A User-Friendly Software for Automated Knowledge-Based Virtual Surgical Planning in Mandibular Reconstruction
Abstract
1. Introduction
1.1. Related Works
1.2. Contributions
- -
- Description of an automated VSP workflow with standardized planning activities in tumor-related mandibular reconstruction.
- -
- Introduction of an easy-to-use self-developed software that permits surgeons to plan mandibular reconstructions via free fibula flaps swiftly and without relying on external planning services.
- -
- Automated planning assistance for knowledge-based calculation of reconstruction proposals, which can be easily parameterized via the user interface.
- -
- Evaluation of 21 heterogeneous clinical cases to assess the usability and to demonstrate the feasibility of automated planning with high quality using our software.
2. Materials and Methods
2.1. Preliminary Work
2.2. Software for Knowledge-Based Planning
2.2.1. Registration of a Mandibular Reference
2.2.2. Tumor Resection Planning
2.2.3. Configuration and Automatic Calculation of Reconstruction Proposals
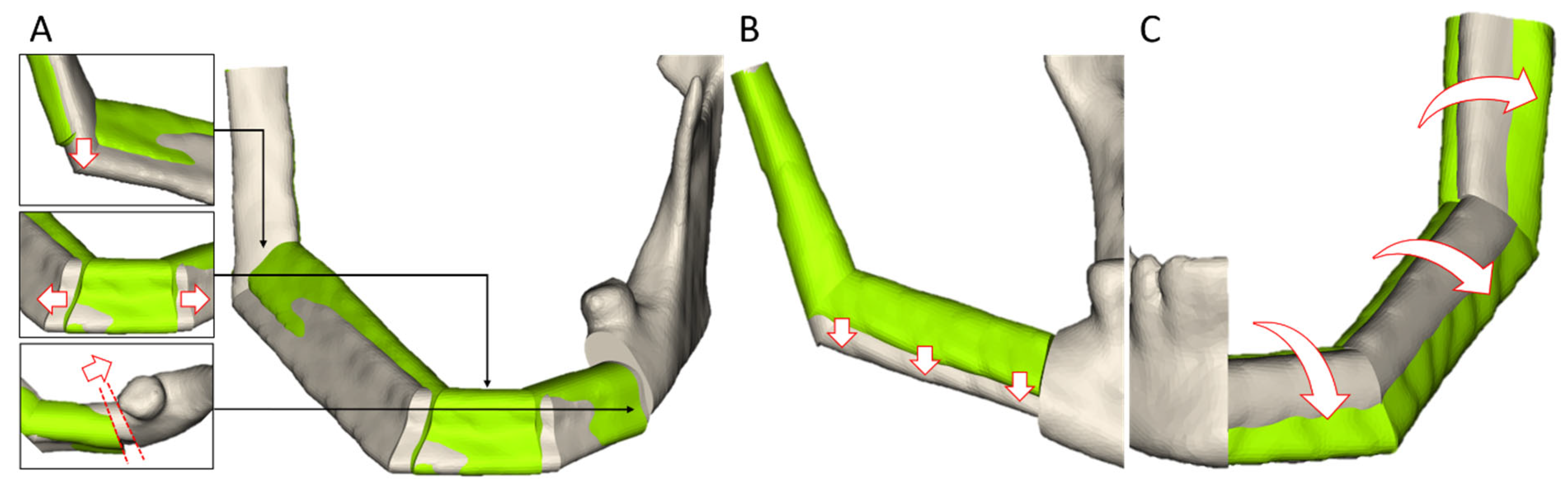
2.2.4. Appraisal and Manual Refinement of the Proposals
- -
- The segment edges can be interactively manipulated in the 2D and 3D views of the head-and-neck region. At the junction with the residual mandible, only a displacement on the planned cut edge is permitted to ensure a seamless connection.
- -
- To adjust the transplant height, the segments in the tooth-bearing part of the mandibular body can be moved by a slider on the UI.
- -
- The transplant can be rotated around the mid-axis via a slider to adjust its lateral surface.
- -
- As with tumor resection planning, the cut planes can be re-positioned and re-oriented afterwards.
2.3. Data Selection
2.4. Pre-Processing
2.5. Virtual Surgical Planning of Retrospective Clinical Cases
- -
- Complexity: I consider the complexity of planning this case to be high.
- -
- Proposal: I consider the proposed reconstruction to be suitable.
