Colorectal Cancer Risk in Korean Patients with Inflammatory Bowel Disease: A Nationwide Big Data Study of Subtype and Socioeconomic Disparities
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
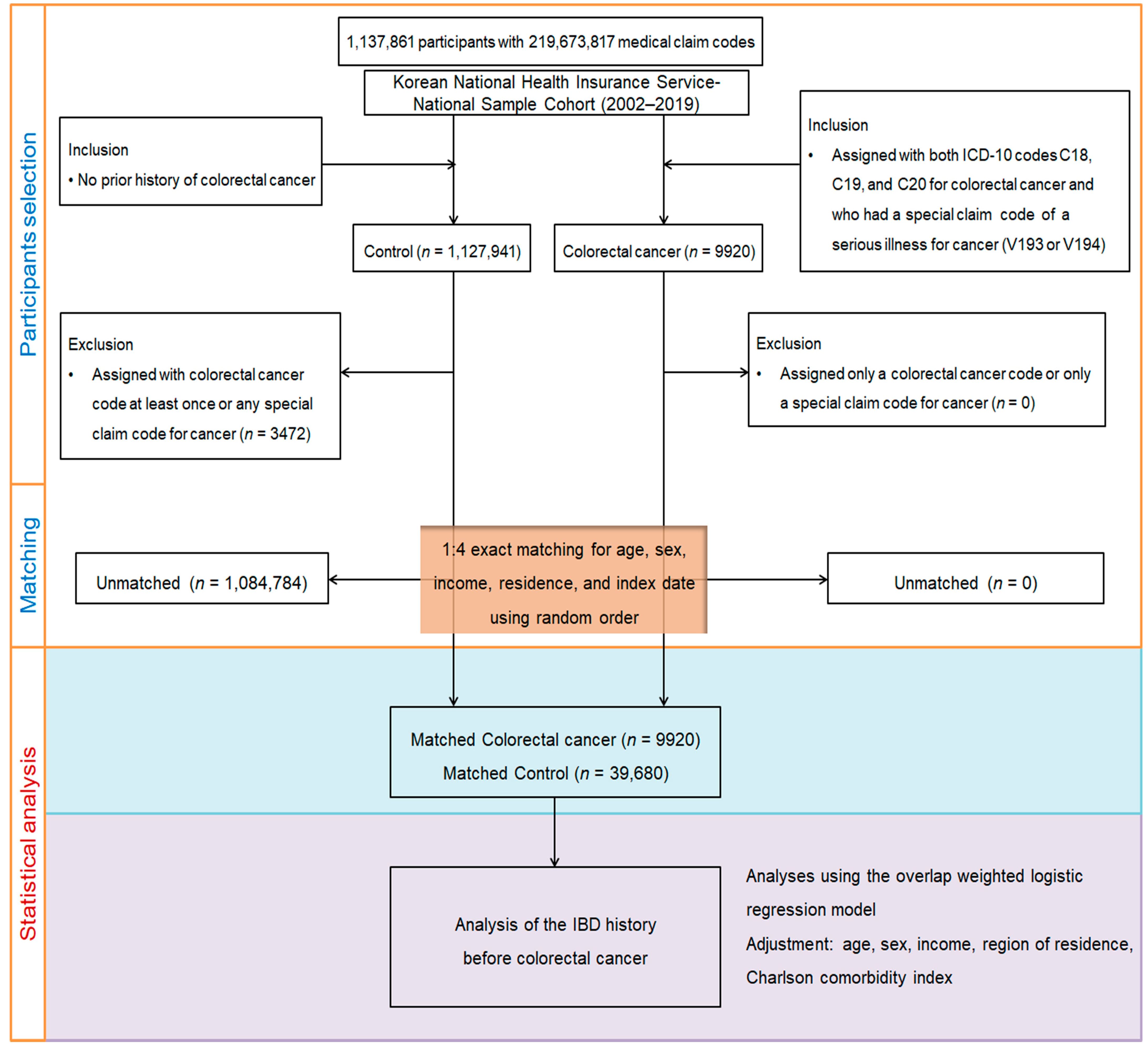
2.2. Research Design and Participant Enrollment
2.3. Definition of Colorectal Cancer (Outcome)
2.4. Definition of Inflammatory Bowel Disease (Exposure)
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
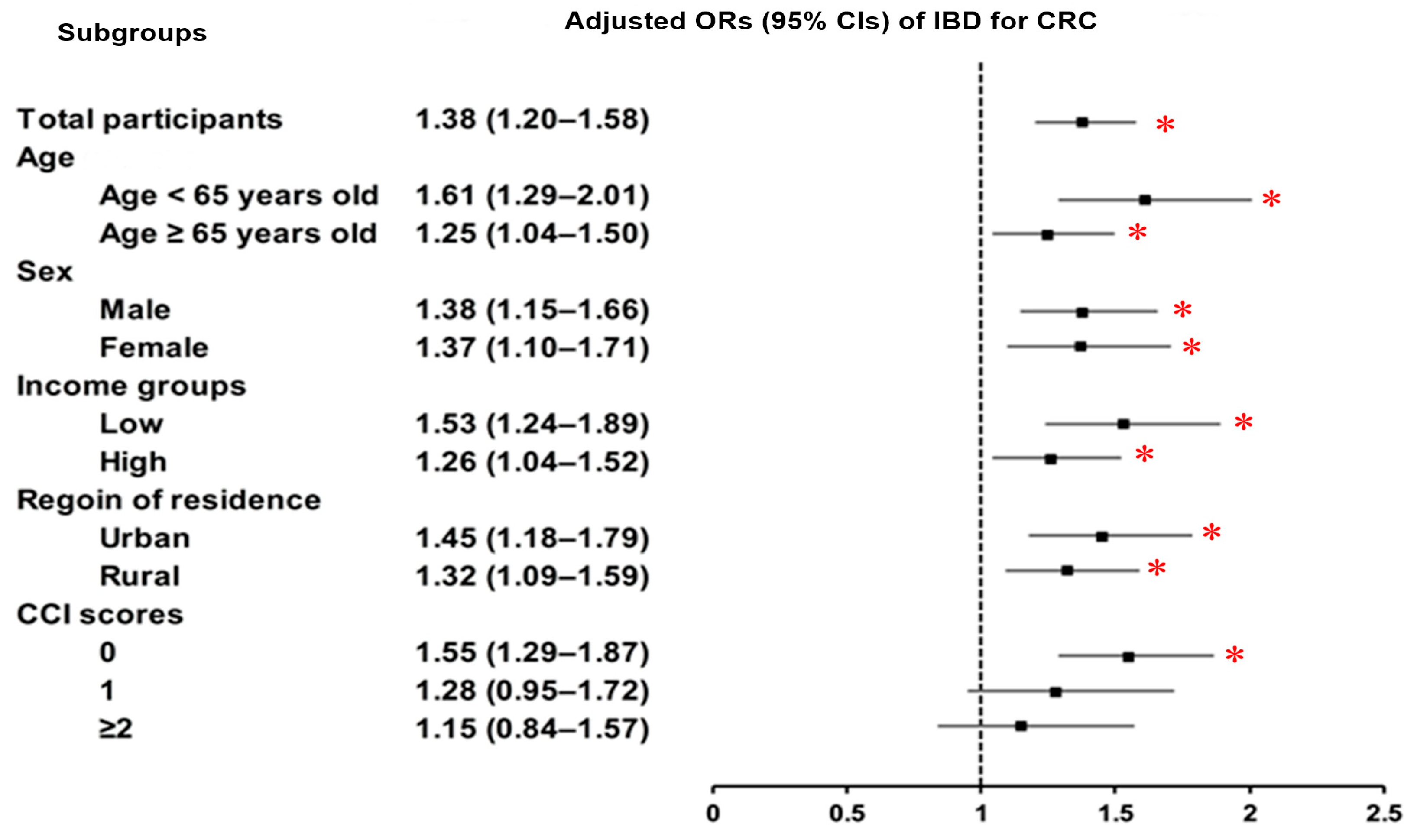
3.2. Relationship Between Inflammatory Bowel Diseases and Colorectal Cancer
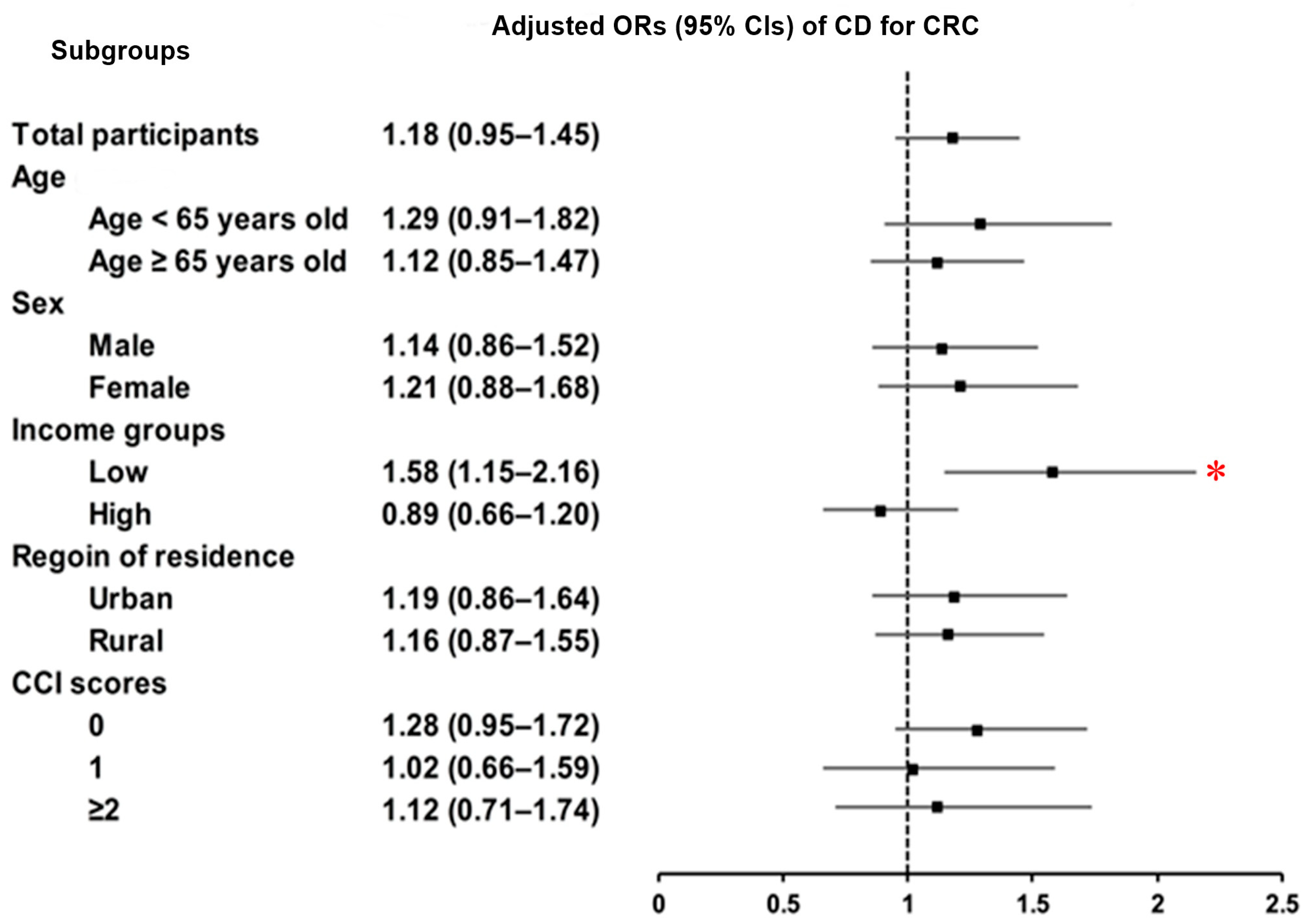
3.3. Relationship Between Crohn’s Disease and Colorectal Cancer
3.4. Relationship Between Ulcerative Colitis and Colorectal Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Khil, H.; Kim, S.M.; Hong, S.; Gil, H.M.; Cheon, E.; Lee, D.H.; Kim, Y.A.; Keum, N. Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea. Sci. Rep. 2021, 11, 2413. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.M.; Soerjomataram, I.; Bray, F. A global view on cancer incidence and national levels of the human development index. Int. J. Cancer 2016, 139, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Association of the inflammatory balance of diet and lifestyle with colorectal cancer among Korean adults: A case-control study. Epidemiol. Health 2022, 44, e2022084. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, J.; Oh, J.H.; Shin, A.; Kim, J. Dietary patterns and colorectal cancer risk in a Korean population: A case-control study. Medicine 2016, 95, e3759. [Google Scholar] [CrossRef]
