Transcatheter Aortic Valve Implantation in Cardiogenic Shock: Current Evidence, Clinical Challenges, and Future Directions
Abstract
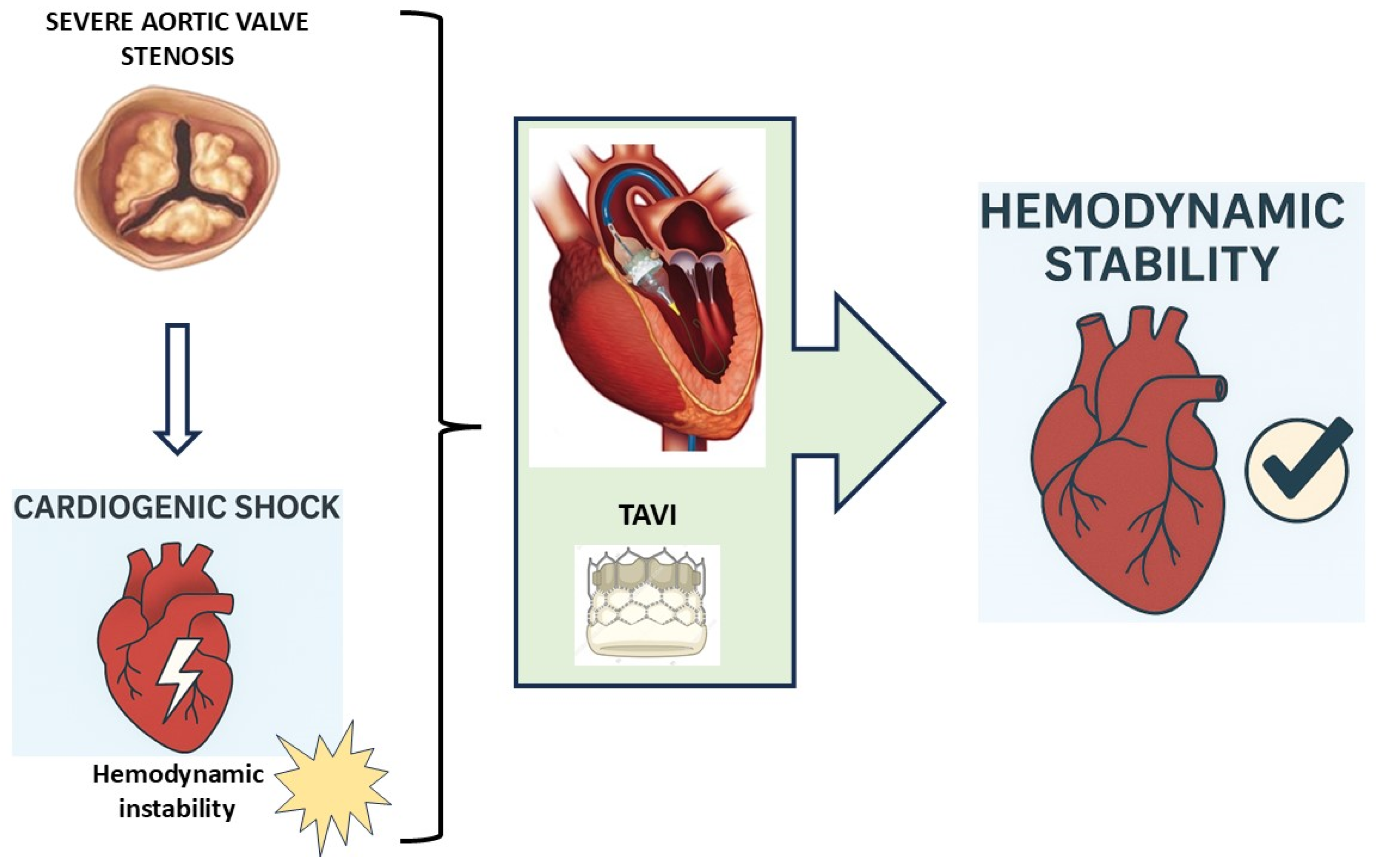
1. Introduction
2. Pathophysiology of Cardiogenic Shock in Aortic Stenosis
3. Clinical Evidence of TAVI in Cardiogenic Shock
3.1. Methodology
3.2. Study and Patient Characteristics
3.3. Clinical Outcomes
3.3.1. Evidence from Large Registries
3.3.2. Evidence from Observational Studies and Small Cohorts
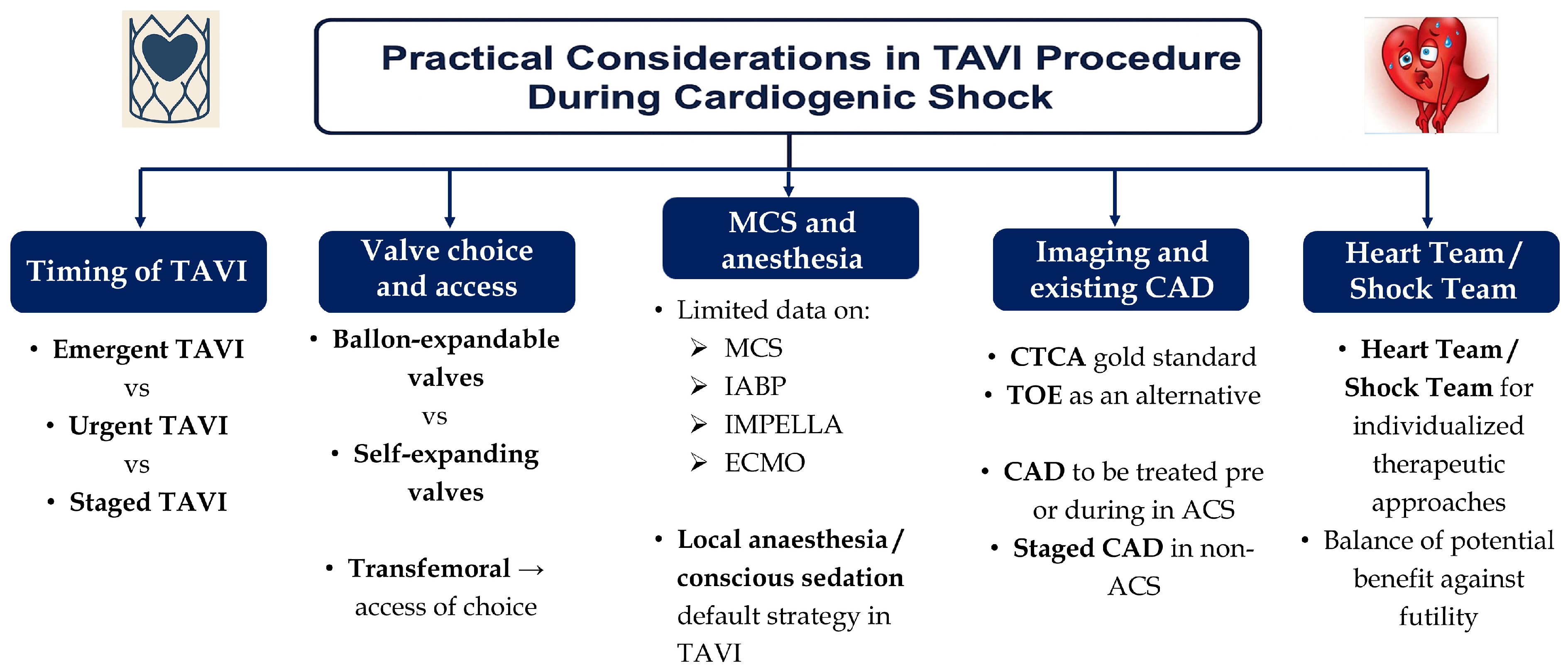
4. Practical Considerations in TAVI Procedure During Cardiogenic Shock
4.1. Role of Balloon Aortic Valvuloplasty and Contraindications to TAVI in Cardiogenic Shock
4.2. Timing of Intervention
4.3. Valve Choice and Access Point
4.4. Use of Mechanical Circulatory Support and Anesthetic Approach
4.5. Imaging in TAVI and Existing Coronary Artery Disease
4.6. Individualized Approach via the Heart Team/Shock Team
5. Challenges and Limitations
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AS | Aortic Stenosis |
| ACC | American College of Cardiology |
| ACS | Acute Coronary Syndrome. |
| BAV | Ballon Aortic Valvuloplasty |
| CAD | Coronary Artery Disease |
| CS | Cardiogenic Shock |
| CTCA | Computed Tomography Coronary Angiography |
| ECMO | Extracorporeal Membrane Oxygenation |
| IABP | Intra-Aortic Balloon Pump |
| KCCQ | Kansas City Cardiomyopathy Questionnaire |
| LV | Left Ventricle/Left Ventricular |
| MCS | Mechanical Circulatory Support |
| MACE | Major Adverse Cardiovascular Events |
| PCI | Percutaneous Coronary Intervention |
| TAVI | Transcatheter Aortic Valve Implantation |
| VA | Venoarterial |
| TV | Transvalvular |
| TA | Transapical |
| TOE | Transoesophageal Echocardiography |
| SAVR | Surgical Aortic Valve Replacement |
| STS | Society of Thoracic Surgeons |
| TVT | Transcatheter Valve Therapy |
References
- Vahdatpour, C.; Collins, D.; Goldberg, S. Cardiogenic Shock. J. Am. Heart Assoc. 2019, 8, e011991. [Google Scholar] [CrossRef]
- Lüsebrink, E.; Binzenhöfer, L.; Adamo, M.; Lorusso, R.; Mebazaa, A.; Morrow, D.A.; Price, S.; Jentzer, J.C.; Brodie, D.; Combes, A.; et al. Cardiogenic shock. Lancet 2024, 404, 2006–2020. [Google Scholar] [CrossRef]
- Yuen, T.; Senaratne, J.M. Definition, Classification, and Management of Primary Noncardiac Causes of Cardiogenic Shock. Can. J. Cardiol. 2025, 41, 587–604. [Google Scholar] [CrossRef]
- Jones, T.L.; Nakamura, K.; McCabe, J.M. Cardiogenic shock: Evolving definitions and future directions in management. Open Heart 2019, 6, e000960. [Google Scholar] [CrossRef]
- Hochman, J.S.; Buller, C.E.; Sleeper, L.A.; Boland, J.; Dzavik, V.; Sanborn, T.A.; Godfrey, E.; White, H.D.; Lim, J.; LeJemtel, T. Cardiogenic shock complicating acute myocardial infarction--etiologies, management and outcome: A report from the SHOCK Trial Registry. J. Am. Coll. Cardiol. 2000, 36 (Suppl. S3), 1063–1070. [Google Scholar] [CrossRef]
- Iung, B.; Vahanian, A. Epidemiology of acquired valvular heart disease. Can. J. Cardiol. 2014, 30, 962–970. [Google Scholar] [CrossRef]
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Fontana, G.P.; Jilaihawi, H.; Kapadia, S.; Pichard, A.D.; Douglas, P.S.; Thourani, V.H.; Babaliaros, V.C.; Webb, J.G.; Herrmann, H.C.; et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N. Engl. J. Med. 2012, 366, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Goody, P.R.; Hosen, M.R.; Christmann, D.; Niepmann, S.T.; Zietzer, A.; Adam, M.; Bönner, F.; Zimmer, S.; Nickenig, G.; Jansen, F. Aortic Valve Stenosis: From Basic Mechanisms to Novel Therapeutic Targets. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 885–900. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Gonnah, A.R.; Abdelwahab, M.; Taylor, R.; Labib, A.; Masoud, O.; Debski, M.; Abdelaziz, H.K.; Roberts, D.H. Health-related quality of life following TAVI or cardiac surgery in patients at intermediate and low risk: A systematic review and meta-analysis. Clin. Med. 2023, 23, 594–605. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.S.; Redwood, S.R.; Allen, C.J.; Hurrell, H.; Chehab, O.; Rajani, R.; Prendergast, B.; Patterson, T. A 20-year journey in transcatheter aortic valve implantation: Evolution to current eminence. Front. Cardiovasc. Med. 2022, 9, 971762. [Google Scholar] [CrossRef]
