Gastroesophageal Reflux Disease 10 Years After Bariatric Surgery—Is It a Problem? A Multicenter Study (BARI-10-POL)
Abstract
1. Introduction
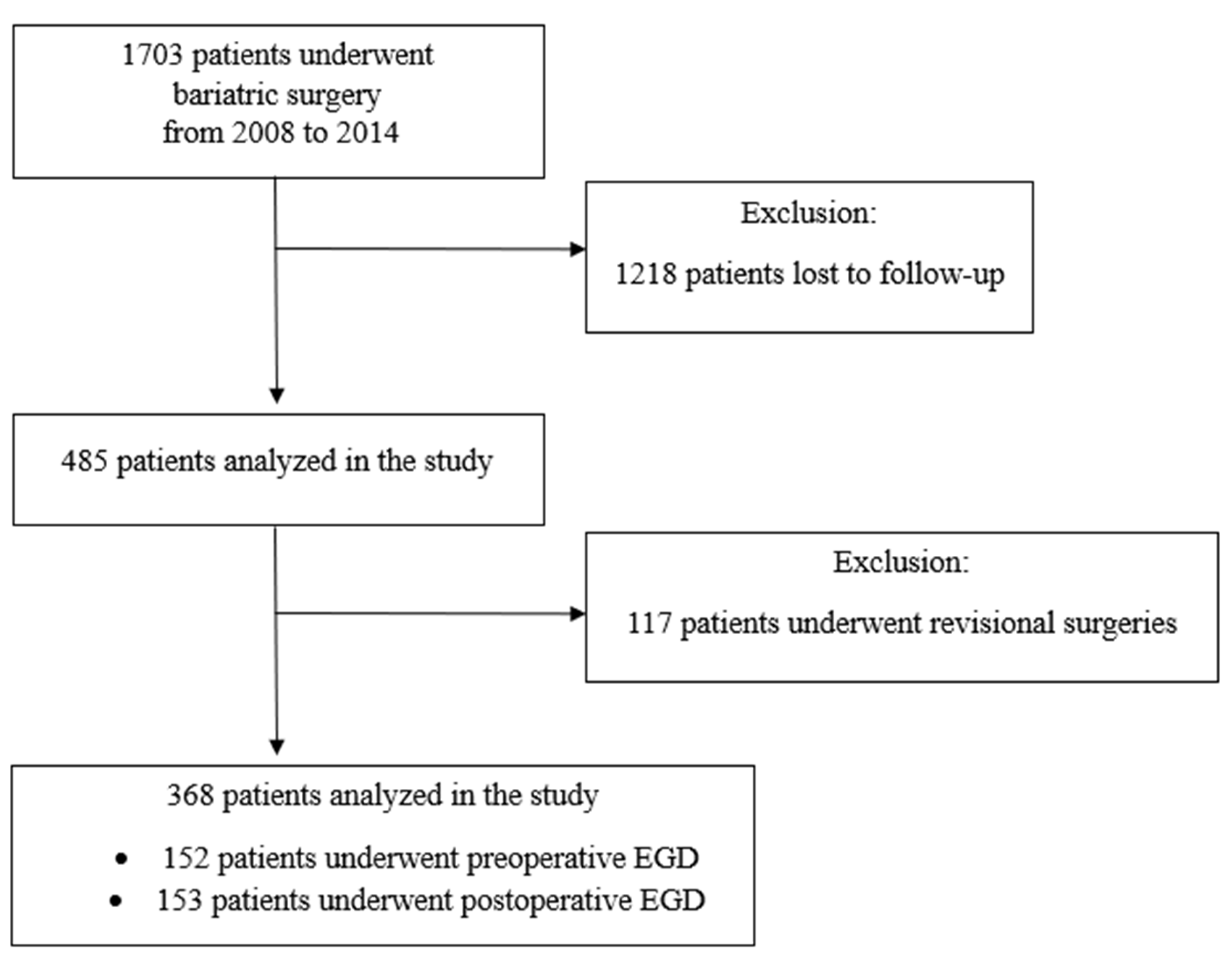
2. Materials and Methods
2.1. Surgical Techniques and Perioperative Care
2.2. Statistical Analysis
2.3. Ethical Considerations
3. Results
3.1. Symptoms of GERD Before Surgery
3.2. Symptoms of GERD After Surgery
3.3. EGD Findings
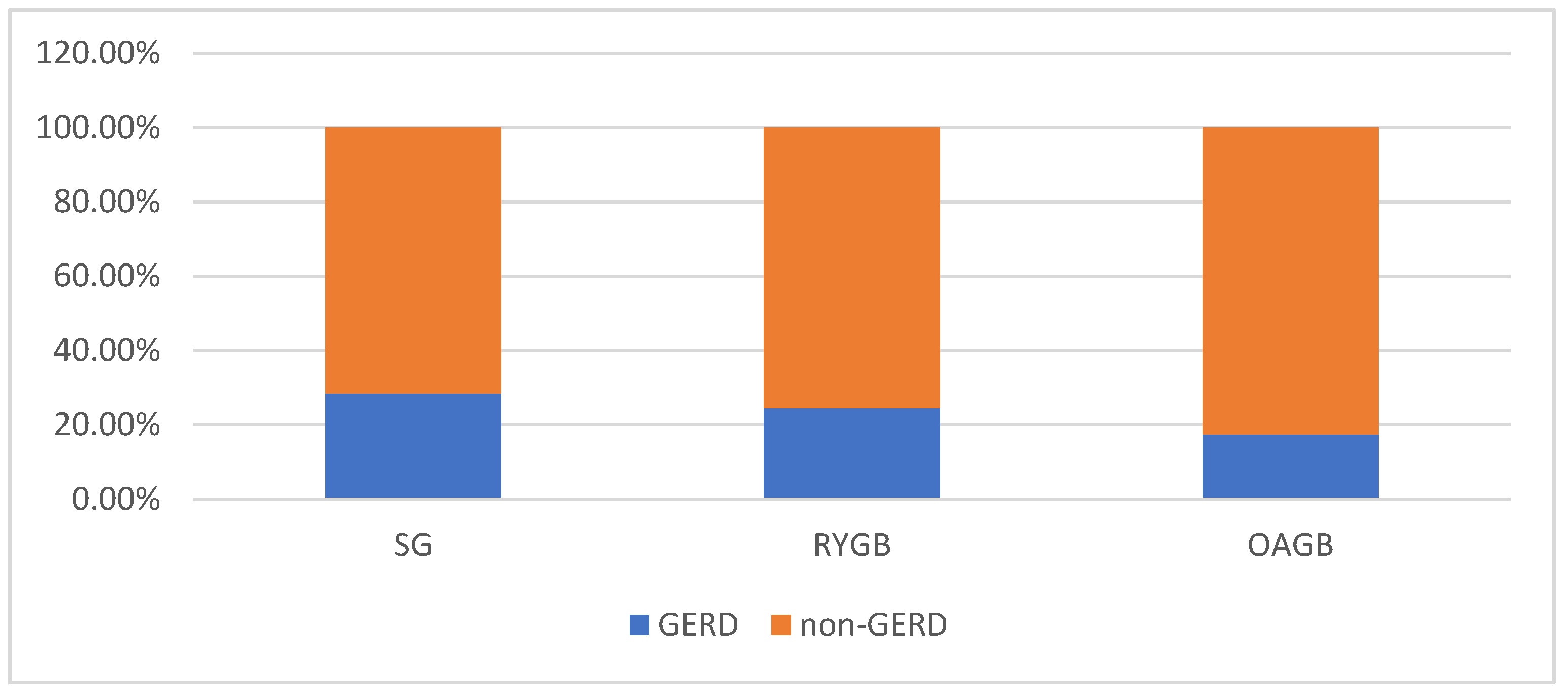
3.4. Evolution of Symptoms of GERD Depending on the Surgical Procedure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janik, M.R.; Sroczyński, P.; Major, P. Bariatric surgery in Poland, 2023: Growth, trends, and impact of the KOS-BAR program. Wideochir. Inne Tech. Maloinwazyjne 2024, 19, 454–459. [Google Scholar] [CrossRef]
- Konttinen, H.; Sjöholm, K.; Carlsson, L.M.S.; Peltonen, M.; Svensson, P.A. Fifteen-year changes in health-related quality of life after bariatric surgery and non-surgical obesity treatment. Int. J. Obes. 2024, 48, 1447–1456. [Google Scholar] [CrossRef]
- Hussein, A.; Awashra, A.; Rajab, I.; Bdair, M.; Hamdan, D.; Nouri, A.; Khatib, E.; Khatib, G.; Latt, N. Comparative effectiveness of bariatric surgery versus GLP-1 receptor agonists in reducing the risk of new-onset of NASH: A retrospective multinational cohort study from North America and Europe. Endocrinol. Diabetes Metab. 2025, 8, e70075. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.E.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-term outcomes after bariatric surgery: A systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes. Surg. 2019, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Abu-Abeid, A.; Dayan, D.; Berardi, G.; Musella, M. Long-term results of laparoscopic sleeve gastrectomy: A review of studies reporting 10+ years outcomes. Obes. Surg. 2023, 33, 3565–3570. [Google Scholar] [CrossRef]
- Mayoral, L.P.; Andrade, G.M.; Mayoral, E.P.; Huerta, T.H.; Canseco, S.P.; Rodal Canales, F.J.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Pérez Santiago, A.D.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [CrossRef]
- Ehlers, A.P.; Thumma, J.R.; Finks, J.F.; Carlin, A.M.; Ghaferi, A.A.; Varban, O.A. Evaluation of Patient Reported Gastroesophageal Reflux Severity at Baseline and at 1-Year After Bariatric Surgery. Ann. Surg. 2022, 275, 1143–1148. [Google Scholar] [CrossRef]
