP > 10 | 0.87 | 0.97 | NR | NR | AUC = 0.92 | r = 0.8 | 50 |
| Hepatic Vein Doppler Flow Pattern—Hepatic Vein Systolic Filling Fraction, % | |||||||||
| Nagueh SF et al. 1996 [28] | Vs/(Vs + Vd) < 55 | >8 | 86 | 92 | NR | NR | NR | r = 0.86–0.92 | 85 |
4.5. Right Ventricular–Pulmonary Arterial Coupling and Uncoupling
5. Exercise Hemodynamics and Right Ventricular Functional Reserve
6. Alternative Noninvasive Imaging Modalities
7. Selected Right Ventricular Failure-Associated Conditions
7.1. Acute Right-Sided Heart Failure
7.2. Chronic Right Ventricular Failure
7.2.1. Pulmonary Hypertension
7.2.2. Chemotherapy
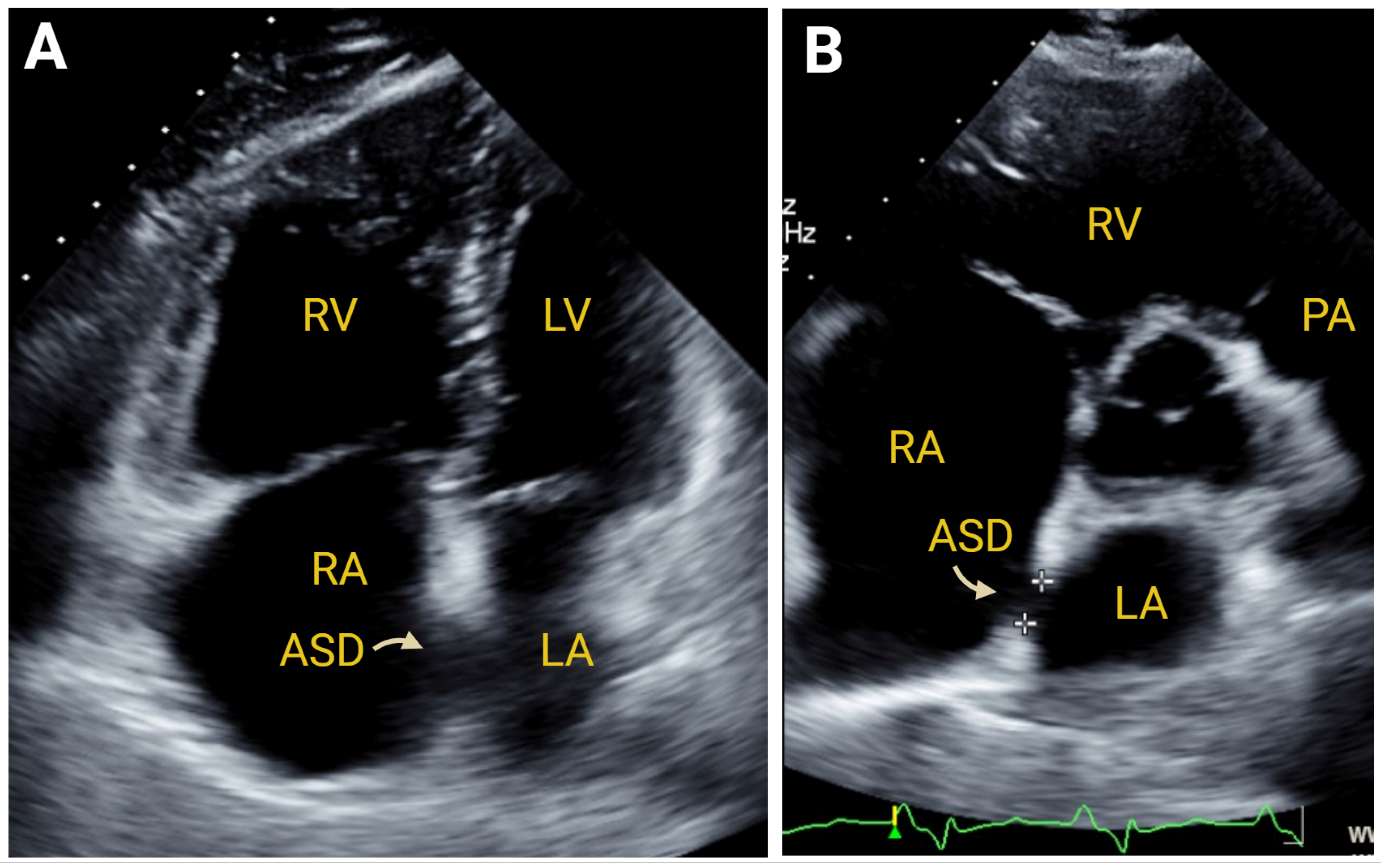
7.2.3. Simple Congenital Heart Defects
7.2.4. Pulmonic and Tricuspid Valvular Disease
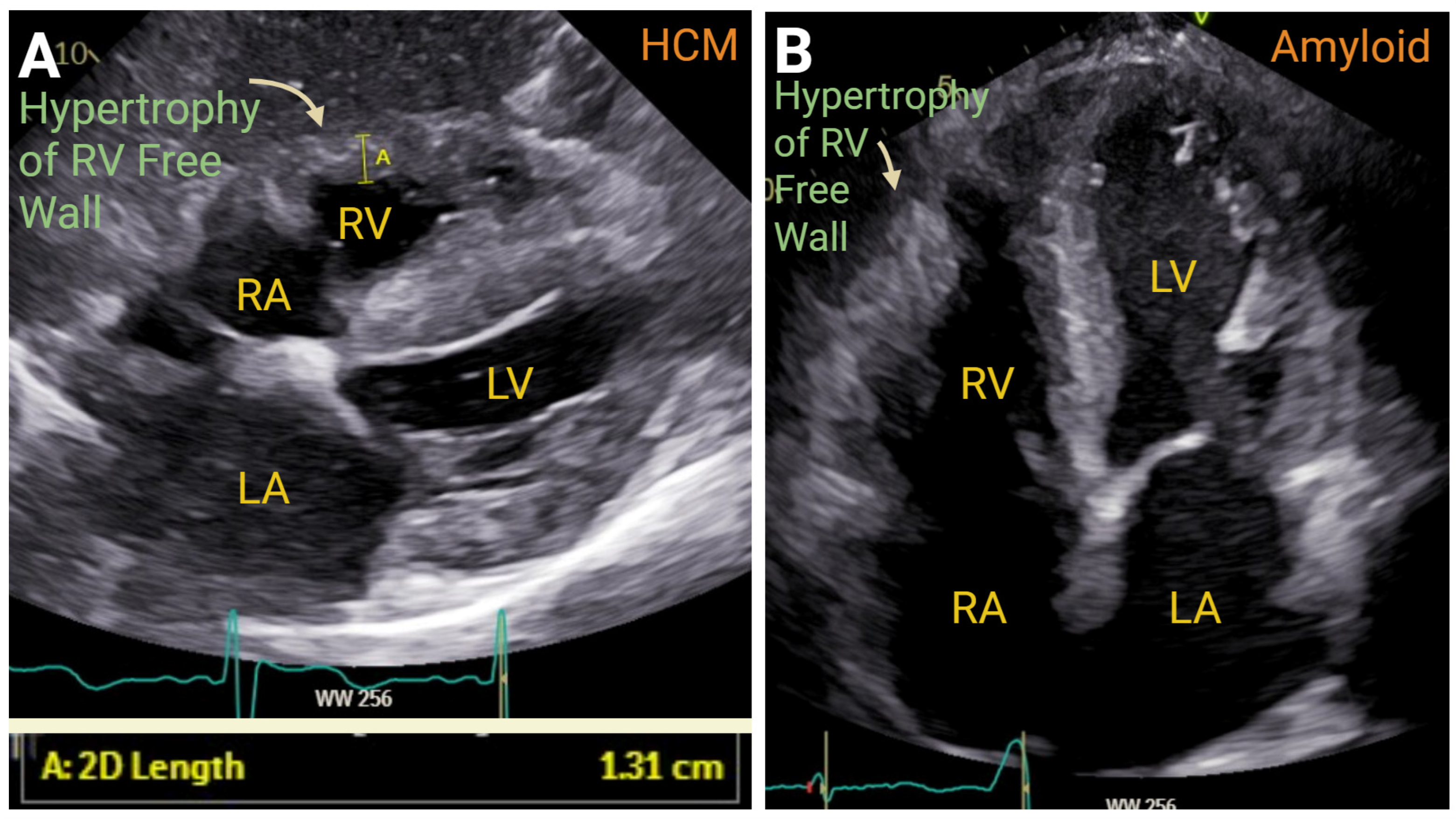
7.3. Cardiomyopathies
8. Conclusions
9. Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanz, J.; Sanchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M.R.; Maron, B.A. The Right Ventricle: From Embryologic Development to RV Failure. Curr. Heart Fail. Rep. 2022, 19, 325–333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walker, L.A.; Buttrick, P.M. The right ventricle: Biologic insights and response to disease: Updated. Curr. Cardiol. Rev. 2013, 9, 73–81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Rudski, L.G.; Addetia, K.; Afilalo, J.; D’Alto, M.; Freed, B.H.; Friend, L.B.; Gargani, L.; Grapsa, J.; Hassoun, P.M.; et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults and Special Considerations in Pulmonary Hypertension: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2025, 38, 141–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Srinivasan, A.; Seoane, T.; Di Franco, A.; Peskin, C.S.; McQueen, D.M.; Paul, T.K.; Feher, A.; Geevarghese, A.; Rozenstrauch, M.; et al. Echocardiographic Linear Dimensions for Assessment of Right Ventricular Chamber Volume as Demonstrated by Cardiac Magnetic Resonance. J. Am. Soc. Echocardiogr. 2016, 29, 861–870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rothstein, E.S.; Palac, R.T.; O’Rourke, D.J.; Venkataraman, P.; Gemignani, A.S.; Friedman, S.E. Evaluation of echocardiographic derived parameters for right ventricular size and function using cardiac magnetic resonance imaging. Echocardiography 2021, 38, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Genovese, D.; Rashedi, N.; Weinert, L.; Narang, A.; Addetia, K.; Patel, A.R.; Prater, D.; Goncalves, A.; Mor-Avi, V.; Lang, R.M. Machine Learning-Based Three-Dimensional Echocardiographic Quantification of Right Ventricular Size and Function: Validation Against Cardiac Magnetic Resonance. J. Am. Soc. Echocardiogr. 2019, 32, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Greiner, S.; Andre, F.; Heimisch, M.; Aurich, M.; Steen, H.; Katus, H.A.; Mereles, D. A closer look at right ventricular 3D volume quantification by transthoracic echocardiography and cardiac MRI. Clin. Radiol. 2019, 74, 490.e7–490.e14. [Google Scholar] [CrossRef] [PubMed]
- Kochav, J.; Chen, J.; Nambiar, L.; Mitlak, H.W.; Kushman, A.; Sultana, R.; Horn, E.; RouChoudhury, A.; Devereux, R.B.; Weinsaft, J.W.; et al. Novel Echocardiographic Algorithm for Right Ventricular Mass Quantification: Cardiovascular Magnetic Resonance and Clinical Prognosis Validation. J. Am. Soc. Echocardiogr. 2021, 34, 839–850.e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krittanawong, C.; Maitra, N.S.; Hassan Virk, H.U.; Farrell, A.; Hamzeh, I.; Arya, B.; Pressman, G.S.; Wang, Z.; Marwick, T.H. Normal Ranges of Right Atrial Strain: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2023, 16, 282–294. [Google Scholar] [CrossRef] [PubMed]