- Sattar, Y.; Hamza, M.; Yasmin, F.; Jabeen, S.; Patel, N.; Ishaq, S.; Alyami, B.; Ul Hussain, H.; Rehan, S.T.; Shuja, S.H.; et al. Cardiovascular outcomes of emergent vs elective transcatheter aortic valve replacement in severe aortic stenosis: Regression matched meta-analysis. Am. J. Cardiovasc. Dis. 2024, 14, 54–69. [Google Scholar] [CrossRef]
- Ishaq, M.; Khan, M.S.; Shahbaz, A.; Vedre, J.G.; Yarkoni, A.; Malovrh, R.; Mesa, J. Successful emergency transcatheter aortic valve replacement in the setting of hemodynamically unstable prosthetic aortic valve stenosis. J. Cardiovasc. Thorac. Res. 2022, 14, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Khera, S.; Vemulapalli, S.; Dai, D.; Heo, S.; Goldsweig, A.M.; Aronow, H.D.; Elmariah, S.; Inglessis, I.; Palacios, I.F.; et al. Outcomes Following Urgent/Emergent Transcatheter Aortic Valve Replacement: Insights from the STS/ACC TVT Registry. JACC Cardiovasc. Interv. 2018, 11, 1175–1185. [Google Scholar] [CrossRef]
- Nair, R.M.; Chawla, S.; Abdelghaffar, B.; Alkhalaieh, F.; Bansal, A.; Puri, R.; Yun, J.; Krishnaswamy, A.; Kapadia, S.; Menon, V.; et al. Comparison of Contemporary Treatment Strategies in Patients With Cardiogenic Shock Due to Severe Aortic Stenosis. J. Am. Heart Assoc. 2024, 13, e033601. [Google Scholar] [CrossRef]
- Czarny, M.J.; Resar, J.R. Diagnosis and management of valvular aortic stenosis. Clin. Med. Insights Cardiol. 2014, 8 (Suppl. S1), 15–24. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Blumer, V.; Burkhoff, D.; Pahuja, M.; Sinha, S.S.; Rosner, C.; Vorovich, E.; Grafton, G.; Bagnola, A.; Hernandez-Montfort, J.A.; et al. Heart Failure-Related Cardiogenic Shock: Pathophysiology, Evaluation and Management Considerations: Review of Heart Failure-Related Cardiogenic Shock. J. Card. Fail. 2021, 27, 1126–1140. [Google Scholar] [CrossRef]
- Ross, J., Jr.; Braunwald, E. Aortic stenosis. Circulation 1968, 38 (Suppl. S1), 61–67. [Google Scholar] [CrossRef]
- Chioncel, O.; Adamo, M.; Nikolaou, M.; Parissis, J.; Mebazaa, A.; Yilmaz, M.B.; Hassager, C.; Moura, B.; Bauersachs, J.; Harjola, V.P.; et al. Acute heart failure and valvular heart disease: A scientific statement of the Heart Failure Association, the Association for Acute CardioVascular Care and the European Association of Percutaneous Cardiovascular Interventions of the European Society of Cardiology. Eur. J. Heart Fail. 2023, 25, 1025–1048. [Google Scholar]
- Tandar, A.; Drakos, S.G. Cardiogenic shock in aortic stenosis patients: Balancing between complexity and simplicity. Hellenic J. Cardiol. 2019, 60, 182–184. [Google Scholar] [CrossRef]
- Badiani, S.; van Zalen, J.; Treibel, T.A.; Bhattacharyya, S.; Moon, J.C.; Lloyd, G. Aortic Stenosis, a Left Ventricular Disease: Insights from Advanced Imaging. Curr. Cardiol. Rep. 2016, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Ross, J., Jr. Afterload mismatch in aortic and mitral valve disease: Implications for surgical therapy. J. Am. Coll. Cardiol. 1985, 5, 811–826. [Google Scholar] [CrossRef]
- Rajah, M.R.; Doubell, A.; Herbst, P. High afterload rather than myocardial fibrosis predicts reduced ejection fraction in severe aortic stenosis with afterload mismatch. Open Heart 2025, 12, e003345. [Google Scholar] [CrossRef] [PubMed]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; et al. Guidelines on the management of valvular heart disease (version 2012). Eur. Heart J. 2012, 33, 2451–2496. [Google Scholar] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- D’Ancona, G.; Pasic, M.; Buz, S.; Drews, T.; Dreysse, S.; Kukucka, M.; Hetzer, R.; Unbehaun, A. Transapical transcatheter aortic valve replacement in patients with cardiogenic shock. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 426–430. [Google Scholar] [CrossRef]