- Park, J.; Cheon, J.H. Incidence and Prevalence of Inflammatory Bowel Disease across Asia. Yonsei Med. J. 2021, 62, 99–108. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, Y.J.; Rhee, K.H.; Kim, Y.H.; Hong, S.N.; Kim, K.H.; Seo, S.I.; Cha, J.M.; Park, S.Y.; Jeong, S.K.; et al. A 30-year Trend Analysis in the Epidemiology of Inflammatory Bowel Disease in the Songpa-Kangdong District of Seoul, Korea in 1986–2015. J. Crohn’s Colitis 2019, 13, 1410–1417. [Google Scholar] [CrossRef]
- Zhiqin, W.; Palaniappan, S.; Raja Ali, R.A. Inflammatory Bowel Disease-related Colorectal Cancer in the Asia-Pacific Region: Past, Present, and Future. Intest. Res. 2014, 12, 194–204. [Google Scholar] [CrossRef]
- Kochhar, R.; Goenka, M.K.; Kaushik, S.P.; Gupta, N.M.; Nagi, B.; Mehta, S.K. Colorectal carcinoma in Indian patients with idiopathic ulcerative colitis. Eur. J. Cancer Prev. 1992, 1, 293–296. [Google Scholar] [CrossRef]
- Watanabe, T.; Konishi, T.; Kishimoto, J.; Kotake, K.; Muto, T.; Sugihara, K. Ulcerative colitis-associated colorectal cancer shows a poorer survival than sporadic colorectal cancer: A nationwide Japanese study. Inflamm. Bowel Dis. 2011, 17, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Eun, C.S. Inflammatory bowel disease in Korea: Epidemiology and pathophysiology. Korean J. Intern. Med. 2022, 37, 885–894. [Google Scholar] [CrossRef]
- Xie, J.; Itzkowitz, S.H. Cancer in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Tsujinaka, S.; Miura, T.; Kitamura, Y.; Suzuki, H.; Shibata, C. Inflammatory Bowel Disease and Colorectal Cancer: Epidemiology, Etiology, Surveillance, and Management. Cancers 2023, 15, 4154. [Google Scholar] [CrossRef] [PubMed]
- de Dombal, F.T. Ulcerative colitis. Epidemiology and aetiology, course and prognosis. Br. Med. J. 1971, 1, 649–650. [Google Scholar] [CrossRef]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef]