- D’Alto, M.; D’Andrea, A.; Di Salvo, G.; Scognamiglio, G.; Argiento, P.; Romeo, E.; Di Marco, G.M.; Mattera Iacono, A.; Bossone, E.; Sarubbi, B.; et al. Right atrial function and prognosis in idiopathic pulmonary arterial hypertension. Int. J. Cardiol. 2017, 248, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Querejeta Roca, G.; Campbell, P.; Claggett, B.; Solomon, S.D.; Shah, A.M. Right Atrial Function in Pulmonary Arterial Hypertension. Circ. Cardiovasc. Imaging 2015, 8, e003521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alenezi, F.; Mandawat, A.; Il’Giovine, Z.J.; Shaw, L.K.; Siddiqui, I.; Tapson, V.F.; Arges, K.; Rivera, D.; Romano, M.M.D.; Velazquez, E.J.; et al. Clinical Utility and Prognostic Value of Right Atrial Function in Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2018, 11, e006984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bai, Y.; Yang, J.; Liu, J.; Ning, H.; Zhang, R. Right atrial function for the prediction of prognosis in connective tissue disease-associated pulmonary arterial hypertension: A study with two-dimensional speckle tracking. Int. J. Cardiovasc. Imaging 2019, 35, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Vitarelli, A.; Mangieri, E.; Gaudio, C.; Tanzilli, G.; Miraldi, F.; Capotosto, L. Right atrial function by speckle tracking echocardiography in atrial septal defect: Prediction of atrial fibrillation. Clin. Cardiol. 2018, 41, 1341–1347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruscio, E.; Gabrielli, F.A.; Pinnacchio, G.; Spera, F.R.; Giordano, F.; Scacciavillani, R.; Narducci, M.L.; Bencardino, G.; Perna, F.; Crea, F.; et al. Right atrial strain in atrial fibrillation: The hidden side of the moon. Front. Cardiovasc. Med. 2025, 12, 1578524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gentile, F.; Chianca, M.; Bazan, L.; Sciarrone, P.; Chubuchny, V.; Taddei, C.; Poggianti, E.; Passino, C.; Emdin, M.; Giannoni, A. Incremental Prognostic Value of Echocardiography Measures of Right Ventricular Systolic Function in Patients with Chronic Heart Failure. J. Am. Heart Assoc. 2025, 14, e038616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaul, S.; Tei, C.; Hopkins, J.M.; Shah, P.M. Assessment of right ventricular function using two-dimensional echocardiography. Am. Heart J. 1984, 107, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Aloia, E.; Cameli, M.; D’Ascenzi, F.; Sciaccaluga, C.; Mondillo, S. TAPSE: An old but useful tool in different diseases. Int. J. Cardiol. 2016, 225, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Pislaru, S.; Borlaug, B.A. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur. Heart J. 2019, 40, 689–697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sugawara, Y.; Yoshihisa, A.; Takeishi, R.; Ohara, H.; Anzai, F.; Hotsuki, Y.; Watanabe, K.; Sato, Y.; Abe, S.; Misaka, T.; et al. Prognostic Effects of Changes in Right Ventricular Fractional Area Change in Patients With Heart Failure. Circ. J. 2022, 86, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, E.; Biagioli, P.; Lauciello, R.; Zuchi, C.; Mengoni, A.; Bardelli, G.; Alunni, G.; Gronda, E.G.; Ambrosio, G. Superior Prognostic Value of Right Ventricular Free Wall Compared to Global Longitudinal Strain in Patients With Heart Failure. J. Am. Soc. Echocardiogr. 