- Frerker, C.; Schewel, J.; Schlüter, M.; Schewel, D.; Ramadan, H.; Schmidt, T.; Thielsen, T.; Kreidel, F.; Schlingloff, F.; Bader, R.; et al. Emergency transcatheter aortic valve replacement in patients with cardiogenic shock due to acutely decompensated aortic stenosis. EuroIntervention 2016, 11, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Landes, U.; Orvin, K.; Codner, P.; Assali, A.; Vaknin-Assa, H.; Schwartznberg, S.; Levi, A.; Shapira, Y.; Sagie, A.; Kornowski, R. Urgent Transcatheter Aortic Valve Implantation in Patients with Severe Aortic Stenosis and Acute Heart Failure: Procedural and 30-Day Outcomes. Can. J. Cardiol. 2016, 32, 726–731. [Google Scholar] [CrossRef]
- Bongiovanni, D.; Kühl, C.; Bleiziffer, S.; Stecher, L.; Poch, F.; Greif, M.; Mehilli, J.; Massberg, S.; Frey, N.; Lange, R.; et al. Emergency treatment of decompensated aortic stenosis. Heart 2018, 104, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fraccaro, C.; Campante Teles, R.; Tchétché, D.; Saia, F.; Bedogni, F.; Montorfano, M.; Fiorina, C.; Meucci, F.; De Benedictis, M.; Leonzi, O.; et al. Transcatheter aortic valve implantation (TAVI) in cardiogenic shock: TAVI-Shock registry results. Catheter. Cardiovasc. Interv. 2020, 96, 1128–1135. [Google Scholar] [CrossRef]
- Huang, H.; Kovach, C.P.; Bell, S.; Reisman, M.; Aldea, G.; McCabe, J.M.; Dvir, D.; Don, C. Outcomes of Emergency Transcatheter Aortic Valve Replacement. J. Interv. Cardiol. 2019, 2019, 7598581. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Chakraborty, S.; Amgai, B.; Patel, N.; Hajra, A.; Ghosh, R.K.; Kolte, D.; Fonarow, G.C.; Ramee, S.R.; Lavie, C.J.; et al. Urgent Balloon Aortic Valvuloplasty or Urgent TAVR in Patients with Severe Aortic Stenosis: A Propensity-Matched Analysis. JACC Cardiovasc. Interv. 2020, 13, 274–275. [Google Scholar] [CrossRef]
- Masha, L.; Vemulapalli, S.; Manandhar, P.; Balan, P.; Shah, P.; Kosinski, A.S.; Stewart, G. Demographics, Procedural Characteristics, and Clinical Outcomes When Cardiogenic Shock Precedes TAVR in the United States. JACC Cardiovasc. Interv. 2020, 13, 1314–1325. [Google Scholar] [CrossRef]
- Piriou, P.G.; Manigold, T.; Letocart, V.; Le Ruz, R.; Schurtz, G.; Vincent, F.; Van Belle, É.; Guérin, P.; Plessis, J. Outcomes of emergency transcatheter aortic valve replacement in patients with cardiogenic shock: A multicenter retrospective study. Catheter. Cardiovasc. Interv. 2022, 99, 2117–2124. [Google Scholar] [CrossRef]
- Steffen, J.; Stocker, A.; Scherer, C.; Haum, M.; Fischer, J.; Doldi, P.M.; Theiss, H.; Braun, D.; Rizas, K.; Peterß, S.; et al. Emergency transcatheter aortic valve implantation for acute heart failure due to severe aortic stenosis in critically ill patients with or without cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Castelo, A.; Teixeira, B.; Grazina, A.; Mendonça, T.; Rodrigues, I.; Garcia Brás, P.; Ferreira, V.V.; Ramos, R.; Fiarresga, A.; Cruz Ferreira, R.; et al. Urgent versus Non-Urgent Transcatheter Aortic Valve Implantation Outcomes. Cardiology 2023, 148, 469–477. [Google Scholar] [CrossRef]
- Goel, K.; Shah, P.; Jones, B.M.; Korngold, E.; Bhardwaj, A.; Kar, B.; Barker, C.; Szerlip, M.; Smalling, R.; Dhoble, A. Outcomes of transcatheter aortic valve replacement in patients with cardiogenic shock. Eur. Heart J. 2023, 44, 3181–3195. [Google Scholar] [CrossRef]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef]
- Piriou, P.G.; Plessis, J.; Guerin, P. Transcatheter aortic valve replacement in patients with cardiogenic shock: Safe and effective, but the most critical patients require further investigations. Eur. Heart J. 2024, 45, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.W.; Möllmann, H.; Holzhey, D.; Beckmann, A.; Veit, C.; Figulla, H.R.; Cremer, J.; Kuck, K.H.; Lange, R.; Zahn, R.; et al. The German Aortic Valve Registry (GARY): In-Hospital outcome. Eur. Heart J. 2014, 35, 1588–1598. [Google Scholar] [CrossRef]
- Llah, S.T.; Sharif, S.; Ullah, S.; Sheikh, S.A.; Shah, M.A.; Shafi, O.M.; Dar, T. TAVR vs balloon aortic valvotomy for severe aortic stenosis and cardiogenic shock: An insight from the National Inpatient Sample database. Cardiovasc. Revasc. Med. 2023, 55, 1–7. [Google Scholar] [CrossRef]
