SGLT2 Inhibitors and the Risk of Arrhythmias in Heart Failure: A Network Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Data Collection Process
2.2. Study Selection
2.3. Data Extraction
2.4. Data Analysis
2.4.1. Pairwise Meta-Analysis
2.4.2. Network Meta-Analysis
2.5. Inconsistency Assessment
2.6. Treatment Ranking
2.7. League Table Synthesis
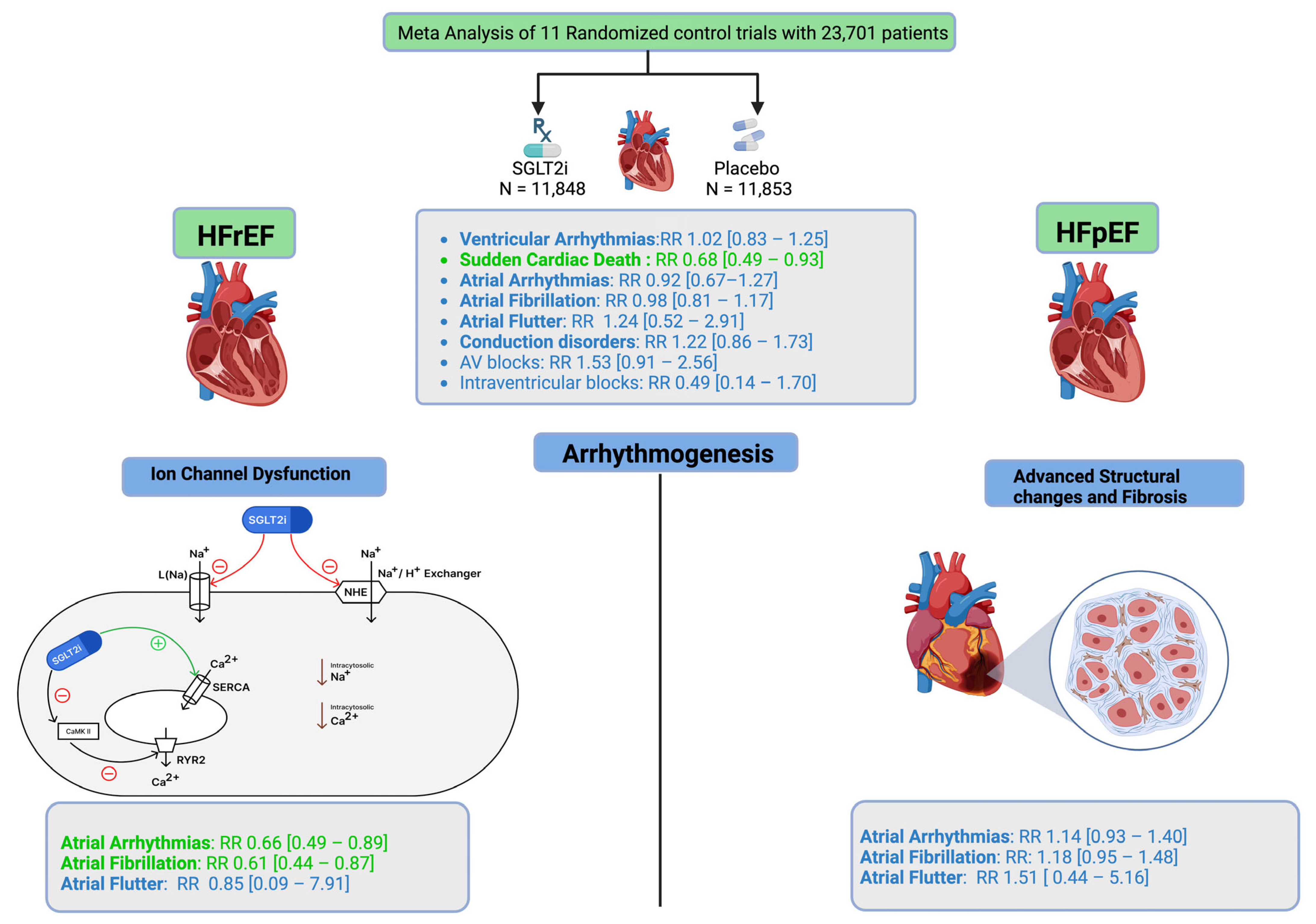
3. Results
3.1. Heart Failure Phenotype-Specific Effects
3.1.1. Heart Failure with Preserved Ejection Fraction (HFpEF)
3.1.2. Heart Failure with Reduced Ejection Fraction (HFrEF)
3.2. Drug-Specific Effects (Table 4)
3.2.1. Dapagliflozin
3.2.2. Empagliflozin
3.2.3. Sotagliflozin
3.2.4. Canagliflozin
3.3. Network Meta-Analysis (NMA) Results
3.4. NMA Consistency and Heterogeneity
4. Discussion