2019, 32, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, G.; Carerj, S.; Di Bella, G.; Manganaro, R.; Pizzino, F.; Restelli, D.; Pelaggi, G.; Lofrumento, F.; Licordari, R.; Taverna, G.; et al. Clinical Applications of Myocardial Work in Echocardiography: A Comprehensive Review. J. Cardiovasc. Echogr. 2024, 34, 99–113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Landra, F.; Sciaccaluga, C.; Pastore, M.C.; Gallone, G.; Barilli, M.; Fusi, C.; Focardi, M.; Cavigli, L.; D’Ascenzi, F.; Natali, B.M.; et al. Right ventricular myocardial work for the prediction of early right heart failure and long-term mortality after left ventricular assist device implant. Eur. Heart J. Cardiovasc. Imaging. 2023, 25, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Butcher, S.C.; Feloukidis, C.; Kamperidis, V.; Yedidya, I.; Stassen, J.; Fortuni, F.; Vrana, E.; Mouratoglou, S.A.; Boutou, A.; Giannakoulas, G.; et al. Right Ventricular Myocardial Work Characterization in Patients With Pulmonary Hypertension and Relation to Invasive Hemodynamic Parameters and Outcomes. Am. J. Cardiol. 2022, 177, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Ciozda, W.; Kedan, I.; Kehl, D.W.; Zimmer, R.; Khandwalla, R.; Kimchi, A. The efficacy of sonographic measurement of inferior vena cava diameter as an estimate of central venous pressure. Cardiovasc. Ultrasound. 2016, 14, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagueh, S.F.; Kopelen, H.A.; Zoghbi, W.A. Relation of mean right atrial pressure to echocardiographic and Doppler parameters of right atrial and right ventricular function. Circulation 1996, 93, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Knight, D.S.; Augustine, D.X.; Harkness, A.; Oxborough, D.; Pearce, K.; Ring, L.; Robinson, S.; Stout, M.; Willis, J.; et al. Echocardiographic assessment of the right heart in adults: A practical guideline from the British Society of Echocardiography. Echo Res. Pract. 2020, 7, G19–G41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brennan, J.M.; Blair, J.E.; Goonewardena, S.; Ronan, A.; Shah, D.; Vasaiwala, S.; Kirkpatrick, J.N.; Spencer, K.T. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J. Am. Soc. Echocardiogr. 2007, 20, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Egbe, A.C.; Connolly, H.M.; Pellikka, P.A.; Anderson, J.H.; Miranda, W.R. Role of Inferior Vena Cava Dynamics for Estimating Right Atrial Pressure in Congenital Heart Disease. Circ. Cardiovasc. Imaging 2022, 15, e014308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moreno, F.L.; Hagan, A.D.; Holmen, J.R.; Pryor, T.A.; Strickland, R.D.; Castle, C.H. Evaluation of size and dynamics of the inferior vena cava as an index of right-sided cardiac function. Am. J. Cardiol. 1984, 53, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Prekker, M.E.; Scott, N.L.; Hart, D.; Sprenkle, M.D.; Leatherman, J.W. Point-of-care ultrasound to estimate central venous pressure: A comparison of three techniques. Crit. Care Med. 2013, 41, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Ohtani, T.; Nakatani, S.; Hayashi, K.; Yamaguchi, O.; Komuro, I.; Sakata, Y. Impact of Body Size on Inferior Vena Cava Parameters for Estimating Right Atrial Pressure: A Need for Standardization? J. Am. Soc. Echocardiogr. 