Added Value of SPECT/CT in Radio-Guided Occult Localization (ROLL) of Non-Palpable Pulmonary Nodules Treated with Uniportal Video-Assisted Thoracoscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Techniques
2.4. Surgical Procedure
2.5. Data Collection
2.6. Outcome Measure
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, S.J.; Stone, E.; Baldwin, D.R.; Vliegenthart, R.; Lee, P.; Fintelmann, F.J. Lung Cancer Screening. Lancet 2023, 401, 390–408. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Tang, T.; Liu, I.-L.A.; Lee, J.; Zheng, C.; Danforth, K.N.; Kosco, A.E.; Di Fiore, J.L.; Suh, D.E. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am. J. Respir. Crit. Care Med. 2015, 192, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, M.; Du, Q.; Han, H.; Ge, Y.; Park, H. The Value of Positron Emission Tomography Computed Tomography in Predicting Invasiveness of Ground Glass Nodules: A Protocol for Systematic Review and Meta-Analysis. Medicine 2021, 100, e27507. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J.; Lam, L. Evaluating the Patient with a Pulmonary Nodule: A Review. JAMA 2022, 327, 264–273. [Google Scholar] [CrossRef]
- Montagne, F.; Guisier, F.; Venissac, N.; Baste, J.-M. The Role of Surgery in Lung Cancer Treatment: Present Indications and Future Perspectives-State of the Art. Cancers 2021, 13, 3711. [Google Scholar] [CrossRef]
- Li, Y.; Dai, T. Meta-Analysis Comparing the Perioperative Efficacy of Single-Port Versus two and Multi-Port Video-Assisted Thoracoscopic Surgical Anatomical Lung Resection for Lung Cancer. Medicine 2023, 102, e32636. [Google Scholar] [CrossRef]
- Tamura, M.; Oda, M.; Fujimori, H.; Shimizu, Y.; Matsumoto, I.; Watanabe, G. New Indication for Preoperative Marking of Small Peripheral Pulmonary Nodules in Thoracoscopic Surgery. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 590–593. [Google Scholar] [CrossRef]