- -
- Final Surgical Plan: I consider that the mandibular reconstruction can be planned as desired (if necessary, after manual refinement of the proposal).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VSP | virtual surgical planning |
| PSI | patient-specific implants |
| FEA | finite element analysis |
| AI | artificial intelligence |
| DL | deep learning |
| CNN | convolutional neural network |
| CAD | computer-aided design |
| CAM | computer-aided manufacturing |
| MITK | Medical Imaging Interaction Toolkit |
| CT | computer tomography |
| DVT | digital volume tomography |
| UI | user interface |
| PACS | Picture Archiving and Communication System |
| RAM | Random-Access Memory |
| GB | Gigabyte |
| GPU | graphics processing unit |
| SUS | System Usability Scale |
References
- Zavattero, E.; Bolzoni, A.; Dell’Aversana, G.; Santagata, M.; Massarelli, O.; Ferri, A.; Della Monaca, M.; Copelli, C.; Gessaroli, M.; Valsecchi, S.; et al. Accuracy of Fibula Reconstruction Using Patient-Specific Cad/Cam Plates: A Multicenter Study on 47 Patients. Laryngoscope 2021, 131, E2169–E2175. [Google Scholar] [CrossRef]
- Cornelius, C.-P.; Smolka, W.; Giessler, G.A.; Wilde, F.; Probst, F.A. Patient-specific reconstruction plates are the missing link in computer-assisted mandibular reconstruction: A showcase for technical description. J. Craniomaxillofac. Surg. 2015, 43, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Seier, T.; Hingsammer, L.; Schumann, P.; Gander, T.; Rücker, M.; Lanzer, M. Virtual planning, simultaneous dental implantation and CAD/CAM plate fixation: A paradigm change in maxillofacial reconstruction. Int. J. Oral Maxillofac. Surg. 2020, 49, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Succo, G.; Berrone, M.; Battiston, B.; Tos, P.; Goia, F.; Appendino, P.; Crosetti, E. Step-by-step surgical technique for mandibular reconstruction with fibular free flap: Application of digital technology in virtual surgical planning. Eur. Arch. Otorhinolaryngol. 2015, 272, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Tarsitano, A.; Battaglia, S.; Crimi, S.; Ciocca, L.; Scotti, R.; Marchetti, C. Is a computer-assisted design and computer-assisted manufacturing method for mandibular reconstruction economically viable? J. Craniomaxillofac. Surg. 2016, 44, 795–799. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, H.Q.; Fan, S.; Zhang, D.M.; Huang, Z.Q.; Chen, W.L.; Ye, J.T.; Li, J.S. Mandibular reconstruction with the vascularized fibula flap: Comparison of virtual planning surgery and conventional surgery. Int. J. Oral Maxillofac. Surg. 2016, 45, 1400–1405. [Google Scholar] [CrossRef]
- Yang, W.-F.; Zhang, C.-Y.; Choi, W.S.; Zhu, W.-Y.; Li, D.T.S.; Chen, X.-S.; Du, R.; Su, Y.-X. A novel ‘surgeon-dominated’ approach to the design of 3D-printed patient-specific surgical plates in mandibular reconstruction: A proof-of-concept study. Int. J. Oral Maxillofac. Surg. 2020, 49, 13–21. [Google Scholar] [CrossRef]
- Probst, F.A.; Liokatis, P.; Mast, G.; Ehrenfeld, M. Virtual planning for mandible resection and reconstruction. Innov. Surg. Sci. 2023, 8, 137–148. [Google Scholar] [CrossRef]
- Foley, B.D.; Thayer, W.P.; Honeybrook, A.; McKenna, S.; Press, S. Mandibular reconstruction using computer-aided design and computer-aided manufacturing: An analysis of surgical results. J. Oral Maxillofac. Surg. 2013, 71, e111–e119. [Google Scholar] [CrossRef]
- Levine, J.P.; Bae, J.S.; Soares, M.; Brecht, L.E.; Saadeh, P.B.; Ceradini, D.J.; Hirsch, D.L. Jaw in a day: Total maxillofacial reconstruction using digital technology. Plast. Reconstr. Surg. 2013, 131, 1386–1391. [Google Scholar] [CrossRef]