- Debry, N.; Kone, P.; Vincent, F.; Lemesle, G.; Delhaye, C.; Schurtz, G.; Spillemaeker, H.; Porouchani, S.; Coisne, A.; Auffray, J.L.; et al. Urgent balloon aortic valvuloplasty in patients with cardiogenic shock related to severe aortic stenosis: Time matters. EuroIntervention 2018, 14, e519–e525. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.X.; O’Neill, B.P.; Wang, D.D.; Giustino, G.; von Buchwald, C.L.; Lee, J.C.; Engel Gonzalez, P.; Frisoli, T.M.; O’Neill, W.W.; Villablanca, P.A. Feasibility and technicality of aortic valve lithotripsy-facilitate balloon valvuloplasty in patients with severe aortic stenosis unsuitable for immediate valvular replacement. Cardiovasc. Revasc. Med. 2024, 67, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Patel, P.; Wahab, A.; Das, A.; Blackman, D.J.; Cunnington, M.S.; Malkin, C.J. A cohort study examining urgent and emergency treatment for decompensated severe aortic stenosis. J. Cardiovasc. Med. 2021, 22, 126–132. [Google Scholar] [CrossRef]
- Chakraborty, S.; Patel, N.; Bandyopadhyay, D.; Hajra, A.; Amgai, B.; Zaid, S.; Sharedalal, P.; Ahmad, H.; Cohen, M.B.; Abbott, J.D.; et al. Readmission following urgent transcatheter aortic valve implantation versus urgent balloon aortic valvuloplasty in patients with decompensated heart failure or cardiogenic shock. Catheter. Cardiovasc. Interv. 2021, 98, 607–612. [Google Scholar] [CrossRef]
- Wernly, B.; Jirak, P.; Lichtenauer, M.; Veulemans, V.; Zeus, T.; Piayda, K.; Hoppe, U.C.; Lauten, A.; Frerker, C.; Jung, C. Systematic Review and Meta-Analysis of Interventional Emergency Treatment of Decompensated Severe Aortic Stenosis. J. Invasive Cardiol. 2020, 32, 30–36. [Google Scholar] [CrossRef]
- Fraccaro, C.; Karam, N.; Möllmann, H.; Bleiziffer, S.; Bonaros, N.; Teles, R.C.; Carrilho Ferreira, P.; Chieffo, A.; Czerny, M.; Donal, E.; et al. Transcatheter interventions for left-sided valvular heart disease complicated by cardiogenic shock: A consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) in collaboration with the Association for Acute Cardiovascular Care (ACVC) and the ESC Working Group on Cardiovascular Surgery. EuroIntervention 2023, 19, 634–651. [Google Scholar] [CrossRef]
- Scolari, F.L.; Schneider, D.; Fogazzi, D.V.; Gus, M.; Rover, M.M.; Bonatto, M.G.; de Araújo, G.N.; Zimerman, A.; Sganzerla, D.; Goldraich, L.A.; et al. Association between serum lactate levels and mortality in patients with cardiogenic shock receiving mechanical circulatory support: A multicenter retrospective cohort study. BMC Cardiovasc. Disord. 2020, 20, 496. [Google Scholar] [CrossRef]
- Pop, C.F.; Coadă, C.A.; Lupu, M.; Ferenț, I.F.; Hodas, R.I.; Pintilie, A.; Ursu, M.Ş. Factors Associated with Mortality Risk in Patients with Cardiogenic Shock Post-ST-Elevation Myocardial Infarction: Insights from a Regional Centre in Northwest Romania. Medicina 2025, 61, 725. [Google Scholar] [CrossRef]
- Kataria, R.; Sinha, S.S.; Li, S.; Kong, Q.; Kanwar, M.; Hernandez-Montfort, J.; Garan, A.R.; Abraham, J.; Zweck, E.; Ton, V.K.; et al. Worsening Renal Function Is Common and Associated With Higher Mortality in Cardiogenic Shock: A Cardiogenic Shock Working Group Report. J. Card. Fail. 2025. ahead of print. [Google Scholar] [CrossRef]
- Bjørn, M.; Kunkel, J.B.; Helgestad, O.; Josiassen, J.; Jeppesen, K.K.; Holmvang, L.; Jensen, L.O.; Schmidt, H.; Fosbøl, E.; Hassager, C.; et al. Long-term outcomes after acute kidney injury in myocardial infarction complicated by cardiogenic shock—A retrospective, observational study. Eur. Heart J. Acute Cardiovasc. Care 2025, zuaf048. [Google Scholar] [CrossRef]
- Jäntti, T.; Tarvasmäki, T.; Harjola, V.P.; Parissis, J.; Pulkki, K.; Javanainen, T.; Tolppanen, H.; Jurkko, R.; Hongisto, M.; Kataja, A.; et al. Hypoalbuminemia is a frequent marker of increased mortality in cardiogenic shock. PLoS ONE 2019, 14, e0217006. [Google Scholar] [CrossRef] [PubMed]