- Mattioni, G.; Palleschi, A.; Mendogni, P.; Tosi, D. Approaches and Outcomes of Robotic-assisted Thoracic Surgery (RATS) for Lung Cancer: A Narrative Review. J. Robot. Surg. 2022, 17, 797–809. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Y.; Wang, Y. Intraoperative Identification of Pulmonary Nodules During Minimally Invasive Thoracic Surgery: A Narrative Review. Quant. Imaging Med. Surg. 2022, 12, 5271–5287. [Google Scholar] [CrossRef]
- Tsoumakidou, G.; Saltiel, S.; Villard, N.; Duran, R.; Meuwly, J.Y.; Denys, A. Image-Guided Marking Techniques in Interventional Radiology: A review of Current Evidence. Diagn. Interv. Imaging 2021, 102, 699–707. [Google Scholar] [CrossRef]
- Lin, M.-W.; Chen, J.-S. Image-Guided Techniques for Localizing Pulmonary Nodules in Thoracoscopic Surgery. J. Thorac. Dis. 2016, 8, S749–S755. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gu, C. Expert consensus Workshop Report: Guidelines for Preoperative Assisted Localization of Small Pulmonary Nodules. J. Cancer Res. Ther. 2020, 16, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Imperatori, A.; Nardecchia, E.; Cattoni, M.; Mohamed, S.; Di Natale, D.; Righi, I.; Mendogni, P.; Diotti, C.; Rotolo, N.; Dominioni, L.; et al. Perioperative Identifications of Non-Palpable Pulmonary Nodules: A Narrative Review. J. Thorac. Dis. 2021, 13, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Chella, A.; Lucchi, M.; Ambrogi, M.C.; Menconi, G.; Melfi, F.M.A.; Gonfiotti, A.; Boni, G.; Angeletti, C.A. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur. J. Cardio Thoracic Surg. 2000, 18, 17–21. [Google Scholar] [CrossRef]
- Bellomi, M.; Veronesi, G.; Trifirò, G.; Brambilla, S.; Bonello, L.; Preda, L.; Casiraghi, M.; Borri, A.; Paganelli, G.; Spaggiari, L. Computed tomography-guided preoperative radiotracer localization of nonpalpable lung nodules. Ann. Thorac. Surg. 2010, 90, 1759–1764. [Google Scholar] [CrossRef]
- Grogan, E.L.; Jones, D.R.; Kozower, B.D.; Simmons, W.D.; Daniel, T.M. Identification of Small Lung Nodules: Technique of Radiotracer-Guided Thoracoscopic Biopsy. Ann. Thorac. Surg. 2008, 85, S772–S777. [Google Scholar] [CrossRef]