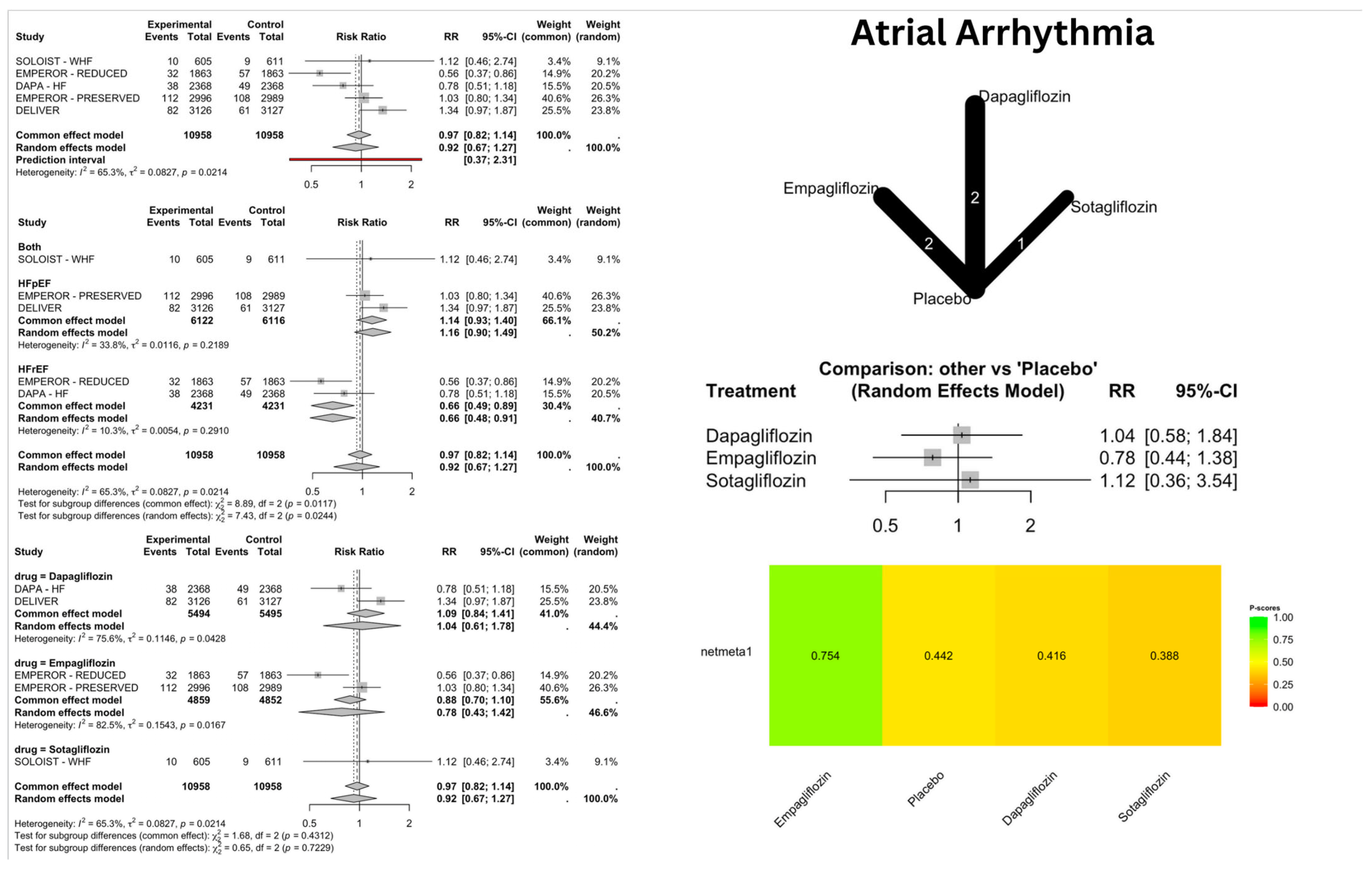
4.1. Atrial Arrhythmias
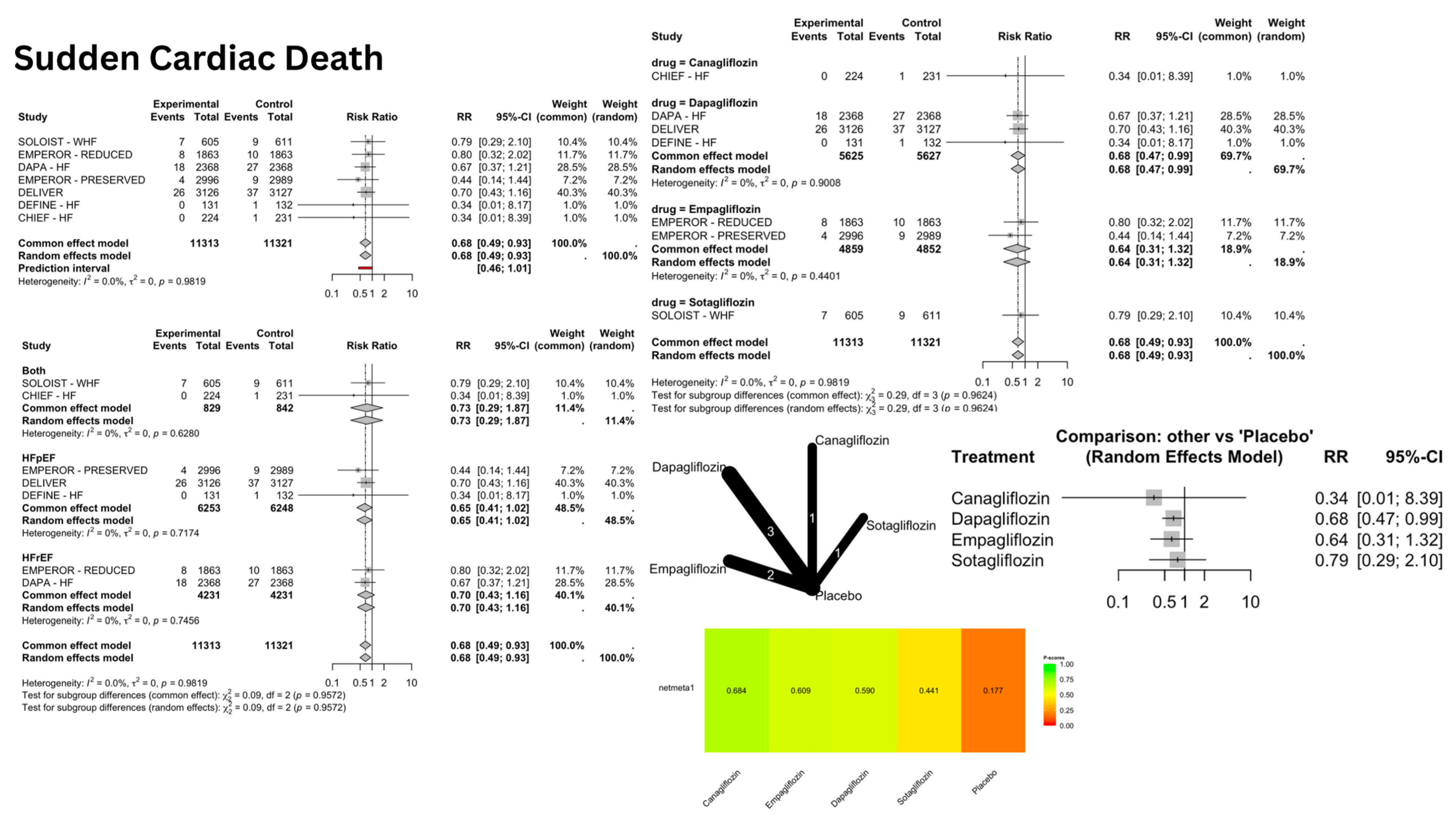
4.2. Ventricular Arrhythmias and SCD
4.3. Bradyarrhythmias and Conduction Disorders
4.4. Clinical Implications
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Heart Federation. Heart Failure. Available online: https://world-heart-federation.org/what-we-do/heart-failure/ (accessed on 25 June 2025).
- Bozkurt, B.; Ahmad, T.; Alexander, K.M.; Baker, W.L.; Bosak, K.; Breathett, K.; Fonarow, G.C.; Heidenreich, P.; Ho, J.E.; Hsich, E.; et al. Heart failure epidemiology and outcomes statistics: A report of the Heart Failure Society of America. J. Card. Fail. 2023, 29, 1412–1451. [Google Scholar] [CrossRef]