- Champion, S. The double-edged sword of mechanical ventilation for patients with cardiogenic shock. Cardiol. J. 2014, 21, 449. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Mackenhauer, J.; Roberts, J.C.; Berg, K.M.; Cocchi, M.N.; Donnino, M.W. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin. Proc. 2013, 88, 1127–1140. [Google Scholar] [CrossRef]
- Bombardini, T.; Zagatina, A.; Ciampi, Q.; Arbucci, R.; Merlo, P.M.; Haber, D.M.L.; Morrone, D.; D’Andrea, A.; Djordjevic-Dikic, A.; Beleslin, B.; et al. Hemodynamic Heterogeneity of Reduced Cardiac Reserve Unmasked by Volumetric Exercise Echocardiography. J. Clin. Med. 2021, 10, 2906. [Google Scholar] [CrossRef] [PubMed]
- Dauerman, H.L.; Lahoud, R. The miracle of left ventricular recovery after transcatheter aortic valve implantation. EuroIntervention 2024, 20, e463–e464. [Google Scholar] [CrossRef]
- Akchurin, R.; Imaev, T.; Lepilin, P.; Kolegaev, A.; Komlev, A.; Pokidkin, I. Mid-term results of TAVI in high-risk patients: Data from a single center study. J. Cardiothorac. Surg. 2013, 8 (Suppl. S1), O315. [Google Scholar] [CrossRef][Green Version]
- Pyrpyris, N.; Dimitriadis, K.; Theofilis, P.; Iliakis, P.; Beneki, E.; Pitsiori, D.; Tsioufis, P.; Shuvy, M.; Aznaouridis, K.; Tsioufis, K. Transcatheter Structural Heart Interventions in the Acute Setting: An Emerging Indication. J. Clin. Med. 2024, 13, 3528. [Google Scholar] [CrossRef]
- Ozdemir, E.; Esin, F.K.; Tuluce, S.Y.; Karaca, M. Emergent Transcatheter Aortic Valve Implantation as a Life-saving Procedure: A Primary Treatment Approach. J. Coll. Physicians Surg. Pak. 2019, 29, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Bianco, V.; Habertheuer, A.; Kilic, A.; Aranda-Michel, E.; Serna-Gallegos, D.; Schindler, J.; Kliner, D.; Toma, C.; Zalewski, A.; Sultan, I. Urgent transcatheter aortic valve replacement may be performed with acceptable long-term outcomes. J. Card. Surg. 2021, 36, 206–215. [Google Scholar] [CrossRef]
- Sá, M.P.; Van den Eynde, J.; Jacquemyn, X.; Tasoudis, P.; Erten, O.; Dokollari, A.; Torregrossa, G.; Sicouri, S.; Ramlawi, B. Late outcomes of transcatheter aortic valve implantation in bicuspid versus tricuspid valves: Meta-analysis of reconstructed time-to-event data. Trends Cardiovasc. Med. 2023, 33, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar]
- Jose, J.; Richardt, G.; Abdel-Wahab, M. Balloon- or Self-Expandable TAVI: Clinical Equipoise? Interv. Cardiol. 2015, 10, 103–108. [Google Scholar]
- Lee, M.; Modine, T.; Piazza, N.; Mylotte, D. TAVI device selection: Time for a patient-specific approach. EuroIntervention 2016, 12, Y37–Y41. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, H.; Samaee, M.; Sathananthan, J.; Sellers, S.; Kuetting, M.; Lilly, S.M.; Ihdayhid, A.R.; Blanke, P.; Leipsic, J.; Thourani, V.H.; et al. Comparison of performance of self-expanding and balloon-expandable transcatheter aortic valves. JTCVS Open 2022, 10, 128–139. [Google Scholar] [CrossRef]
- Okuno, T.; Tomii, D.; Lanz, J.; Heg, D.; Praz, F.; Stortecky, S.; Reineke, D.; Windecker, S.; Pilgrim, T. 5-Year Outcomes With Self-Expanding vs Balloon-Expandable Transcatheter Aortic Valve Replacement in Patients With Small Annuli. JACC Cardiovasc. Interv. 2023, 16, 429–440. [Google Scholar] [CrossRef]
- Huynh, K. Self-expanding valves more beneficial than balloon-expandable valves in patients with a small aortic annulus. Nat. Rev. Cardiol. 2024, 21, 356. [Google Scholar] [CrossRef]
- Stortecky, S.; O’Sullivan, C.J.; Buellesfeld, L.; Windecker, S.; Wenaweser, P. Transcatheter aortic valve implantation: The transfemoral access route is the default access. EuroIntervention 2013, 9, S14–S18. [Google Scholar] [CrossRef]
- Compagnone, M.; Dall’Ara, G.; Grotti, S.; Mambelli, G.; Fabbri, E.; Savini, C.; Balducelli, M.; Santarelli, A.; Iorio, E.; Vaquerizo, B.; et al. Transfemoral Transcatheter Aortic Valve Implantation at Hospitals Without On-Site Cardiac Surgery (TAVI at Home): A Multicenter Prospective Interventional Study. J. Cardiovasc. Dev. Dis. 2025, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Scotti, A.; Leone, P.P.; Sturla, M.; Curio, J.; Spring, A.M.; Ressa, G.; Ludwig, S.; Sugiura, T.; Assafin, M.; Granada, J.F.; et al. Prophylactic intra-aortic balloon pump in transfemoral transcatheter aortic valve implantation. EuroIntervention 2023, 19, e188–e190. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.S.K.; Sin, S.W.C. Short-term mechanical circulatory support (intra-aortic balloon pump, Impella, extracorporeal membrane oxygenation, TandemHeart): A review. Ann. Transl. Med. 2020, 8, 829. [Google Scholar] [CrossRef]