- Venkataraman, S.; Mohan, V.; Ramakrishna, B.S.; Peter, S.; Chacko, A.; Chandy, G.; Kurian, G.; Kurian, S.; Mathan, M.; Mathan, V.I.; et al. Risk of colorectal cancer in ulcerative colitis in India. J. Gastroenterol. Hepatol. 2005, 20, 705–709. [Google Scholar] [CrossRef]
- Kim, H.J.; Ryoo, S.-B.; Choi, J.S.; Lim, H.-K.; Kim, M.J.; Park, J.W.; Jeong, S.-Y.; Park, K.J. Ulcerative colitis-associated colorectal neoplasm is increasing as a surgical indication in the biologics era: A retrospective observational study of 20 years of experience in a single tertiary center. Ann. Surg. Treat. Res. 2025, 108, 150–157. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, S.H.; Yang, S.K.; Ye, B.D.; Kim, J.H.; Kim, S.O.; Soh, J.S.; Lee, S.; Bae, J.H.; Lee, H.J.; et al. The risk of colorectal cancer in inflammatory bowel disease: A hospital-based cohort study from Korea. Scand. J. Gastroenterol. 2015, 50, 188–196. [Google Scholar] [CrossRef]
- So, J.; Tang, W.; Leung, W.K.; Li, M.; Lo, F.H.; Wong, M.T.L.; Sze, A.S.F.; Leung, C.M.; Tsang, S.W.C.; Shan, E.H.S.; et al. Cancer Risk in 2621 Chinese Patients with Inflammatory Bowel Disease: A Population-based Cohort Study. Inflamm. Bowel Dis. 2017, 23, 2061–2068. [Google Scholar] [CrossRef]
- Wang, L.H.; Yang, Y.J.; Cheng, W.C.; Wang, W.M.; Lin, S.H.; Shieh, C.C. Higher Risk for Hematological Malignancies in Inflammatory Bowel Disease: A Nationwide Population-based Study in Taiwan. Am. J. Gastroenterol. 2016, 111, 1313–1319. [Google Scholar] [CrossRef]
- Canavan, C.; Abrams, K.R.; Mayberry, J. Meta-analysis: Colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2006, 23, 1097–1104. [Google Scholar] [CrossRef]
- Jess, T.; Rungoe, C.; Peyrin-Biroulet, L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef]