- Diaz, J.; Martinez, F.; Calderon, J.M.; Fernandez, A.; Sauri, I.; Uso, R.; Trillo, J.L.; Redon, J.; Forner, M.J. Incidence and impact of atrial fibrillation in heart failure patients: Real-world data in a large community. ESC Heart Fail. 2022, 9, 4230–4239. [Google Scholar] [CrossRef]
- Gutierrez, A.; Ash, J.; Akdemir, B.; Alexy, T.; Cogswell, R.; Chen, J.; Adabag, S. Nonsustained ventricular tachycardia in heart failure with preserved ejection fraction. Pacing Clin. Electrophysiol. 2020, 43, 1126–1131. [Google Scholar] [CrossRef]
- Masarone, D.; Limongelli, G.; Ammendola, E.; Verrengia, M.; Gravino, R.; Pacileo, G. Risk stratification of sudden cardiac death in patients with heart failure: An update. J. Clin. Med. 2018, 7, 436. [Google Scholar] [CrossRef]
- Gao, J.; Xue, G.; Zhan, G.; Wang, X.; Li, J.; Yang, X.; Xia, Y. Benefits of SGLT2 inhibitors in arrhythmias. Front. Cardiovasc. Med. 2022, 9, 1011429. [Google Scholar] [CrossRef]
- Effect of Sotagliflozin on Cardiovascular Events in Participants with Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF Trial) (ClinicalTrials.gov Identifier NCT03521934). Available online: https://clinicaltrials.gov/ct2/show/NCT03521934 (accessed on 25 June 2025).
- EMPagliflozin outcomE tRial in Patients with chrOnic heaRt Failure with Reduced Ejection Fraction (EMPEROR-Reduced) (ClinicalTrials.gov Identifier NCT03057977). Available online: https://clinicaltrials.gov/ct2/show/NCT03057977 (accessed on 25 June 2025).
- Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients with Chronic Heart Failure (DAPA-HF) (ClinicalTrials.gov Identifier NCT03036124). Available online: https://clinicaltrials.gov/ct2/show/NCT03036124 (accessed on 25 June 2025).