2015, 28, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Nakatani, S.; Nishihira, M.; Kanzaki, H.; Kyotani, S.; Nakanishi, N.; Kihara, Y.; Kitakaze, M. Value of estimated right ventricular filling pressure in predicting cardiac events in chronic pulmonary arterial hypertension. J. Am. Soc. Echocardiogr. 2009, 22, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Kircher, B.J.; Himelman, R.B.; Schiller, N.B. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am. J. Cardiol. 1990, 66, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Arbo, J.E.; Maslove, D.M.; Beraud, A.S. Bedside assessment of right atrial pressure in critically ill septic patients using tissue Doppler ultrasonography. J. Crit. Care. 2013, 28, 1112. [Google Scholar] [CrossRef] [PubMed]
- Sade, L.E.; Gulmez, O.; Eroglu, S.; Sezgin, A.; Muderrisoglu, H. Noninvasive estimation of right ventricular filling pressure by ratio of early tricuspid inflow to annular diastolic velocity in patients with and without recent cardiac surgery. J. Am. Soc. Echocardiogr. 2007, 20, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Said, K.; Shehata, A.; Ashour, Z.; El-Tobgi, S. Value of conventional and tissue Doppler echocardiography in the noninvasive measurement of right atrial pressure. Echocardiography 2012, 29, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Sundereswaran, L.; Nagueh, S.F.; Vardan, S.; Middleton, K.J.; Zoghbi, W.A.; Quinones, M.A.; Torre-Amione, G. Estimation of left and right ventricular filling pressures after heart transplantation by tissue Doppler imaging. Am. J. Cardiol. 1998, 82, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Amsallem, M.; Sternbach, J.M.; Adigopula, S.; Kobayashi, Y.; Vu, T.A.; Zamanian, R.; Liang, D.; Dhillon, G.; Schnittger, I.; McConnell, M.V.; et al. Addressing the Controversy of Estimating Pulmonary Arterial Pressure by Echocardiography. J. Am. Soc. Echocardiogr. 2016, 29, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Platts, D.G.; Vaishnav, M.; Burstow, D.J.; Craig, C.H.; Chan, J.; Sedgwick, J.L.; Scalia, G.M. Contrast microsphere enhancement of the tricuspid regurgitant spectral Doppler signal-Is it still necessary with contemporary scanners? Int. J. Cardiol. Heart Vasc. 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jankowich, M.; Maron, B.A.; Choudhary, G. Mildly elevated pulmonary artery systolic pressure on echocardiography: Bridging the gap in current guidelines. Lancet Respir. Med. 2021, 9, 1185–1191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Q.; Zhang, M. Echocardiography assessment of right ventricular-pulmonary artery coupling: Validation of surrogates and clinical utilities. Int. J. Cardiol. 2024, 394, 131358. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.L.; Arena, R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1373–H1381. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Gargani, L.; Armstrong, W.F.; Lancellotti, P.; Lester, S.J.; Grunig, E.; D’Alto, M.; Astrom Aneq, M.; Ferrara, F.; Saggar, R.; et al. Stressing the Cardiopulmonary Vascular System: The Role of Echocardiography. J. Am. Soc. Echocardiogr. 2018, 31, 527–550. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023, 61, 2200879. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R.; Vanderpool, R.; Dhakal, B.P.; Saggar, R.; Saggar, R.; Vachiery, J.L.; Lewis, G.D. Exercise-induced pulmonary hypertension: Physiological basis and methodological concerns. Am. J. Respir. Crit. Care Med. 2013, 187, 576–583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gargani, L.; Pugliese, N.R.; De Biase, N.; Mazzola, M.; Agoston, G.; Arcopinto, M.; Argiento, P.; Armstrong, W.F.; Bandera, F.; Cademartiri, F.; et al. Exercise Stress Echocardiography of the Right Ventricle and Pulmonary Circulation. J. Am. Coll. Cardiol. 2023, 82, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Kurzyna, M.; Torbicki, A.; Pruszczyk, P.; Burakowska, B.; Fijalkowska, A.; Kober, J.; Oniszh, K.; Kuca, P.; Tomkowski, W.; Burakowski, J.; et al. Disturbed right ventricular ejection pattern as a new Doppler echocardiographic sign of acute pulmonary embolism. Am. J. Cardiol. 2002, 90, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Kurnicka, K.; Lichodziejewska, B.; Goliszek, S.; Dzikowska-Diduch, O.; Zdonczyk, O.; Kozlowska, M.; Kostrubiec, M.; Ciurzynski, M.; Palczewski, P.; Grudzka, K.; et al. Echocardiographic Pattern of Acute Pulmonary Embolism: Analysis of 511 Consecutive Patients. J. Am. Soc. Echocardiogr. 2016, 29, 907–913. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.V.; Solomon, S.D.; Rayan, M.E.; Come, P.C.; Goldhaber, S.Z.; Lee, R.T. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am. J. Cardiol. 1996, 78, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Piazza, G.; Rigatelli, G.; Bilato, C.; Bongarzoni, A.; Henkin, S.; Zonzin, P.; Casazza, F.; Roncon, L. Prognostic Role of Tricuspid Annular Plane Systolic Excursion to Systolic Pulmonary Artery Pressure Ratio for the Identification of Early Clinical Deterioration in Intermediate-High-Risk Pulmonary Embolism Patients. Am. J. Cardiol. 2024, 214, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Davis, J.; Liu, R.; Gupta, V.; Dziura, J.; Moore, C.L. Point-of-care focused cardiac ultrasound for prediction of pulmonary embolism adverse outcomes. J. Emerg. Med. 2013, 45, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V.; Vandelli, R.; Mattioli, G. Doppler echocardiographic evaluation of right ventricular function in patients with right ventricular infarction. J. Ultrasound Med. 2000, 19, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Pretre, R.; Chilcott, M. Blunt trauma to the heart and great vessels. N. Engl. J. Med. 1997, 336, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, M.A.; Lopez-Perez, J.M.; Perez-Castellano, N.; Quero, L.F.; Virgos-Lamela, A.; Otero-Ferreiro, A.; Lasara, A.M.; Vega, M.; Moreno, M.; Pastor-Benavent, J.A.; et al. Role of transesophageal echocardiography in the assessment of patients with blunt chest trauma: Correlation of echocardiographic findings with the electrocardiogram and creatine kinase monoclonal antibody measurements. Am. Heart J. 1998, 135, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M. Heart-lung interactions in COVID-19: Prognostic impact and usefulness of bedside echocardiography for monitoring of the right ventricle involvement. Heart Fail. Rev. 2022, 27, 1325–1339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bleakley, C.; Singh, S.; Garfield, B.; Morosin, M.; Surkova, E.; Mandalia, M.S.; Dias, B.; Androulakis, E.; Price, L.C.; McCabe, C.; et al. Right ventricular dysfunction in critically ill COVID-19 ARDS. Int. J. Cardiol. 