- Conte, M.; De Feo, M.S.; Frantellizzi, V.; Tomaciello, M.; Marampon, F.; Evangelista, L.; Filippi, L.; De Vincentis, G. Radio-Guided Lung Surgery: A Feasible Approach for a Cancer Precision Medicine. Diagnostics 2023, 13, 2628. [Google Scholar] [CrossRef]
- Durmo, R.; Lechiara, M.; Benetti, D.; Rodella, C.; Camoni, L.; Albano, D.; Bertagna, F.; Giubbini, R. Radioguided lung lesion localization: Introducing a fluoroscopy system in a SPECT/CT scan. Nucl. Med. Commun. 2019, 40, 597–603. [Google Scholar] [CrossRef]
- Aricò, D.; Macrì, P.; Bambaci, M.; Leone, G.; Romano, D.; Barbagallo, F.; Maria, S.; Picone, A.; Carnaghi, C.; Piazza, D.; et al. Non-palpable Pulmonary Nodules and Uniportal-VATS: Radio-Guided Localization (ROLL) Experience of a Lung Multidisciplinary Team. Anticancer Res. 2024, 44, 3507–3514. [Google Scholar] [CrossRef]
- Yue, X.; Cui, J.; Huang, S.; Liu, W.; Qi, J.; He, K.; Li, T. An interpretable radiomics-based machine learning model for predicting reverse left ventricular remodeling in STEMI patients using late gadolinium enhancement of myocardial scar. Eur. Radiol. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Song, F.; Zhang, W.; Gong, T.; Zhao, S.; Lv, F. Prediction of benign and malignant pulmonary nodules using preoperative CT features: Using PNI-GARS as a predictor. Front. Immunol. 2024, 15, 1446511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ricciardi, S.; Davini, F.; Manca, G.; De Liperi, A.; Romano, G.; Zirafa, C.C.; Melfi, F. Radioguided Surgery, a Cost-Effective Strategy for Treating Solitary Pulmonary Nodules: 20-Year Experience of a Single Center. Clin. Lung Cancer 2020, 21, e417–e422. [Google Scholar] [CrossRef]
- Davini, F.; Ricciardi, S.; Zirafa, C.C.; Cavaliere, I.; Romano, G.; Melfi, F. Treatment of Pulmonary Nodule: From VATS to RATS. J. Vis. Surg. 2018, 4, 36. [Google Scholar] [CrossRef]
- Manca, G.; Davini, F.; Tardelli, E.; De Liperi, A.; Falaschi, F.; Melfi, F.; Colletti, P.M.; Rubello, D.; Volterrani, D.; Boni, G.; et al. Clinical Impact of Radioguided Localization in the Treatment of Solitary Pulmonary Nodule: A 20-Year Retrospective Analysis. Clin. Nucl. Med. 2018, 43, 317–322. [Google Scholar] [CrossRef]