- M’Koma, A.E. Inflammatory bowel disease: An expanding global health problem. Clin. Med. Insights Gastroenterol. 2013, 6, 33–47. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [PubMed]
- Abu-Freha, N.; Cohen, B.; Gordon, M.; Weissmann, S.; Kestenbaum, E.H.; Vosko, S.; Abu-Tailakh, M.; Ben-Shoshan, L.; Cohen, D.L.; Shirin, H. Colorectal cancer among inflammatory bowel disease patients: Risk factors and prevalence compared to the general population. Front. Med. 2023, 10, 1225616. [Google Scholar] [CrossRef]
- Ramsey, M.; Krishna, S.G.; Stanich, P.P.; Husain, S.; Levine, E.J.; Conwell, D.; Hinton, A.; Zhang, C. Inflammatory Bowel Disease Adversely Impacts Colorectal Cancer Surgery Short-term Outcomes and Health-Care Resource Utilization. Clin. Transl. Gastroenterol. 2017, 8, e127. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Blanchard, J.F.; Kliewer, E.; Wajda, A. Cancer risk in patients with inflammatory bowel disease: A population-based study. Cancer 2001, 91, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Sandborn, W.J.; Gupta, S. Colorectal Cancer and Dysplasia in Inflammatory Bowel Disease: A Review of Disease Epidemiology, Pathophysiology, and Management. Cancer Prev. Res. 2016, 9, 887–894. [Google Scholar] [CrossRef]
- Jess, T.; Horváth-Puhó, E.; Fallingborg, J.; Rasmussen, H.H.; Jacobsen, B.A. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: A Danish population-based cohort study. Am. J. Gastroenterol. 2013, 108, 1869–1876. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Mattar, M.C.; Lough, D.; Pishvaian, M.J.; Charabaty, A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest. Cancer Res. 2011, 4, 53–61. [Google Scholar]