2021, 327, 251–258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shafiabadi Hassani, N.; Shojaee, A.; Khodaprast, Z.; Sepahvandi, R.; Shahrestanaki, E.; Rastad, H. Echocardiographic Features of Cardiac Injury Related to COVID-19 and Their Prognostic Value: A Systematic Review. J. Intensive Care Med. 2021, 36, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Martha, J.W.; Pranata, R.; Wibowo, A.; Lim, M.A. Tricuspid annular plane systolic excursion (TAPSE) measured by echocardiography and mortality in COVID-19: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 105, 351–356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johri, A.M.; Galen, B.; Kirkpatrick, J.N.; Lanspa, M.; Mulvagh, S.; Thamman, R. ASE Statement on Point-of-Care Ultrasound during the 2019 Novel Coronavirus Pandemic. J. Am. Soc. Echocardiogr. 2020, 33, 670–673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clement, A.; Tomaselli, M.; Badano, L.P.; Hadareanu, D.R.; Radu, N.; Penso, M.; Caravita, S.; Baratto, C.; Fisicaro, S.; Delcea, C.; et al. Association With Outcome of the Regurgitant-Volume Adjusted Right Ventricular Ejection Fraction in Secondary Tricuspid Regurgitation. J. Am. Soc. Echocardiogr. 2025, 38, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Axmann, J.; Ghofrani, H.A.; Naeije, R.; Narcin, N.; Rieth, A.; Seeger, W.; Gall, H.; Richter, M.J. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int. J. Cardiol. 2018, 266, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Abdar Esfahani, M.; Mokarian, F.; Karimipanah, M. Alterations in the echocardiographic variables of the right ventricle in asymptomatic patients with breast cancer during anthracycline chemotherapy. Postgrad. Med. J. 2017, 93, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Gorgiladze, N.; Shavdia, M.; Gaprindashvili, T.; Gogua, E.; Gachechiladze, L.; Gujabidze, M.; Pagava, Z. Detection of Cardiotoxicity Using Right Ventricular Free Wall Longitudinal Strain in Low Cardiovascular Risk Breast Cancer Patients Receiving Low-Dose Anthracycline Treatment. Cureus 2024, 16, e63138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fischer-Bacca, C.O.; Huntermann, R.; de Oliveira, J.P.; Alexandrino, F.B.; Sato, M.Y.; Cardoso, R.; Gomes, R.; Melo, E.S. Systematic review and meta-analysis of right ventricular changes in cancer-therapy—The forgotten ventricle in cardio-oncology. Curr. Probl. Cardiol. 2025, 50, 103039. [Google Scholar] [CrossRef] [PubMed]
- Tikoff, G.; Schmidt, A.M.; Kuida, H.; Hecht, H.H. Heart failure in atrial septal defect. Am. J. Med. 1965, 39, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Ramcharan, T.K.W.; Goff, D.A.; Greenleaf, C.E.; Shebani, S.O.; Salazar, J.D.; Corno, A.F. Ebstein’s Anomaly: From Fetus to Adult-Literature Review and Pathway for Patient Care. Pediatr. Cardiol. 2022, 43, 1409–1428. [Google Scholar] [CrossRef] [PubMed]
- Dice, J.E.; Bhatia, J. Patent ductus arteriosus: An overview. J. Pediatr. Pharmacol. Ther. 2007, 12, 138–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhattacharyya, S.; Davar, J.; Dreyfus, G.; Caplin, M.E. Carcinoid heart disease. Circulation 2007, 116, 2860–2865. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Brener, M.I.; Cox, Z.L.; Pinney, S.; Lindenfeld, J. Tricuspid Regurgitation Management for Heart Failure. JACC Heart Fail. 2023, 11, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, Y.L.; Debonnaire, P.; Bootsma, M.