- Telukuntla, K.S.; Estep, J.D. Acute Mechanical Circulatory Support for Cardiogenic Shock. Methodist. Debakey Cardiovasc. J. 2020, 16, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Giao, D.M.; Giugliano, R.P. Left Ventricular Mechanical Circulatory Support Devices for Cardiogenic Shock After Myocardial Infarction. Cardiol. Ther. 2025, 14, 123–139. [Google Scholar] [CrossRef]
- Zeymer, U.; Morrow, D.A. Intra-aortic balloon pump in patients with heart failure-related cardiogenic shock: The Altshock-2 trial in perspective. Eur. Heart J. Acute Cardiovasc. Care 2025, 14, 237–239. [Google Scholar] [CrossRef]
- Gillespie, L.E.; Lane, B.H.; Shaw, C.R.; Gorder, K.; Grisoli, A.; Lavallee, M.; Gobble, O.; Vidosh, J.; Deimling, D.; Ahmad, S.; et al. The Intra-aortic Balloon Pump: A Focused Review of Physiology, Transport Logistics, Mechanics, and Complications. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101337. [Google Scholar] [CrossRef]
- Burzotta, F.; Nerla, R.; Trani, C. Bail-Out Use of Impella CP as a Bridge to TAVI in a Cardiogenic Shock Patient: The “Pump-Rewiring” Technique. J. Invasive Cardiol. 2016, 28, E1–E5. [Google Scholar]
- Almajed, M.R.; Mahmood, S.; Obri, M.; Nona, P.; Gonzalez, P.E.; Chiang, M.; Wang, D.D.; Frisoli, T.; Lee, J.; Basir, M.; et al. Application of Impella Mechanical Circulatory Support Devices in Transcatheter Aortic Valve Replacement and Balloon Aortic Valvuloplasty: A Single-Center Experience. Cardiovasc. Revasc. Med. 2023, 53, 1–7. [Google Scholar] [CrossRef]
- Koziol, K.J.; Isath, A.; Rao, S.; Gregory, V.; Ohira, S.; Van Diepen, S.; Lorusso, R.; Krittanawong, C. Extracorporeal Membrane Oxygenation (VA-ECMO) in Management of Cardiogenic Shock. J. Clin. Med. 2023, 12, 5576. [Google Scholar] [CrossRef]
- Mariani, M.V.; Pierucci, N.; Cipollone, P.; Vignaroli, W.; Piro, A.; Compagnucci, P.; Matteucci, A.; Chimenti, C.; Pandozi, C.; Dello Russo, A.; et al. Mechanical Circulatory Support Systems in the Management of Ventricular Arrhythmias: A Contemporary Overview. J. Clin. Med. 2024, 13, 1746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thiele, H.; Kurz, T.; Feistritzer, H.J.; Stachel, G.; Hartung, P.; Lurz, P.; Eitel, I.; Marquetand, C.; Nef, H.; Doerr, O.; et al. General Versus Local Anesthesia With Conscious Sedation in Transcatheter Aortic Valve Implantation: The Randomized SOLVE-TAVI Trial. Circulation 2020, 142, 1437–1447. [Google Scholar] [CrossRef]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Nørgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc. Imaging 2019, 12, 1–24. [Google Scholar] [CrossRef]
- Chiocchi, M.; Ricci, F.; Pasqualetto, M.; D’Errico, F.; Benelli, L.; Pugliese, L.; Cavallo, A.U.; Forcina, M.; Presicce, M.; De Stasio, V.; et al. Role of computed tomography in transcatheter aortic valve implantation and valve-in-valve implantation: Complete review of preprocedural and postprocedural imaging. J. Cardiovasc. Med. 2020, 21, 182–191. [Google Scholar] [CrossRef]
- Tzimas, G.; Meier, D.; Beneki, E.; Antiochos, P.; Auberson, D.; Leboub, S.; Lu, H.; Liabot, Q.; Zimmerli, A.; Salihu, A.; et al. Current Trends and Future Challenges in Transcatheter Aortic Valve Replacement: Utility of Cardiac Computed Tomography Angiography. J. Clin. Med. 2025, 14, 2474. [Google Scholar] [CrossRef] [PubMed]