- EMPagliflozin outcomE tRial in Patients with chrOnic heaRt Failure with Preserved Ejection Fraction (EMPEROR-Preserved) (ClinicalTrials.gov Identifier NCT03057951). Available online: https://clinicaltrials.gov/ct2/show/NCT03057951 (accessed on 25 June 2025).
- Dapagliflozin Evaluation to Improve the LIVEs of Patients with PReserved Ejection Fraction Heart Failure (DELIVER) (ClinicalTrials.gov Identifier NCT03619213). Available online: https://clinicaltrials.gov/ct2/show/NCT03619213 (accessed on 25 June 2025).
- Dapagliflozin Effect on Symptoms and Biomarkers in Patients with Heart Failure (DEFINE-HF) (ClinicalTrials.gov Identifier NCT02653482). Available online: https://clinicaltrials.gov/ct2/show/NCT02653482 (accessed on 25 June 2025).
- A Study on Impact of Canagliflozin on Health Status, Quality of Life, and Functional Status in Heart Failure (CHIEF-HF) (ClinicalTrials.gov Identifier NCT04252287). Available online: https://clinicaltrials.gov/ct2/show/NCT04252287 (accessed on 25 June 2025).
- Dapagliflozin in PRESERVED Ejection Fraction Heart Failure (PRESERVED-HF) (ClinicalTrials.gov Identifier NCT03030235). Available online: https://clinicaltrials.gov/ct2/show/NCT03030235 (accessed on 25 June 2025).
- This Study Tests Empagliflozin in Patients with Chronic Heart Failure with Preserved Ejection Fraction (HFpEF). The Study Looks at How Far Patients Can Walk in 6 Minutes and at Their Heart Failure Symptoms (ClinicalTrials.gov Identifier NCT03448406). Available online: https://clinicaltrials.gov/ct2/show/NCT03448406 (accessed on 25 June 2025).
- This Study Tests Empagliflozin in Patients with Chronic Heart Failure with Reduced Ejection Fraction (HFrEF). The Study Looks at How Far Patients Can Walk in 6 Minutes and at Their Heart Failure Symptoms (ClinicalTrials.gov Identifier NCT03448419). Available online: https://clinicaltrials.gov/ct2/show/NCT03448419 (accessed on 25 June 2025).
- Empagliflozin Impact on Hemodynamics in Patients with Heart Failure (EMBRACE-HF) (ClinicalTrials.gov Identifier NCT03030222). Available online: https://clinicaltrials.gov/ct2/show/NCT03030222 (accessed on 25 June 2025).
- Armillotta, M.; Angeli, F.; Paolisso, P.; Belmonte, M.; Raschi, E.; Di Dalmazi, G.; Amicone, S.; Canton, L.; Fedele, D.; Suma, N.; et al. Cardiovascular therapeutic targets of sodium-glucose co-transporter 2 (SGLT2) inhibitors beyond heart failure. Pharmacol. Ther. 2025, 270, 108861. [Google Scholar] [CrossRef]
- Liao, J.; Ebrahimi, R.; Ling, Z.; Meyer, C.; Martinek, M.; Sommer, P.; Futyma, P.; Di Vece, D.; Schratter, A.; Acou, W.J.; et al. Effect of SGLT-2 inhibitors on arrhythmia events: Insight from an updated secondary analysis of > 80,000 patients (the SGLT2i-Arrhythmias and Sudden Cardiac Death). Cardiovasc. Diabetol. 2024, 23, 78. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhang, W.; Li, J.; Weng, W.; Li, Q. Association of Sodium-Glucose Cotransporter 2 Inhibitors (SGLT2i) with Cardiac Arrhythmias: A Systematic Review and Meta-Analysis of Cardiovascular Outcome Trials. Rev. Cardiovasc. Med. 2023, 24, 258. [Google Scholar] [CrossRef]