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Prevalence and Prognostic Implications of Right Ventricular Dysfunction in Patients With Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2019, 124, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Knowles, J.W.; Pavlovic, A.; Perez, M.; Magavern, E.; Sinagra, G.; Haddad, F.; Ashley, E.A. Prevalence and clinical correlates of right ventricular dysfunction in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2014, 113, 361–367. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Limongelli, G.; Baldini, L.; Verrengia, M.; Carbone, A.; Di Palma, E.; Vastarella, R.; Masarone, D.; Tagliamonte, G.; Riegler, L.; et al. Exercise speckle-tracking strain imaging demonstrates impaired right ventricular contractile reserve in hypertrophic cardiomyopathy. Int. J. Cardiol. 2017, 227, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.P.; Li, Y.D.; Wang, Y.D.; Zhang, M.; Zhu, W.W.; Cai, Q.Z.; Jiang, W.; Sun, L.L.; Ding, X.Y.; Ye, X.G.; et al. Impaired Right Ventricular Mechanics at Rest and During Exercise Are Associated With Exercise Capacity in Patients With Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2019, 8, e011269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monivas Palomero, V.; Durante-Lopez, A.; Sanabria, M.T.; Cubero, J.S.; Gonzalez-Mirelis, J.; Lopez-Ibor, J.V.; Navarro Rico, S.M.; Krsnik, I.; Dominguez, F.; Mingo, A.M.; et al. Role of Right Ventricular Strain Measured by Two-Dimensional Echocardiography in the Diagnosis of Cardiac Amyloidosis. J. Am. Soc. Echocardiogr. 2019, 32, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, S.; Henein, M.Y.; Wikstrom, G.; Suhr, O.B.; Lindqvist, P. Right ventricular involvement in transthyretin amyloidosis. Amyloid 2018, 25, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Tomasoni, D.; Adamo, M.; Porcari, A.; Aimo, A.; Bonfioli, G.B.; Castiglione, V.; Franzini, M.; Inciardi, R.M.; Khalil, A.; Lombardi, C.M.; et al. Right ventricular to pulmonary artery coupling and outcome in patients with cardiac amyloidosis. Eur. Heart J. Cardiovasc. Imaging. 2023, 24, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation 2010, 121, 1533–1541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malik, N.; Mukherjee, M.; Wu, K.C.; Zimmerman, S.L.; Zhan, J.; Calkins, H.; James, C.A.; Gilotra, N.A.; Sheikh, F.H.; Tandri, H.; et al. Multimodality Imaging in Arrhythmogenic Right Ventricular Cardiomyopathy. Circ. Cardiovasc. Imaging 2022, 15, e013725. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Qin, X.; Zhang, J.; Guo, X. Artificial intelligence: The future for multimodality imaging of right ventricle. Int. J. Cardiol. 2024, 404, 131970. [Google Scholar] [CrossRef] [PubMed]
- Hsia, B.C.; Lai, A.; Singh, S.; Samtani, R.; Bienstock, S.; Liao, S.; Stern, E.; LaRocca, G.; Sanz, J.; Lerakis, S.; et al. Validation of American Society of Echocardiography Guideline-Recommended Parameters of Right Ventricular Dysfunction Using Artificial Intelligence Compared With Cardiac Magnetic Resonance Imaging. J. Am. Soc. Echocardiogr. 2023, 36, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bao, Y.; Zheng, K.; Zhou, W.; Zhang, J.; Sun, R.; Deng, Y.; Xia, L.; Liu, Y. Quantitative assessment of right ventricular size and function with multiple parameters from artificial intelligence-based three-dimensional echocardiography: A comparative study with cardiac magnetic resonance. Echocardiography 2022, 39, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bose, R.; Ahmed, A.; Maslow, A.; Feng, Y.; Sharkey, A.; Baribeau, Y.; Mahmood, F.; Matyal, R.; Khabbaz, K. Artificial Intelligence-Based Assessment of Indices of Right Ventricular Function. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2698–2702. [Google Scholar] [CrossRef] [PubMed]