- Lutgens, M.W.; Oldenburg, B.; Siersema, P.D.; van Bodegraven, A.A.; Dijkstra, G.; Hommes, D.W.; de Jong, D.J.; Stokkers, P.C.; van der Woude, C.J.; Vleggaar, F.P. Colonoscopic surveillance improves survival after colorectal cancer diagnosis in inflammatory bowel disease. Br. J. Cancer 2009, 101, 1671–1675. [Google Scholar] [CrossRef]
- Mizushima, T.; Ohno, Y.; Nakajima, K.; Kai, Y.; Iijima, H.; Sekimoto, M.; Nishida, T.; Nezu, R.; Ito, T.; Doki, Y.; et al. Malignancy in Crohn’s disease: Incidence and clinical characteristics in Japan. Digestion 2010, 81, 265–270. [Google Scholar] [CrossRef]

| Characteristics | Before PS Overlap Weighting Adjustment | After PS Overlap Weighting Adjustment | ||||
|---|---|---|---|---|---|---|
| CRC | Control | Standardized Difference | CRC | Control | Standardized Difference | |
| Age (y), n (%) | 0.00 | 0.00 | ||||
| 0–4 | 1 (0.01) | 4 (0.01) | 1 (0.01) | 1 (0.01) | ||
| 5–9 | N/A | N/A | N/A | N/A | ||
| 10–14 | 3 (0.03) | 12 (0.03) | 2 (0.03) | 2 (0.03) | ||
| 15–19 | 1 (0.01) | 4 (0.01) | 1 (0.01) | 1 (0.01) | ||
| 20–24 | 8 (0.08) | 32 (0.08) | 6 (0.08) | 6 (0.08) | ||
| 25–29 | 26 (0.26) | 104 (0.26) | 21 (0.26) | 21 (0.26) | ||
| 30–34 | 94 (0.95) | 376 (0.95) | 75 (0.95) | 75 (0.95) | ||
| 35–39 | 180 (1.81) | 720 (1.81) | 144 (1.81) | 144 (1.81) | ||
| 40–44 | 359 (3.62) | 1436 (3.62) | 287 (3.61) | 287 (3.61) | ||
| 45–49 | 578 (5.83) | 2312 (5.83) | 462 (5.82) | 462 (5.82) | ||
| 50–54 | 968 (9.76) | 3872 (9.76) | 772 (9.75) | 772 (9.75) | ||
| 55–59 | 1242 (12.52) | 4968 (12.52) | 992 (12.51) | 992 (12.51) | ||
| 60–64 | 1393 (14.04) | 5572 (14.04) | 1112 (14.02) | 1112 (14.02) | ||
| 65–69 | 1488 (15.00) | 5952 (15.00) | 1189 (15.00) | 1189 (15.00) | ||
| 70–74 | 1471 (14.83) | 5884 (14.83) | 1176 (14.84) | 1176 (14.84) | ||