- Dowgiałło-Gornowicz, N.; Lech, P. The real occurrence of gastroesophageal reflux disease after sleeve gastrectomy—A prospective pH-monitoring study. Wideochir. Inne Tech. Maloinwazyjne 2024, 19, 205–210. [Google Scholar] [CrossRef]
- Poggi, L.; Bernui, G.M.; Romani, D.A.; Gavidia, A.F.; Poggi, L.A. Persistent and de Novo GERD After Sleeve Gastrectomy: Manometric and pH-Impedance Study Findings. Obes. Surg. 2023, 33, 87–93. [Google Scholar] [CrossRef]
- Rebecchi, F.; Allaix, M.E.; Patti, M.G.; Schlottmann, F.; Morino, M. Gastroesophageal reflux disease and morbid obesity: To sleeve or not to sleeve? World J. Gastroenterol. 2017, 23, 2269–2275. [Google Scholar] [CrossRef]
- Salminen, P.; Grönroos, S.; Helmiö, M.; Hurme, S.; Juuti, A.; Juusela, R.; Peromaa-Haavistoet, P.; Leivonen, M.; Nuutila, P.; Ovaska, J. Effect of Laparoscopic Sleeve Gastrectomy vs Roux-en-Y Gastric Bypass on Weight Loss, Comorbidities, and Reflux at 10 Years in Adult Patients with Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA Surg. 2022, 157, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Szymański, M.; Marek, I.; Wilczyński, M.; Janczy, A.; Bigda, J.; Kaska, Ł.; Proczko-Stepaniak, M. Evaluation of esophageal pathology in a group of patients 2 years after one-anastomosis gastric bypass (OAGB)—Cohort study. Obes. Res. Clin. Pract. 2022, 16, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, D.; Osland, E.; Memon, M.A. Bariatric surgery and gastroesophageal reflux disease. Ann. Transl. Med. 2020, 8 (Suppl. S1), S11. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.A.; Johari Halim Shah, Y.; Balalis, G.; Bashir, A.; Ramos, A.; Kow, L.; Herrera, M.; Shikora, S.; Campos, G.M.; Himpens, J.; et al. IFSO Position Statement on the Role of Esophago-Gastro-Duodenal Endoscopy Prior to and After Bariatric and Metabolic Surgery Procedures. Obes. Surg. 2020, 30, 3135–3153. [Google Scholar] [CrossRef]
- Gyawali, C.P.; Yadlapati, R.; Fass, R.; Katzka, D.; Pandolfino, J.; Savarino, E.; Sifrim, D.; Spechler, S.; Zerbib, F.; Fox, M.K.; et al. Updates to the modern diagnosis of GERD: Lyon consensus 2.0. Gut 2024, 73, 361–371. [Google Scholar] [CrossRef]
- Brethauer, A.S.; Kim, J.; el Chaar, M.; Papasavas, P.; Eisenberg, D.; Rogers, A.; Ballem, N.; Kligman, M.; Kothari, S.; ASMBS Clinical Issues Committee. Standardized outcomes reporting in metabolic and bariatric surgery. Surg. Obes. Relat. Dis. 2015, 11, 489–506. [Google Scholar] [CrossRef]