- Mascha, F.; Winter, K.; Pietzka, S.; Heufelder, M.; Schramm, A.; Wilde, F. Accuracy of computer-assisted mandibular reconstructions using patient-specific implants in combination with CAD/CAM fabricated transfer keys. J. Craniomaxillofac. Surg. 2017, 45, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, R.L.; Mutsvangwa, T.E.M.; van der Merwe, J. Reconstruction of the mandible from partial inputs for virtual surgery planning. Med. Eng. Phys. 2023, 111, 103934. [Google Scholar] [CrossRef]
- Kraeima, J.; Glas, H.H.; Merema, B.B.J.; Vissink, A.; Spijkervet, F.K.L.; Witjes, M.J.H. Three-dimensional virtual surgical planning in the oncologic treatment of the mandible. Oral Dis. 2021, 27, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Moe, J.; Foss, J.; Herster, R.; Powell, C.; Helman, J.; Ward, B.B.; VanKoevering, K. An In-House Computer-Aided Design and Computer-Aided Manufacturing Workflow for Maxillofacial Free Flap Reconstruction is Associated With a Low Cost and High Accuracy. J. Oral Maxillofac. Surg. 2021, 79, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Villarmé, A.; Pace-Loscos, T.; Schiappa, R.; Poissonnet, G.; Dassonville, O.; Chamorey, E.; Bozec, A.; Culié, D. Impact of virtual surgical planning and three-dimensional modeling on time to surgery in mandibular reconstruction by free fibula flap. Eur. J. Surg. Oncol. 2024, 50, 108008. [Google Scholar] [CrossRef]
- Fatima, A.; Hackman, T.G.; Wood, J.S. Cost-Effectiveness Analysis of Virtual Surgical Planning in Mandibular Reconstruction. Plast. Reconstr. Surg. 2019, 143, 1185–1194. [Google Scholar] [CrossRef]
- Zhou, K.X.; Patel, M.; Shimizu, M.; Wang, E.; Prisman, E.; Thang, T. Development and validation of a novel craniofacial statistical shape model for the virtual reconstruction of bilateral maxillary defects. Int. J. Oral Maxillofac. Surg. 2024, 53, 146–155. [Google Scholar] [CrossRef]
- Klop, C.; Schreurs, R.; Jong GAde Klinkenberg, E.T.; Vespasiano, V.; Rood, N.L.; Niehe, V.G.; Soerdjbalie-Maikoe, V.; van Goethem, A.; Bakker BSde Maal, T.J.; Nolte, J.W.; et al. An open-source, three-dimensional growth model of the mandible. Comput. Biol. Med. 2024, 175, 108455. [Google Scholar] [CrossRef]
- Han, B.; Jie, B.; Zhou, L.; Huang, T.; Li, R.; Ma, L.; Zhang, X.; Zhang, Y.; He, Y.; Liao, H. Statistical and individual characteristics-based reconstruction for craniomaxillofacial surgery. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1155–1165. [Google Scholar] [CrossRef]
- Aftabi, H.; Zaraska, K.; Eghbal, A.; McGregor, S.; Prisman, E.; Hodgson, A.; Fels, S. Computational models and their applications in biomechanical analysis of mandibular reconstruction surgery. Comput. Biol. Med. 2024, 169, 107887. [Google Scholar] [CrossRef]
- Dogan, S.E.; Ozturk, C.; Koc, B. Design of patient-specific mandibular reconstruction plates and a hybrid scaffold. Comput. Biol. Med. 2025, 184, 109380. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tang, Y.; Li, J.; Shen, L.; Tian, W.; Tang, W. Establishment of sequential software processing for a biomechanical model of mandibular reconstruction with custom-made plate. Comput. Methods Programs Biomed. 2013, 111, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Aftabi, H.; Sagl, B.; Lloyd, J.E.; Prisman, E.; Hodgson, A.; Fels, S. To what extent can mastication functionality be restored following mandibular reconstruction surgery? A computer modeling approach. Comput. Methods Programs Biomed. 2024, 250, 108174. [Google Scholar] [CrossRef] [PubMed]