| 75–79 | 1060 (10.69) | 4240 (10.69) | 848 (10.70) | 848 (10.70) | ||
| 80–84 | 672 (6.77) | 2688 (6.77) | 539 (6.80) | 539 (6.80) | ||
| 85+ | 376 (3.79) | 1504 (3.79) | 301 (3.80) | 301 (3.80) | ||
| Sex, n (%) | 0.00 | 0.00 | ||||
| Male | 5933 (59.81) | 23,732 (59.81) | 4739 (59.79) | 4739 (59.79) | ||
| Female | 3987 (40.19) | 15,948 (40.19) | 3187 (40.21) | 3187 (40.21) | ||
| Income, n (%) | 0.00 | 0.00 | ||||
| 1 (lowest) | 1990 (20.06) | 7960 (20.06) | 1590 (20.06) | 1590 (20.06) | ||
| 2 | 1253 (12.63) | 5012 (12.63) | 1000 (12.62) | 1000 (12.62) | ||
| 3 | 1562 (15.75) | 6248 (15.75) | 1247 (15.74) | 1247 (15.74) | ||
| 4 | 2059 (20.76) | 8236 (20.76) | 1646 (20.77) | 1647 (20.77) | ||
| 5 (highest) | 3056 (30.81) | 12,224 (30.81) | 2442 (30.81) | 2442 (30.81) | ||
| Region of residence, n (%) | 0.00 | 0.00 | ||||
| Urban | 4447 (44.83) | 17,788 (44.83) | 3553 (44.83) | 3553 (44.83) | ||
| Rural | 5473 (55.17) | 21,892 (55.17) | 4373 (55.17) | 4373 (55.17) | ||
| CCI score, mean (SD) | 0.80 (1.18) | 0.69 (1.18) | 0.09 | 0.77 (1.03) | 0.77 (0.57) | 0.00 |
| IBD, n (%) | 188 (1.90) | 544 (1.37) | 0.04 | 150 (1.89) | 110 (1.38) | 0.04 |
| CD, n (%) | 74 (0.75) | 249 (0.63) | 0.01 | 59 (0.74) | 50 (0.63) | 0.04 |
| UC, n (%) | 117 (1.18) | 306 (0.77) | 0.04 | 94 (1.18) | 62 (0.78) | 0.01 |
| Characteristics | N of Colorectal Cancer | N of Control | OR for Colorectal Cancer (95% CI) | |||
|---|---|---|---|---|---|---|
| (Exposure/Total, %) | (Exposure/Total, %) | Crude | p | Overlap Weighted Model † | p | |
| Inflammatory Bowel Disease | ||||||
| IBD | 188/9920 (1.90) | 544/39,680 (1.37) | 1.39 (1.18–1.64) | <0.001 * | 1.38 (1.20–1.58) | <0.001 * |
| Non-IBD | 9732/9920 (98.1) | 39,136/39,680 (98.63) | 1 | 1 | ||