- Fedele, D.; Casuso Alvarez, M.; Maida, A.; Vasumini, N.; Amicone, S.; Canton, L.; Di Leo, M.; Basile, M.; Manaresi, T.; Angeli, F.; et al. Prevention of atrial fibrillation with SGLT2 inhibitors across the spectrum of cardiovascular disorders: A meta-analysis of randomized controlled trials. Eur. Heart J. Cardiovasc. Pharmacother. 2025, pvaf040. [Google Scholar] [CrossRef]




| Trial | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Outcome Measurement | Selection of Reported Result |
|---|---|---|---|---|---|
| SOLOIST-WHF [7] | Low risk | Low risk | Low risk | Low risk | Low risk |
| EMPEROR- REDUCED [8] | Low risk | Low risk | Low risk | Low risk | Low risk |
| DAPA-HF [9] | Low risk | Low risk | Low risk | Low risk | Low risk |
| EMPEROR- PRESERVED [10] | Low risk | Low risk | Low risk | Low risk | Low risk |
| DELIVER [11] | Low risk | Low risk | Low risk | Low risk | Low risk |
| DEFINE-HF [12] | Some concerns | Low risk | Low risk | Low risk | Low risk |
| CHIEF-HF [13] | Low risk | Low risk | Low risk | Low risk | Low risk |
| PRESERVED-HF [14] | Low risk | Low risk | Low risk | Low risk | Low risk |
| EMPERIAL- PRESERVED [15] | Low risk | Low risk | Low risk | Low risk | Low risk |
| EMPERIAL-REDUCED [16] | Low risk | Low risk | Low risk | Low risk | Low risk |
| EMBRACE-HF [17] | Some concerns | Low risk | Low risk | Low risk | Low risk |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| (a) Human Studies | (a) Animal Studies |
| (b) From 2014–2024 | (b) >10 years old |
| (c) English Text | (c) Non-English Texts |
| (d) Randomized control trials | (d) Case-control, case report, cohort case series, systematic reviews, literature reviews |
| (e) Patients with heart failure without acute decompensation | (e) Studies involving clinical data other than cardiovascular diseases |
| (f) Patients without heart failure/acute decompensated heart failure |
| Trial | Group | Number | Age (Mean ± SD) | Males | LVEF | Beta Blocker | Type of Heart Failure | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| SOLOIST-WHF [7] | Sotagliflozin | 608 | 68.6 ± 9.5 | 410 | 35 ± 14.07 | 564 (92.8%) | HFrEF and HFpEF | 2 years |
| Placebo | 614 | 69.3 ± 8.8 | 400 | 35 ± 12.6 | 561 (91.4%) | |||
| EMPEROR-REDUCED [8] | Empagliflozin | 1863 | 67.2 ± 10.8 | 1426 | 27.7 ± 6.0 | 1765 (94.7%) | HFrEF | 2.85 years |
| Placebo | 1867 | 66.5 ± 11.2 | 1411 | 27.2 ± 6.1 | 1768 (94.7%) | |||
| DAPA-HF [9] | Dapagliflozin | 2373 | 66.2 ± 11 | 564 | 31.2 ± 6.7 | 2278 (96%) | HFrEF | 2.317 years |
| Placebo | 2371 | 66.5 ± 10.8 | 545 | 30.9 ± 6.9 | 2280 (96.2%) | |||
| EMPEROR-PRESERVED [10] | Empagliflozin | 2997 | 71.8 ± 9.3 | 1659 | 54.3 ± 8.8 | - | HFpEF | 2.858 years |