- Bhandari, M.; Fobi, M.A.L.; Buchwald, J.N.; Bariatric Metabolic Surgery Standardization (BMSS) Working Group. Standardization of Bariatric Metabolic Procedures: World Consensus Meeting Statement. Obes. Surg. 2019, 29 (Suppl. S4), 309–345. [Google Scholar] [CrossRef]
- Moayyedi, P.; Lacy, B.E.; Andrews, C.N.; Enns, R.A.; Howden, C.W.; Vakil, N. ACG and CAG Clinical Guideline: Management of Dyspepsia. Am. J. Gastroenterol. 2017, 112, 988–1013. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Desai, M.; Ruan, W.; Thosani, N.C.; Amaris, M.; Scott, J.S.; Saeed, A.; Abu Dayyeh, B.; Canto, M.I.; Abidi, W.; et al. American Society for Gastrointestinal Endoscopy guideline on the diagnosis and management of GERD: Summary and recommendations. Gastrointest. Endosc. 2025, 101, 267–284. [Google Scholar] [CrossRef]
- Felsenreich, D.M.; Kefurt, R.; Schermann, M.; Beckerhinn, P.; Kristo, I.; Krebs, M.; Prager, G.; Langer, F.B. Reflux, Sleeve Dilation, and Barrett’s Esophagus After Laparoscopic Sleeve Gastrectomy: Long-Term Follow-Up. Obes. Surg. 2017, 27, 3092–3101. [Google Scholar] [CrossRef]
- Kristo, I.; Paireder, M.; Jomrich, G.; Felsenreich, D.M.; Fischer, M.; Hennerbichler, F.P.; Schoppmann, S.F. Silent Gastroesophageal Reflux Disease in Patients with Morbid Obesity Prior to Primary Metabolic Surgery. Obes. Surg. 2020, 30, 4885–4891. [Google Scholar] [CrossRef] [PubMed]
- Gormsen, J.; Sanberg, J.; Gögenur, I.; Helgstrand, F. Use of proton pump inhibitors after laparoscopic gastric bypass and sleeve gastrectomy: A nationwide register-based cohort study. Int. J. Obes. 2024, 48, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Barreteau, T.; Frey, S.; de Montrichard, M.; Dreant, A.; Budnik, T.M.; Jacobi, D.; Perrot, B.; Blanchard, C. Effect of sleeve gastrectomy on distal esophagus at 5 and 10 years. Surg. Endosc. 2025, 39, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Coupaye, M.; Cohen-Sors, B.; Gorbatchef, C.; Dior, M.; Nebunu, N.; Latrache, F.; Le Gall, M.; Bado, A.; Ledoux, S.; et al. Do Preoperative Esophageal pH Monitoring and High-Resolution Manometry Predict Symptoms of GERD After Sleeve Gastrectomy? Obes. Surg. 2021, 31, 3490–3497. [Google Scholar] [CrossRef]