- Carvajal, C.; González, F.; Beltrán, R.; Buitrago, R.; Reyes, A.d.L.; Llamas, A.; Beltrán, J.; Carreño, J. Lung nodule radio-guided localization and uniportal video-assisted thoracoscopic surgery resection. Updat. Surg. 2021, 73, 1559–1566. [Google Scholar] [CrossRef]
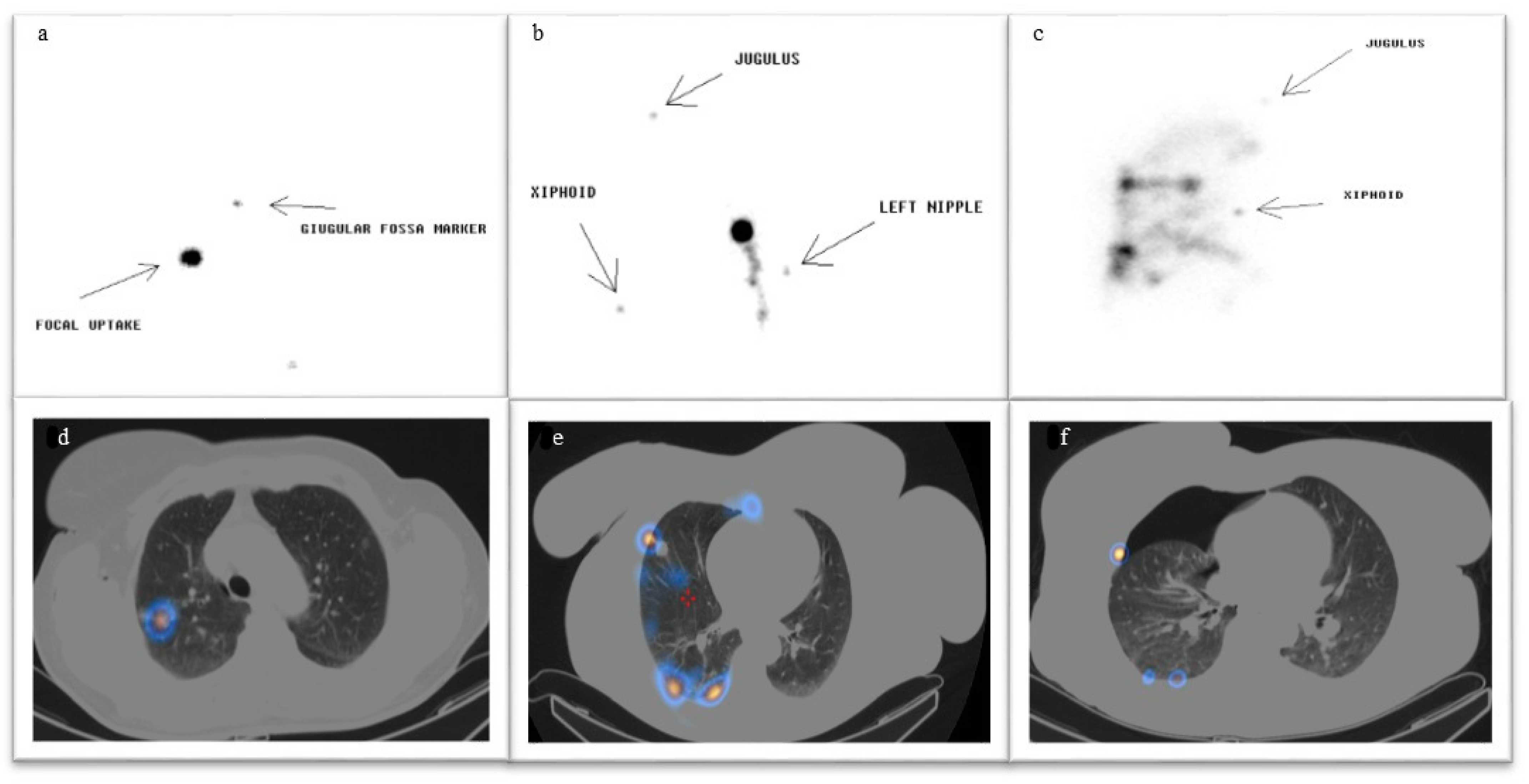
| (A) | |||
| Patients Characteristics | N = 125 | ||
| Median Age (min–max) | 67 (33–84) | ||
| Sex (male/female) | 55/71 | ||
| Nodule Characteristic | |||
| Median Size in mm (range) | 12 (5–30) | ||
| Median Volume in cm3 (range) | 13.5 (6.5–141.3) | ||
| Median Depth in mm (range) | 15 (2–65) | ||
| Median 99mTC-MAA Activity in MBq (range) | 70 (38–210) | ||
| Density, n (%) | |||
| GGO | 14 (11.2%) | ||
| psGGO | 15 (12%) | ||
| Solid | 96 (76.8%) | ||
| Localization, n (%) | |||
| RIL | 28 (22.4%) | ||
| LIL | 23 (18.4%) | ||
| ML | 11 (8.8%) | ||
| RUL | 35 (28%) | ||
| LUL | 28 (22.4%) | ||
| (B) | |||
| Patients Characteristics | Planar Technique Population (N=60) | SPECT Technique Population (N=65) | p-Value |
| Median Age (range) | 66 (44–81) | 68 (33–84) | 0.28 |
| Sex (male/female) | 28/32 | 27/38 | 0.70 |
| Nodule Characteristic | |||
| Median Size in mm | 9 | 12 | <0.001 |
| Median Volume in cm3 | 38.2 | 90.5 | <0.001 |
| Nodule Depth, mean in mm | 16.1 | 13.7 | 0.09 |
| Density, n (%) | |||
| GGO | 4 (6.7%) | 10 (15.4%) | |
| psGGO | 2 (3.3%) | 13 (20%) | |
| Solid | 54 (90%) | 42 (64.6%) | |
| Planar Imaging (n = 60) | SPECT Imaging (n = 65) | Statistical Significance | |
|---|---|---|---|
| Timing (mean hours/minutes) | |||
| Nuclear Medicine Service | 00:30 | 00:39 | p < 0.005 |
| Surgical excision | 1:47 | 2:25 | p < 0.005 |
| Spillage, n (%) | p < 0.0001 | ||
| Pleural | 29 (48%) | 11 (17%) | |
| Subcutaneous | 0 | 1 (1.5%) | |
| Bronchial tract | 1 (1.6%) | 16 (24.6%) | |
| Gastrointestinal tract None | 0 30 (50.4%) | 1 (1.5%) 36 (55.4%) | |
| Nuclear Medicine complications, n (%) | |||