- Fortmeier, V.; Lachmann, M.; Stolz, L.; von Stein, J.; Unterhuber, M.; Kassar, M.; Gercek, M.; Schober, A.R.; Stocker, T.J.; Omran, H.; et al. Artificial intelligence-enabled assessment of right ventricular to pulmonary artery coupling in patients undergoing transcatheter tricuspid valve intervention. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 558–572. [Google Scholar] [CrossRef] [PubMed]
- East, S.A.; Wang, Y.; Yanamala, N.; Maganti, K.; Sengupta, P.P. Artificial Intelligence-Enabled Point-of-Care Echocardiography: Bringing Precision Imaging to the Bedside. Curr. Atheroscler. Rep. 2025, 27, 70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
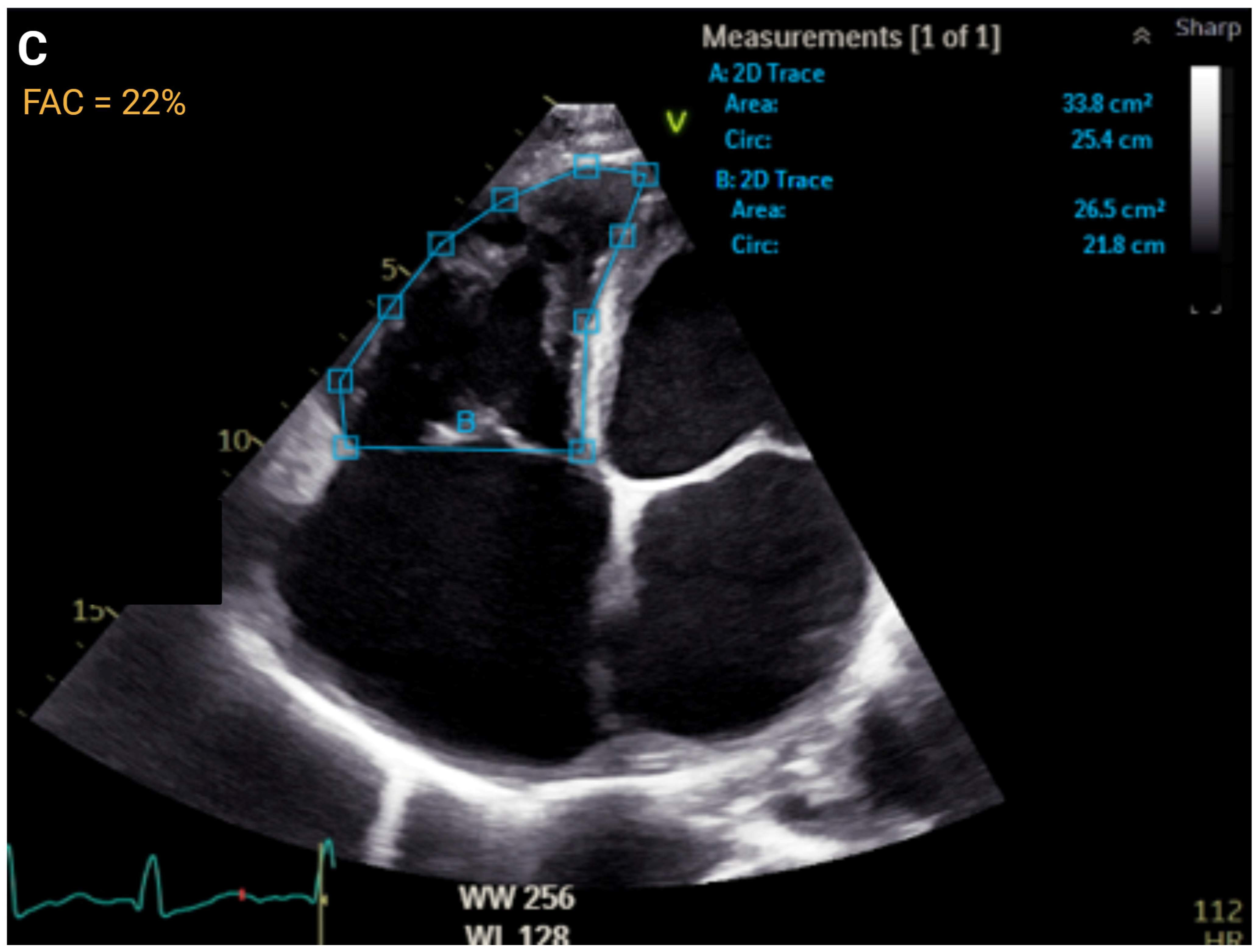

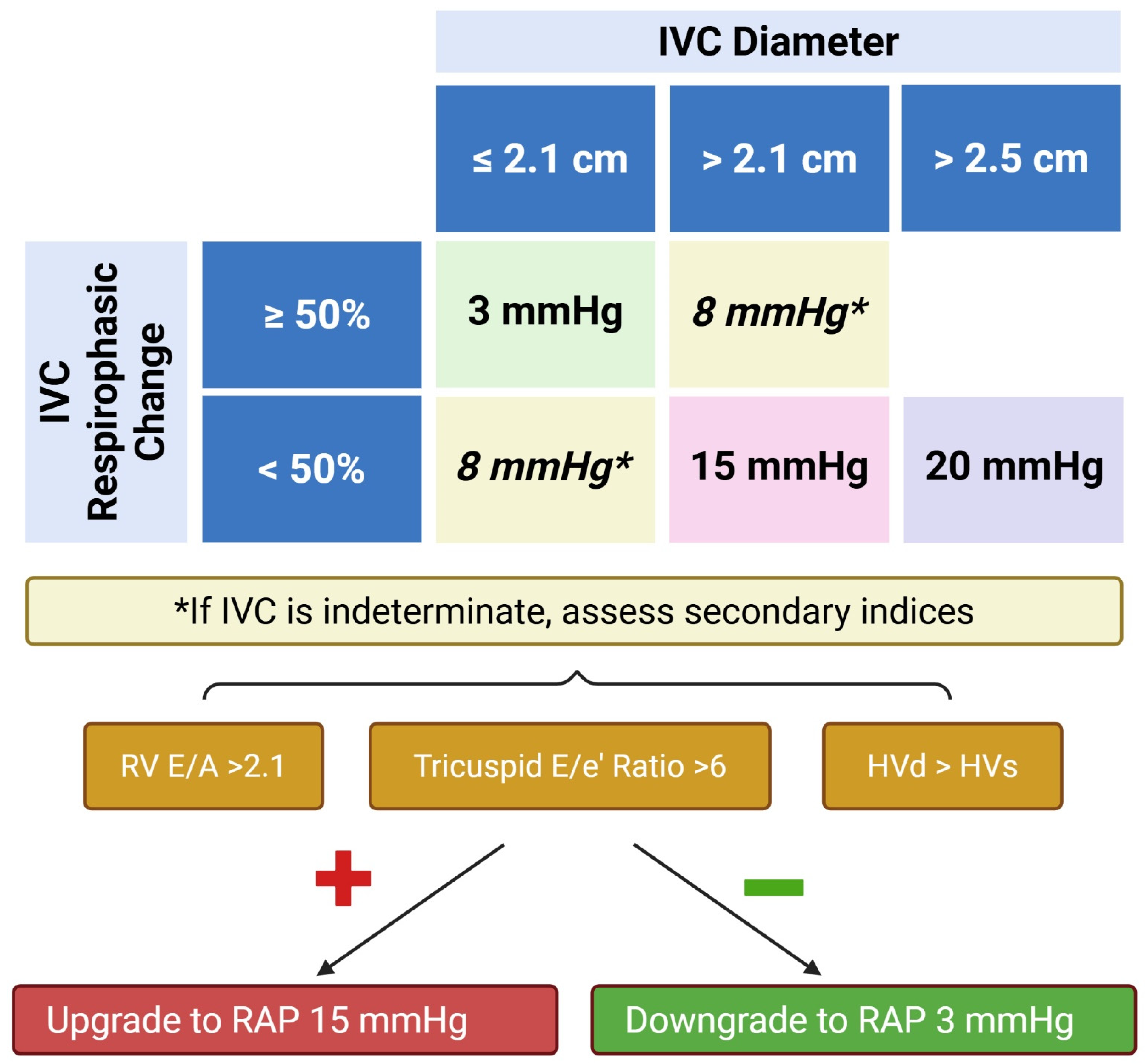

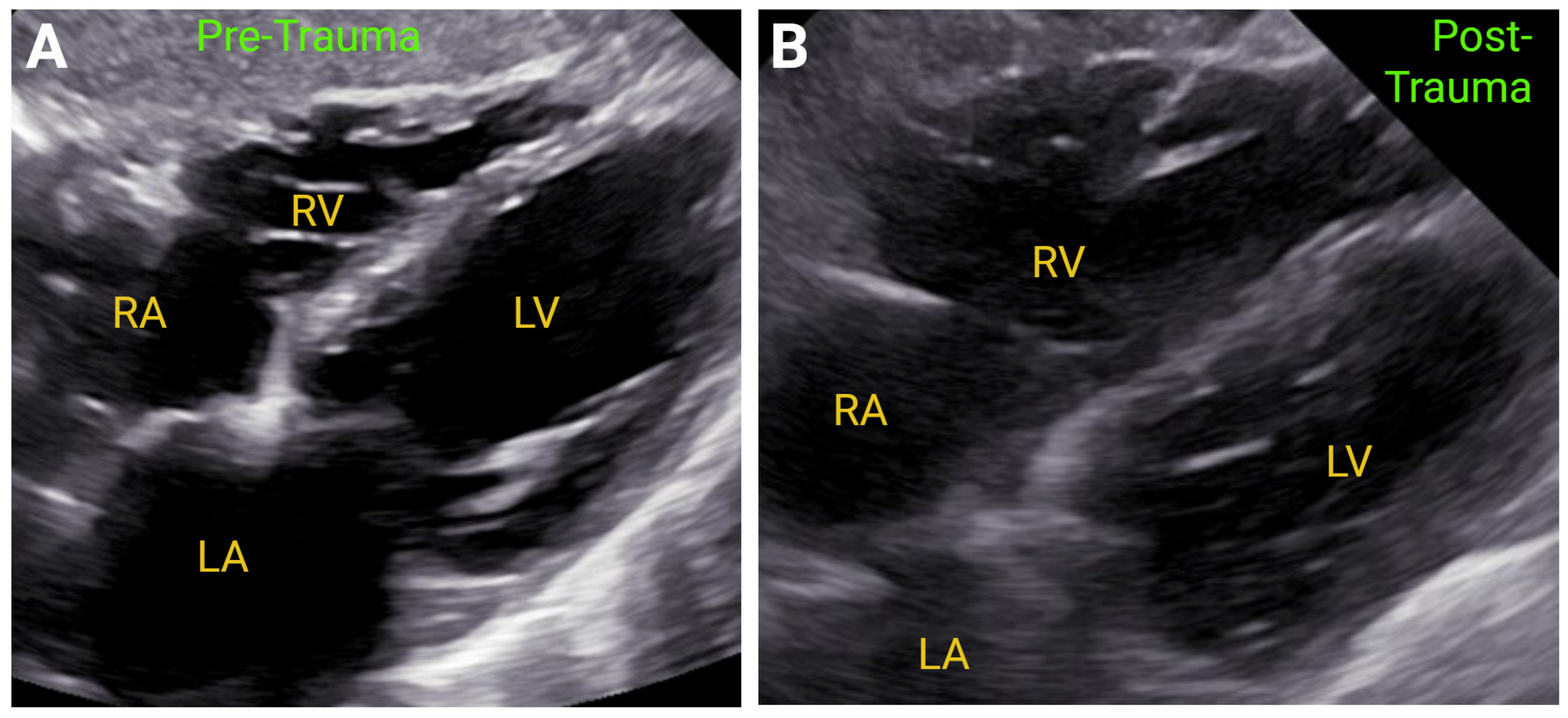
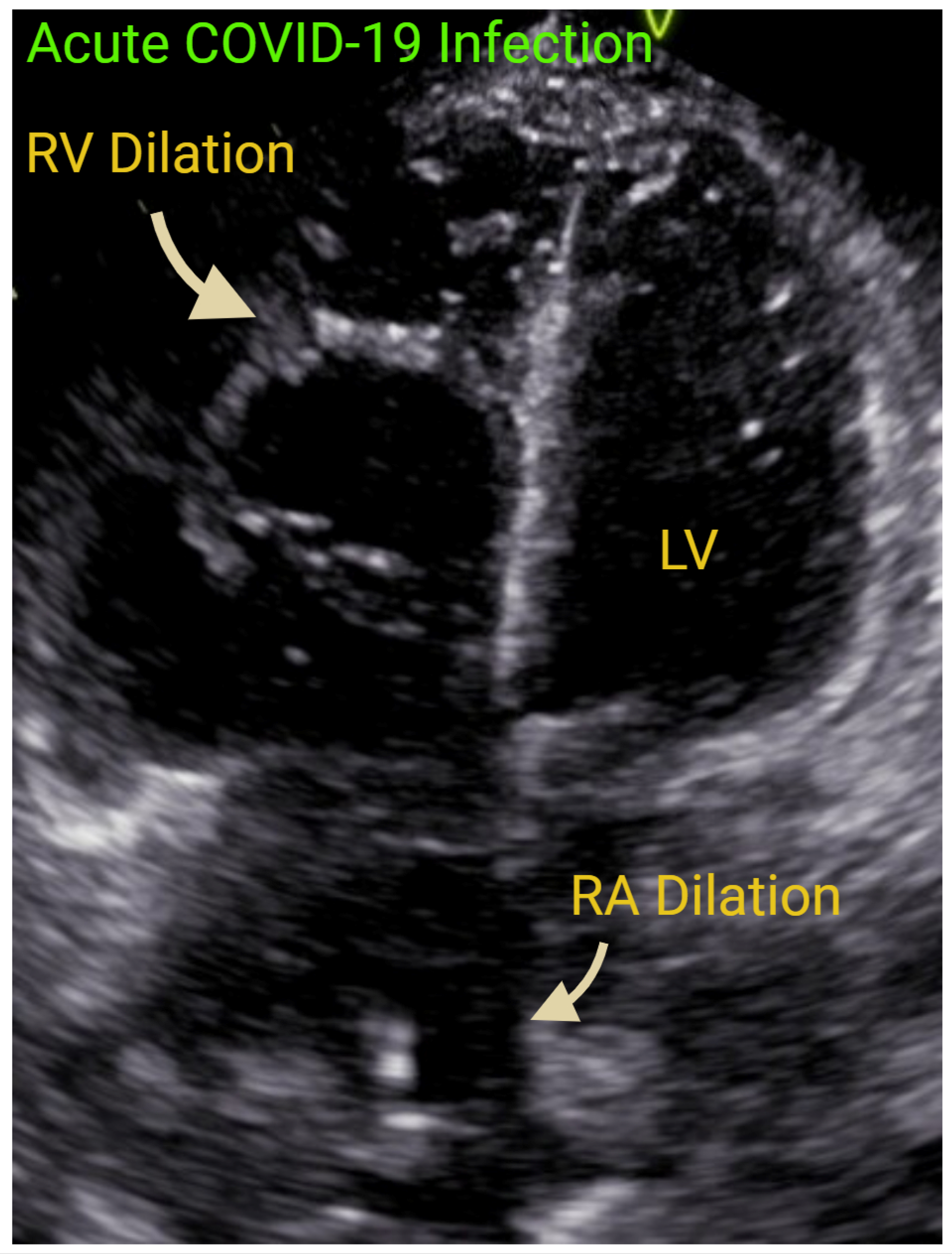

| Measurement | ASE Reference Values | Correlation with MRI | ROC Curve | |||
|---|---|---|---|---|---|---|
| Abnormality Grading | ||||||
| Normal | Mild | Moderate | Severe | |||
| Right Atrial Size | ||||||
| Area, cm2 | <19 | 19 to 22 | 23 to 24 | >24 | ||
| Area to length method, mL/m2 | <30 | 30 to 36 | 37 to 41 | >41 | ||
| Method of disks, mL/m2 | <33 | 33 to 38 | 39 to 44 | >44 | r = 0.96 | |
| Right Ventricular Hypertrophy | ||||||
| RVWT (subcostal), cm | <0.5 | 0.5 to 0.7 | 0.8 to 0.9 | >0.9 | r = 0.71 | |
| Right Ventricular Size | ||||||
| RV basal diameter (A4C), cm | <41 | 41 to 44 | 45 to 49 | >49 | r = 0.69 to 0.73 | AUC = 0.92 |
| Proximal RVOT (PSAX), cm | <34 | 34 to 38 | 39 to 41 | >41 | r = 0.68 | |
| RV end to diastolic area (A4C), cm2 | <25 | 25 to 28 | 29 to 32 | >32 | r = 0.76 | AUC = 0.94 |
| Right Ventricular Systolic Function | ||||||
| TAPSE, cm | >1.7 | ≤1.7 to ≥1.3 | ≤1.3 to >1.0 | ≤1.0 | r = 0.45 to 0.62 | AUC = 0.74 |
| S′, cm/s | >9.5 | ≤9.5 to ≥7.2 | ≤7.2 to >5.0 | ≤5 | r = 0.36 to 0.52 | |
| FAC, % | >35 | ≤35 to >29 | ≤29 to >22 | ≤22 | r = 0.55 to 0.77 | AUC = 0.83 |
| RV FWS, % | >20 | ≤20 to <15 | <15 to ≥11 | <11 | r = 0.69 to 0.92 | |
| 3D RVEF, % | >45 | ≤45 to <39 | ≤39 to ≤32 | <32 | r = 0.56 to 0.95 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noelck, N.J.; Perry, H.A.; Talley, P.L.; Le, D.E. A Narrative Review of the Clinical Applications of Echocardiography in Right Heart Failure. J. Clin. Med. 2025, 14, 5505. https://doi.org/10.3390/jcm14155505
Noelck NJ, Perry HA, Talley PL, Le DE. A Narrative Review of the Clinical Applications of Echocardiography in Right Heart Failure. Journal of Clinical Medicine. 2025; 14(15):5505. https://doi.org/10.3390/jcm14155505
Chicago/Turabian StyleNoelck, North J., Heather A. Perry, Phyllis L. Talley, and D. Elizabeth Le. 2025. "A Narrative Review of the Clinical Applications of Echocardiography in Right Heart Failure" Journal of Clinical Medicine 14, no. 15: 5505. https://doi.org/10.3390/jcm14155505
APA StyleNoelck, N. J., Perry, H. A., Talley, P. L., & Le, D. E. (2025). A Narrative Review of the Clinical Applications of Echocardiography in Right Heart Failure. Journal of Clinical Medicine, 14(15), 5505. https://doi.org/10.3390/jcm14155505