- Muratori, M.; Mancini, M.E.; Tamborini, G.; Mushtaq, S.; Annoni, A.; Fusini, L.; Celeste, F.; Baggiano, A.; Fazzari, F.; Mantegazza, V.; et al. Approach to the Patient with Acute Aortic Syndromes in Light of the New Consensus Statement on Multimodality Imaging in Thoracic Aortic Diseases. J. Cardiovasc. Echogr. 2023, 33, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, C.; Eskandari, M.; Monaghan, M. 3D transoesophageal echocardiography in the TAVI sizing arena: Should we do it and how do we do it? Echo. Res. Pract. 2017, 4, R21–R32. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.P.; Parissis, J.; Bauersachs, J.; Brunner-La Rocca, H.P.; Bueno, H.; Čelutkienė, J.; Chioncel, O.; Coats, A.J.S.; Collins, S.P.; de Boer, R.A.; et al. Acute coronary syndromes and acute heart failure: A diagnostic dilemma and high-risk combination. A statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1298–1314. [Google Scholar] [CrossRef]
- Cao, D.; Chiarito, M.; Pagnotta, P.; Reimers, B.; Stefanini, G.G. Coronary Revascularisation in Transcatheter Aortic Valve Implantation Candidates: Why, Who, When? Interv. Cardiol. 2018, 13, 69–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McHugh, S.; Allaham, H.; Chahal, D.; Gupta, A. Coronary Artery Revascularization in Patients Undergoing Transcatheter Aortic Valve Replacement. Interv. Cardiol. Clin. 2025, 14, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.P.; Michail, M.; Treibel, T.A.; Rathod, K.; Jones, D.A.; Ozkor, M.; Kennon, S.; Forrest, J.K.; Mathur, A.; Mullen, M.J.; et al. Coronary Revascularization in Patients Undergoing Aortic Valve Replacement for Severe Aortic Stenosis. JACC Cardiovasc. Interv. 2021, 14, 2083–2096. [Google Scholar] [CrossRef] [PubMed]
- Karamasis, G.V.; Polyzogopoulou, E.; Varlamos, C.; Frantzeskaki, F.; Dragona, V.M.; Boultadakis, A.; Bistola, V.; Fountoulaki, K.; Pappas, C.; Kolokathis, F.; et al. Implementation of a cardiogenic shock team in a tertiary academic center. Hell. J. Cardiol. 2024. [Google Scholar] [CrossRef]
- Senman, B.; Jentzer, J.C.; Barnett, C.F.; Bartos, J.A.; Berg, D.D.; Chih, S.; Drakos, S.G.; Dudzinski, D.M.; Elliott, A.; Gage, A.; et al. Need for a Cardiogenic Shock Team Collaborative-Promoting a Team-Based Model of Care to Improve Outcomes and Identify Best Practices. J. Am. Heart Assoc. 2024, 13, e031979. [Google Scholar] [CrossRef] [PubMed]
- Alviar, C.L.; Li, B.K.; Keller, N.M.; Bohula-May, E.; Barnett, C.; Berg, D.D.; Burke, J.A.; Chaudhry, S.P.; Daniels, L.B.; DeFilippis, A.P.; et al. Prognostic performance of the IABP-SHOCK II Risk Score among cardiogenic shock subtypes in the critical care cardiology trials network registry. Am. Heart J. 2024, 270, 1–12. [Google Scholar] [CrossRef]
- Schmidt, M.; Burrell, A.; Roberts, L.; Bailey, M.; Sheldrake, J.; Rycus, P.T.; Hodgson, C.; Scheinkestel, C.; Cooper, D.J.; Thiagarajan, R.R.; et al. Predicting survival after ECMO for refractory cardiogenic shock: The survival after veno-arterial-ECMO (SAVE)-score. Eur. Heart J. 2015, 36, 2246–2256. [Google Scholar] [CrossRef]
- Amin, F.; Lombardi, J.; Alhussein, M.; Posada, J.D.; Suszko, A.; Koo, M.; Fan, E.; Ross, H.; Rao, V.; Alba, A.C.; et al. Predicting Survival After VA-ECMO for Refractory Cardiogenic Shock: Validating the SAVE Score. CJC Open 2020, 3, 71–81. [Google Scholar] [CrossRef]
- Duchnowski, P.; Hryniewiecki, T.; Kuśmierczyk, M.; Szymanski, P. Performance of the EuroSCORE II and the Society of Thoracic Surgeons score in patients undergoing aortic valve replacement for aortic stenosis. J. Thorac. Dis. 2019, 11, 2076–2081. [Google Scholar] [CrossRef]
- Gerfer, S.; Großmann, C.; Gablac, H.; Elderia, A.; Wienemann, H.; Krasivskyi, I.; Mader, N.; Lee, S.; Mauri, V.; Djordjevic, I.; et al. Low Left-Ventricular Ejection Fraction as a Predictor of Intraprocedural Cardiopulmonary Resuscitation in Patients Undergoing Transcatheter Aortic Valve Implantation. Life 2024, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Sinning, J.M.; Ghanem, A.; Steinhäuser, H.; Adenauer, V.; Hammerstingl, C.; Nickenig, G.; Werner, N. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2010, 3, 1141–1149. [Google Scholar] [CrossRef] [PubMed]

| Authors | Year | Design | Setting | Number of Patients | Mean Age (Years) | Mean LVEF (%) | 30-Day Mortality (%) | 1-Year Survival (%) |
|---|---|---|---|---|---|---|---|---|
| D’Ancona et al. [31] | 2012 | Prospective | Single center | 21 | 74.5 ± 11.1 | 26.0 ± 13.1 | 19.0 | 46.0 |