- Lee, Y.M.; Barazanchi, A.; Robertson, J.; Murphy, R.; Booth, M.W.C. Long-term effect of Roux-en-Y gastric bypass versus sleeve gastrectomy on reflux and Barrett’s oesophagus: A randomized controlled trial. ANZ J. Surg. 2025, 95, 911–918. [Google Scholar] [CrossRef]
- Kapellas, N.; Alkhalil, S.; Senkal, M. Efficacy of One-Anastomosis Gastric Bypass Versus Roux-en-Y Gastric Bypass for Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Obes. Surg. 2024, 34, 4563–4572. [Google Scholar] [CrossRef]
- Bevilacqua, L.A.; Obeid, N.R.; Yang, J.; Zhu, C.; Altieri, M.S.; Spaniolas, K.; Pryor, A.D. Incidence of GERD, esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma after bariatric surgery. Surg. Obes. Relat. Dis. 2020, 16, 1828–1836. [Google Scholar] [CrossRef]
- Kraljević, M.; Süsstrunk, J.; Wölnerhanssen, B.K.; Peters, T.; Bueter, M.; Gero, D.; Peterli, R. Long-Term Outcomes of Laparoscopic Roux-en-Y Gastric Bypass vs Laparoscopic Sleeve Gastrectomy for Obesity: The SM-BOSS Randomized Clinical Trial. JAMA Surg. 2025, 160, 369–377. [Google Scholar] [CrossRef]
- Crozet, J.; Denneval, A.; Brosse, M.; Pelascini, E.; Pasquer, A.; Robert, M. Conversion of Sleeve Gastrectomy to Roux-en-Y Gastric Bypass: Is Intrathoracic Migration of the Sleeve of High Incidence? Obes. Surg. 2024, 34, 2907–2913. [Google Scholar] [CrossRef]
- Karila-Cohen, P.; Pelletier, A.L.; Saker, L.; Laouénan, C.; Bachelet, D.; Khalil, A.; Arapis, K. Staple Line Intrathoracic Migration After Sleeve Gastrectomy: Correlation Between Symptoms, CT Three-Dimensional Stomach Analysis, and 24-h pH Monitoring. Obes. Surg. 2022, 32, 1–9. [Google Scholar] [CrossRef]
- Hutopila, I.; Ciocoiu, M.; Paunescu, L.; Copaescu, C. Reconstruction of the phreno-esophageal ligament (R-PEL) prevents the intrathoracic migration (ITM) after concomitant sleeve gastrectomy and hiatal hernia repair. Surg. Endosc. 2023, 37, 3747–3759. [Google Scholar] [CrossRef]
- Małczak, P.; Pisarska-Adamczyk, M.; Zarzycki, P.; Wysocki, M.; Major, P. Hiatal Hernia Repair During Laparoscopic Sleeve Gastrectomy: Systematic Review and Meta-Analysis on Gastroesophageal Reflux Disease Symptoms Changes. Pol. J. Surg. 2021, 93, 1–6. [Google Scholar] [CrossRef]
| GERD n = 63 | Non-GERD n = 305 | p-Value | |
|---|---|---|---|
| Characteristics | |||
| Sex, female/male | 44/19 | 209/96 | 0.837 |
| Age, median (IQR) | 42.0 (36.0–50.0) | 43.0 (35.0–53.0) | 0.939 |