| Crohn’s Disease | ||||||
| CD | 74/9920 (0.75) | 249/39,680 (0.63) | 1.19 (0.92–1.54) | 0.190 | 1.18 (0.95–1.45) | 0.139 |
| Non-CD | 9846/9920 (99.25) | 39,431/39,680 (99.37) | 1 | 1 | ||
| Ulcerative Colitis | ||||||
| UC | 117/9920 (1.18) | 306/39,680 (0.77) | 1.54 (1.24–1.90) | <0.001 * | 1.52 (1.27–1.83) | <0.001 * |
| Non-UC | 9803/9920 (98.82) | 39,374/39,680 (99.23) | 1 | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, K.M.; Kang, H.S.; Kim, J.-H.; Choi, H.G.; Yoo, D.M.; Kim, N.Y.; Park, H.Y.; Kwon, M.J. Colorectal Cancer Risk in Korean Patients with Inflammatory Bowel Disease: A Nationwide Big Data Study of Subtype and Socioeconomic Disparities. J. Clin. Med. 2025, 14, 5503. https://doi.org/10.3390/jcm14155503
Han KM, Kang HS, Kim J-H, Choi HG, Yoo DM, Kim NY, Park HY, Kwon MJ. Colorectal Cancer Risk in Korean Patients with Inflammatory Bowel Disease: A Nationwide Big Data Study of Subtype and Socioeconomic Disparities. Journal of Clinical Medicine. 2025; 14(15):5503. https://doi.org/10.3390/jcm14155503
Chicago/Turabian StyleHan, Kyeong Min, Ho Suk Kang, Joo-Hee Kim, Hyo Geun Choi, Dae Myoung Yoo, Nan Young Kim, Ha Young Park, and Mi Jung Kwon. 2025. "Colorectal Cancer Risk in Korean Patients with Inflammatory Bowel Disease: A Nationwide Big Data Study of Subtype and Socioeconomic Disparities" Journal of Clinical Medicine 14, no. 15: 5503. https://doi.org/10.3390/jcm14155503
APA StyleHan, K. M., Kang, H. S., Kim, J.-H., Choi, H. G., Yoo, D. M., Kim, N. Y., Park, H. Y., & Kwon, M. J. (2025). Colorectal Cancer Risk in Korean Patients with Inflammatory Bowel Disease: A Nationwide Big Data Study of Subtype and Socioeconomic Disparities. Journal of Clinical Medicine, 14(15), 5503. https://doi.org/10.3390/jcm14155503