| Placebo | 2991 | 71.9 ± 9.6 | 1653 | 54.3 ± 8.8 | - | |||
| DELIVER [11] | Dapagliflozin | 3131 | 71.8 ± 9.6 | 1767 | 54.0± 8.6 | - | HFpEF and mild reduced | 3.508 years |
| Placebo | 3132 | 71.5 ± 9.5 | 1749 | 54.3 ± 8.9 | - | |||
| DEFINE-HF [12] | Dapaglifozin | 131 | 62.2 ± 11 | 95 | 27.2 ±8.0% | 130 (99.2%) | HFrEF | 12 weeks |
| Placebo | 132 | 60.4 ± 12 | 98 | 25.7 ±8.2% | 124 (93.9%) | |||
| CHIEF-HF [13] | Canagliflozin | 238 | 62.9 ± 13.15 | 119 | - | - | HFrEF and HFpEF | 12 weeks |
| Placebo | 238 | 63.8 ± 13.5 | 132 | - | - | |||
| PRESERVED-HF [14] | Dapagliflozin | 162 | 69 ± 5 | 70 | 60 ± 5 | - | HFpEF | 12 weeks |
| Placebo | 162 | 71 ± 5 | 70 | 60 ± 5 | - | |||
| EMPERIAL-PRESERVED [15] | Empagliflozin | 157 | 73 ± 9 | 87 | - | 140 (89.2%) | HFpEF | 12 weeks |
| Placebo | 158 | 73.9 ± 8.6 | 92 | - | 141 (89.2%) | |||
| EMPERIAL- REDUCED [16] | Empagliflozin | 155 | 68.7 ± 9.9 | 121 | - | 148 (94.9%) | HFrEF | 12 weeks |
| Placebo | 156 | 69.3 ± 10.6 | 111 | - | 147 (94.2%) | |||
| EMBRACE-HF [17] | Empagliflozin | 33 | 69.5 ± 12.0 | 21 | 46.7 ± 14.9 | 29 (87.9%) | HFrEF and HFpEF | 12 weeks |
| Placebo | 32 | 62.9 ± 13.3 | 20 | 40.7± 17.2 | 29 (90.6%) |
| Drug | Outcome | Relative Risk (RR) | 95% CI | p-Value | Clinical Implication |
|---|---|---|---|---|---|
| Dapagliflozin | Sudden Cardiac Death | 0.68 | 0.47–0.99 | 0.047 | Reduced risk |
| Dapagliflozin | Atrial Flutter | 3.03 | 1.18–7.75 | 0.02 | Increased risk |
| Empagliflozin | Bradyarrhythmia and Conduction Disorders | 1.68 | 1.02–2.79 | 0.04 | Increased risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suresh, S.B.; Prasad, A.; Ubaid, M.F.; Farooq, S.; Hajra, A.; Jaiswal, V.; Malik, A.; Fonarow, G.C.; Bandyopadhyay, D. SGLT2 Inhibitors and the Risk of Arrhythmias in Heart Failure: A Network Meta-Analysis. J. Clin. Med. 2025, 14, 5306. https://doi.org/10.3390/jcm14155306
Suresh SB, Prasad A, Ubaid MF, Farooq S, Hajra A, Jaiswal V, Malik A, Fonarow GC, Bandyopadhyay D. SGLT2 Inhibitors and the Risk of Arrhythmias in Heart Failure: A Network Meta-Analysis. Journal of Clinical Medicine. 2025; 14(15):5306. https://doi.org/10.3390/jcm14155306
Chicago/Turabian StyleSuresh, Suchith Boodgere, Aishwarya Prasad, Muhammad Furqan Ubaid, Saad Farooq, Adrija Hajra, Vikash Jaiswal, Aaqib Malik, Gregg C. Fonarow, and Dhrubajyoti Bandyopadhyay. 2025. "SGLT2 Inhibitors and the Risk of Arrhythmias in Heart Failure: A Network Meta-Analysis" Journal of Clinical Medicine 14, no. 15: 5306. https://doi.org/10.3390/jcm14155306
APA StyleSuresh, S. B., Prasad, A., Ubaid, M. F., Farooq, S., Hajra, A., Jaiswal, V., Malik, A., Fonarow, G. C., & Bandyopadhyay, D. (2025). SGLT2 Inhibitors and the Risk of Arrhythmias in Heart Failure: A Network Meta-Analysis. Journal of Clinical Medicine, 14(15), 5306. https://doi.org/10.3390/jcm14155306