| Preoperative BMI, median (IQR) | 40.9 (36.7–45.0) | 42.2 (39.3–47.2) | 0.013 |
| Type of surgery | |||
| SG, n (%) | 42 (66.7) | 198 (64.9) | 0.711 |
| RYGB, n (%) | 9 (14.3) | 56 (18.4) | |
| OAGB, n (%) | 12 (19.0) | 51 (16.7) | |
| T2D, n (%) | 21 (33.3) | 92 (30.2) | 0.620 |
| HT, n (%) | 34 (54.0) | 160 (52.5) | 0.827 |
| OBS, n (%) | 6 (9.5) | 20 (6.6) | 0.403 |
| Outcomes | |||
| Complications, n (%) | 4 (6.3) | 21 (6.9) | 0.878 |
| Postoperative BMI, median (IQR) | 33.1 (27.1–35.4) | 32.5 (28.4–38.4) | 0.100 |
| %EWL, median (IQR) | 62.7 (34.0–82.7) | 57.1 (29.3–80.1) | 0.451 |
| %TWL, median (IQR) | 25.0 (12.7–31.9) | 22.7 (11.7–32.8) | 0.835 |
| Postoperative GERD, n (%) | 17 (27.0) | 78 (25.6) | 0.816 |
| PPI postoperative, n (%) | 13 (20.6) | 68 (22.3) | 0.772 |
| GERD n = 95 | Non-GERD n = 273 | p-Value | |
|---|---|---|---|
| Characteristics | |||
| Sex, female/male | 70/25 | 183/90 | 0.228 |
| Age, median (IQR) | 42.0 (36.0–50.0) | 43.0 (35.0–53.0) | 0.733 |
| Preoperative BMI, median (IQR) | 42.8 (39.4–45.8) | 42.4 (38.6–46.8) | 0.983 |
| Type of surgery | |||
| SG, n (%) | 68 (71.6) | 172 (63.0) | 0.208 |
| RYGB, n (%) | 16 (16.8) | 49 (17.9) | |
| OAGB, n (%) | 11 (11.6) | 52 (19.1) | |
| Preoperative GERD, n (%) | 17 (17.9) | 46 (16.8) | 0.816 |
| Outcomes | |||
| Complications, n (%) | 7 (7.4) | 18 (6.6) | 0.796 |
| Postoperative BMI, median (IQR) | 33.1 (29.1–36.9) | 32.1 (27.7–38.0) | 0.331 |
| %EWL, median (IQR) | 58.9 (28.8–75.3) | 59.1 (29.4–82.1) | 0.465 |
| %TWL, median (IQR) | 24.0 (11.6–32.0) | 22.4 (12.0–32.6) | 0.921 |
| PPI postoperative, n (%) | 81 (85.3) | 0 (0.0) | <0.001 |
| Type of surgery among patients with the use of PPI | |||
| SG, n (%) | 57 (70.4) | 0.513 | |
| RYGB, n (%) | 13 (16.0) | ||
| OAGB, n (%) | 11 (13.6) | ||
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Sex, female | 1.38 | 0.82–2.32 | 0.229 |
| Age | 0.96 | 0.98–1.01 | 0.597 |
| Preoperative BMI | 1.01 | 0.98–1.04 | 0.754 |
| Preoperative GERD | 1.08 | 0.58–1.99 | 0.816 |
| Type of surgery, SG | |||
| RYGB | 0.83 | 0.44–1.55 | 0.552 |
| OAGB | 0.54 | 0.26–1.09 | 0.084 |
| T2D | 0.81 | 0.48–1.35 | 0.413 |
| HT | 0.84 | 0.53–1.34 | 0.462 |
| %TWL | 1.00 | 0.99–1.01 | 0.920 |
| Preoperative | Postoperative | |||||
|---|---|---|---|---|---|---|
| GERD n = 63 | Non-GERD n = 305 | GERD n = 95 | Non-GERD n = 273 | |||
| Preoperative EGD | ||||||