| Frerker et al. [32] | 2016 | Retrospective | Single center | 27 | 78.0 ± 9.0 | 39.5 ± 15.4 | 33.3 | 46.0 |
| Landes et al. [33] | 2016 | Retrospective | Single center | 27 | 80.1 ± 9.7 | Preserved LVEF: 48.1% of patients | 3.7 | NA |
| Bongiovanni et al. [34] | 2018 | Retrospective | Multicenter | 23 | 76.0 ± 11.4 | NA | 23.8 | NA |
| Kolte et al. [18] | 2018 | Retrospective | Multicenter | 3952 | 84 (78–88) | 53.0 (37.0–60.0) | 8.7 | 70.9 |
| Huang et al. [36] | 2019 | Retrospective | Single center | 26 | 73.1 ± 13.9 | 31.8 ±15.3 | 19.4 | 61.0 |
| Bandyopadhya et al. [37] | 2020 | Retrospective | Multicenter | 2136 | 81.4 ± 8.3 | NA | TAVI vs. BAV (p = 0.29) | NA |
| Fraccaro et al. [35] | 2020 | Retrospective | Multicenter | 51 | 75.8 ± 12.9 | ≤35% in 29.4% | 11.8 | 74.3 |
| Masha et al. [38] | 2020 | Retrospective | Multicenter | 2220 | 83 (77–87) | 53 (33–60) | 19.1 | 65.0 |
| Piriou et al. [39] | 2022 | Retrospective | Multicenter | 38 | NA | LVEF < 30% in 78.9% | 7.9 | 78.9 |
| Steffen et al. [40] | 2022 | Retrospective | Single center | 47 | 81.2 (71.4–86.1) | 38.0 (28.5–45.0) | 19.1 | ≈51.1 |
| Castelo et al. [41] | 2023 | Retrospective | Single center | 79 | 86.9 ± 7.5 | 45 | 17.5 | NA |
| Goel et al. [42] | 2023 | Retrospective | Multicenter | 4952 | 75.6 ± 10.9 | 39.9 ± 17.6 | 12.9 | 70.3 |
| Nair et al. [19] | 2024 | Retrospective | Single center | 24 | 79.0 (75.5–84.3) | 33.5 (22.0–41.3) | 4.2 | ≈81.0 |
| Authors | Intervention | Outcomes | Conclusions |
|---|---|---|---|
| D’Ancona et al. [31] | Patients in CS underwent transapical TAVI |
|
|
| Frerker et al. [32] | TAVI in patients with CS due to acutely decompensated AS |
|
|
| Landes et al. [33] | Urgent TAVI in patients with severe AS and acute HF versus elective TAVI |
|
|
| Bongiovanni et al. [34] | Emergency TAVI versus emergency BAV followed by TAVI under elective circumstances in patients with severe AS |
|
|
| Kolte et al. [18] | Patients with severe AS and CS undergoing urgent/emergent TAVI versus elective TAVI |
|
|
| Huang et al. [36] | Patients with decompensated severe AS and/or regurgitation and CS undergoing emergency TAVI |
|
|
| Bandyopadhya et al. [37] | Patients with severe AS and CS undergoing urgent BAV versus urgent TAVI |
|
|
| Fraccaro et al. [35] | Patients with severe AS and CS treated by TAVI |
|
|
| Masha et al. [38] | Patients undergoing TAVI after presenting with CS versus high-risk patients without cardiogenic shock |
|
|
| Piriou et al. [39] | Rescue TAVI in patients with CS and severe aortic disease |
|
|
| Steffen et al. [40] | Patients with acute HF due to severe AS undergoing emergent TAVI versus non-shock versus elective TAVI |
|
|
| Castelo et al. [41] | Patients with severe AS and CS undergoing urgent/emergent TAVI versus elective TAVI |
|
|
| Goel et al. [42] | Patients with severe AS and CS treated by TAVI |
|
|
| Nair et al. [19] | Patients with CS due to severe AS undergoing TAVI versus BAV versus medical therapy |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamasis, G.V.; Kourek, C.; Alexopoulos, D.; Parissis, J. Transcatheter Aortic Valve Implantation in Cardiogenic Shock: Current Evidence, Clinical Challenges, and Future Directions. J. Clin. Med. 2025, 14, 5398. https://doi.org/10.3390/jcm14155398
Karamasis GV, Kourek C, Alexopoulos D, Parissis J. Transcatheter Aortic Valve Implantation in Cardiogenic Shock: Current Evidence, Clinical Challenges, and Future Directions. Journal of Clinical Medicine. 2025; 14(15):5398. https://doi.org/10.3390/jcm14155398
Chicago/Turabian StyleKaramasis, Grigoris V., Christos Kourek, Dimitrios Alexopoulos, and John Parissis. 2025. "Transcatheter Aortic Valve Implantation in Cardiogenic Shock: Current Evidence, Clinical Challenges, and Future Directions" Journal of Clinical Medicine 14, no. 15: 5398. https://doi.org/10.3390/jcm14155398
APA StyleKaramasis, G. V., Kourek, C., Alexopoulos, D., & Parissis, J. (2025). Transcatheter Aortic Valve Implantation in Cardiogenic Shock: Current Evidence, Clinical Challenges, and Future Directions. Journal of Clinical Medicine, 14(15), 5398. https://doi.org/10.3390/jcm14155398