| Pneumothorax | 1 (1.6%) | 28 (43%) | p < 0.0001 |
| Hematoma None | 0 59 (98.7%) | 1 (1.5%) 36 (55.5%) | |
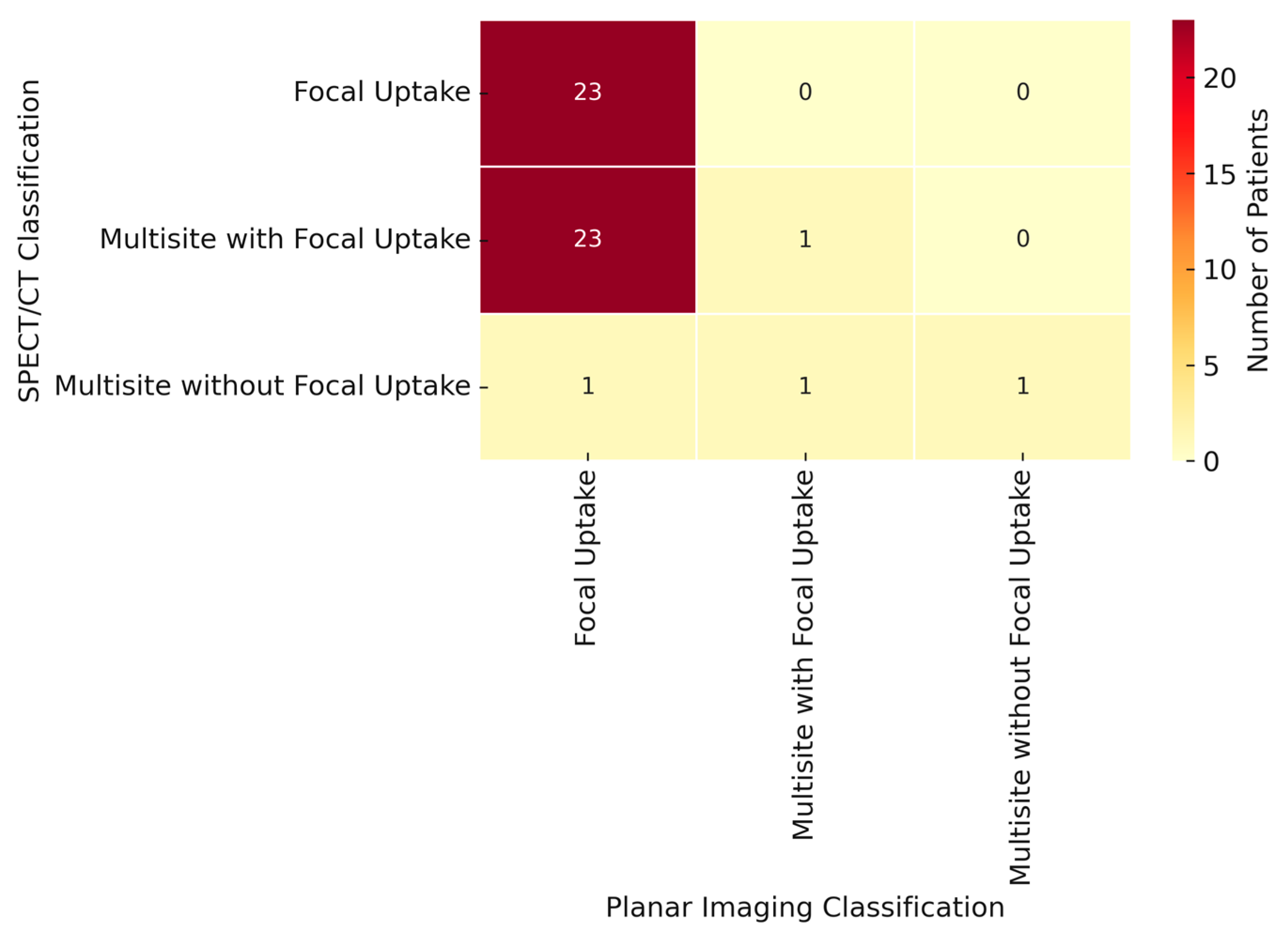
| Radio-guided intraoperative localization detection rate, n (%) No Yes | 1 (1.6%) 59 (98.7%) | 4 (6.1%) 61 (93.9%) | NS |
| Histological Diagnosis, n (%) | |||
| Benign | 8 (13%) | 11 (17%) | NS |
| Malignant | 52 (87%) | 54 (83%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aricò, D.; Motta, L.; Giacoppo, G.; Bambaci, M.; Macrì, P.; Maria, S.; Barbagallo, F.; Ricottone, N.; Marino, L.; Motta, G.; et al. Added Value of SPECT/CT in Radio-Guided Occult Localization (ROLL) of Non-Palpable Pulmonary Nodules Treated with Uniportal Video-Assisted Thoracoscopy. J. Clin. Med. 2025, 14, 5337. https://doi.org/10.3390/jcm14155337
Aricò D, Motta L, Giacoppo G, Bambaci M, Macrì P, Maria S, Barbagallo F, Ricottone N, Marino L, Motta G, et al. Added Value of SPECT/CT in Radio-Guided Occult Localization (ROLL) of Non-Palpable Pulmonary Nodules Treated with Uniportal Video-Assisted Thoracoscopy. Journal of Clinical Medicine. 2025; 14(15):5337. https://doi.org/10.3390/jcm14155337
Chicago/Turabian StyleAricò, Demetrio, Lucia Motta, Giulia Giacoppo, Michelangelo Bambaci, Paolo Macrì, Stefania Maria, Francesco Barbagallo, Nicola Ricottone, Lorenza Marino, Gianmarco Motta, and et al. 2025. "Added Value of SPECT/CT in Radio-Guided Occult Localization (ROLL) of Non-Palpable Pulmonary Nodules Treated with Uniportal Video-Assisted Thoracoscopy" Journal of Clinical Medicine 14, no. 15: 5337. https://doi.org/10.3390/jcm14155337
APA StyleAricò, D., Motta, L., Giacoppo, G., Bambaci, M., Macrì, P., Maria, S., Barbagallo, F., Ricottone, N., Marino, L., Motta, G., Leone, G., Carnaghi, C., Gebbia, V., Caponnetto, D., & Evangelista, L. (2025). Added Value of SPECT/CT in Radio-Guided Occult Localization (ROLL) of Non-Palpable Pulmonary Nodules Treated with Uniportal Video-Assisted Thoracoscopy. Journal of Clinical Medicine, 14(15), 5337. https://doi.org/10.3390/jcm14155337