| No, n (%) | 20 (31.8) | 196 (64.3) | 52 (54.7) | 164 (60.1) | ||
| Yes, n (%) | 43 (68.2) | 109 (35.7) | 43 (45.3) | 109 (39.9) | ||
| No changes | 13 (30.2) | 70 (64.2) | <0.001 | 24 (55.8) | 59 (54.1) | 0.547 |
| HH | 20 (46.5) | 37 (33.9) | 16 (37.2) | 41 (37.6) | ||
| LA-A | 8 (18.6) | 1 (1.0) | 3 (7.0) | 6 (5.5) | ||
| LA-B | 2 (4.7) | 1 (1.0) | 0 (0.0) | 3 (2.8) | ||
| Postoperative EGD | ||||||
| No, n (%) | 33 (52.4) | 182 (59.7) | 27 (28.4) | 188 (68.9) | ||
| Yes, n (%) | 30 (47.6) | 123 (40.3) | 68 (71.6) | 85 (31.1) | ||
| No changes | 19 (63.3) | 80 (65.0) | 0.683 | 26 (38.2) | 73 (85.9) | <0.001 |
| HH | 4 (13.3) | 7 (5.7) | 6 (8.8) | 5 (5.9) | ||
| LA-A | 1 (3.4) | 8 (6.5) | 8 (11.8) | 1 (1.2) | ||
| LA-B | 4 (13.3) | 20 (16.3) | 20 (29.4) | 4 (4.7) | ||
| LA-C/D/Barrett | 2 (6.7) | 8 (6.5) | 8 (11.8) | 2 (2.3) | ||
| Variable, n/N * (%) | SG | RYGB | OAGB | p-Value |
|---|---|---|---|---|
| De novo GERD | 52/198 (26.3) | 16/56 (28.6) | 10/51 (19.6) | 0.074 |
| Remission of GERD | 16/42 (38.1) | 9/9 (100) | 10/11 (90.9) | 0.005 |
| Persistence of GERD | 26/42 (61.9) | 0/9 (0.0) | 1/11 (9.1) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dowgiałło-Gornowicz, N.; Proczko-Stepaniak, M.; Kloczkowska, A.; Jaworski, P.; Major, P. Gastroesophageal Reflux Disease 10 Years After Bariatric Surgery—Is It a Problem? A Multicenter Study (BARI-10-POL). J. Clin. Med. 2025, 14, 5405. https://doi.org/10.3390/jcm14155405
Dowgiałło-Gornowicz N, Proczko-Stepaniak M, Kloczkowska A, Jaworski P, Major P. Gastroesophageal Reflux Disease 10 Years After Bariatric Surgery—Is It a Problem? A Multicenter Study (BARI-10-POL). Journal of Clinical Medicine. 2025; 14(15):5405. https://doi.org/10.3390/jcm14155405
Chicago/Turabian StyleDowgiałło-Gornowicz, Natalia, Monika Proczko-Stepaniak, Anna Kloczkowska, Paweł Jaworski, and Piotr Major. 2025. "Gastroesophageal Reflux Disease 10 Years After Bariatric Surgery—Is It a Problem? A Multicenter Study (BARI-10-POL)" Journal of Clinical Medicine 14, no. 15: 5405. https://doi.org/10.3390/jcm14155405
APA StyleDowgiałło-Gornowicz, N., Proczko-Stepaniak, M., Kloczkowska, A., Jaworski, P., & Major, P. (2025). Gastroesophageal Reflux Disease 10 Years After Bariatric Surgery—Is It a Problem? A Multicenter Study (BARI-10-POL). Journal of Clinical Medicine, 14(15), 5405. https://doi.org/10.3390/jcm14155405