- Pankert, T.; Lee, H.; Peters, F.; Hölzle, F.; Modabber, A.; Raith, S. Mandible segmentation from CT data for virtual surgical planning using an augmented two-stepped convolutional neural network. Int. J. Comput. Assist. Radiol. Surg. 2023, 18, 1479–1488. [Google Scholar] [CrossRef]
- Raith, S.; Pankert, T.; de Souza Nascimento, J.; Jaganathan, S.; Peters, F.; Wien, M.; Hölzle, F.; Modabber, A. Segmentation of the iliac crest from CT-data for virtual surgical planning of facial reconstruction surgery using deep learning. Sci. Rep. 2025, 15, 1097. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Zhang, D.; Zhou, Z.; Zhang, C.; Chen, X. A 3D segmentation network of mandible from CT scan with combination of multiple convolutional modules and edge supervision in mandibular reconstruction. Comput. Biol. Med. 2021, 138, 104925. [Google Scholar] [CrossRef]
- Yang, S.; Yoo, J.-Y.; Lee, S.-J.; Kang, S.-R.; Kim, J.-M.; Kim, J.-E.; Huh, K.-H.; Lee, S.-S.; Heo, M.-S.; Yang, H.J.; et al. MAFNet: A deep multi-scale attentive fusion network for virtual osteotomy of maxillofacial bones in CT images containing metal artifacts. Biomed. Signal Process. Control 2024, 95, 106411. [Google Scholar] [CrossRef]
- Xu, J.; Wei, Y.; Jiang, S.; Zhou, H.; Li, Y.; Chen, X. Intelligent surgical planning for automatic reconstruction of orbital blowout fracture using a prior adversarial generative network. Med. Image Anal. 2025, 99, 103332. [Google Scholar] [CrossRef]
- Wodzinski, M.; Daniol, M.; Socha, M.; Hemmerling, D.; Stanuch, M.; Skalski, A. Deep learning-based framework for automatic cranial defect reconstruction and implant modeling. Comput. Methods Programs Biomed. 2022, 226, 107173. [Google Scholar] [CrossRef]
- Kim, I.-H.; Kim, J.-S.; Jeong, J.; Park, J.-W.; Park, K.; Cho, J.-H.; Hong, M.; Kang, K.-H.; Kim, M.; Kim, S.-J.; et al. Orthognathic surgical planning using graph CNN with dual embedding module: External validations with multi-hospital datasets. Comput. Methods Programs Biomed. 2023, 242, 107853. [Google Scholar] [CrossRef]
- Xiao, D.; Lian, C.; Deng, H.; Kuang, T.; Liu, Q.; Ma, L.; Kim, D.; Lang, Y.; Chen, X.; Gateno, J.; et al. Estimating Reference Bony Shape Models for Orthognathic Surgical Planning Using 3D Point-Cloud Deep Learning. IEEE J. Biomed. Health Inform. 2021, 25, 2958–2966. [Google Scholar] [CrossRef] [PubMed]
- Marin-Montealegre, V.; Cardinali, A.R.; Ríos Borras, V.; Ceballos-Santa, M.C.; Osorio-Orozco, J.J.; Rivero, I.V. 3D surgical planning method for lower jaw osteotomies applied to facial feminization surgery. Ann. 3D Print. Med. 2024, 15, 100164. [Google Scholar] [CrossRef]
- Liang, Y.; Huan, J.; Li, J.-D.; Jiang, C.; Fang, C.; Liu, Y. Use of artificial intelligence to recover mandibular morphology after disease. Sci. Rep. 2020, 10, 16431. [Google Scholar] [CrossRef] [PubMed]
- Volpe, Y.; Furferi, R.; Governi, L.; Uccheddu, F.; Carfagni, M.; Mussa, F.; Scagnet, M.; Genitori, L. Surgery of complex craniofacial defects: A single-step AM-based methodology. Comput. Methods Programs Biomed. 2018, 165, 225–233. [Google Scholar] [CrossRef]
- Block, O.M.; Khromov, T.; Hoene, G.; Schliephake, H.; Brockmeyer, P. In-house virtual surgical planning and guided mandibular reconstruction is less precise, but more economical and time-efficient than commercial procedures. Head Neck 2024, 46, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Lentge, F.; Jehn, P.; Neuhaus, M.-T.; Bettag, S.A.; Gellrich, N.-C.; Korn, P. A Novel Method for Secondary Mandible Reconstruction to Re-Achieve a Native Condyle Position Comprising a New Design for Cutting Guides and New Positioning Devices. J. Pers. Med. 2024, 14, 181. [Google Scholar] [CrossRef]
- Ritschl, L.M.; Kilbertus, P.; Grill, F.D.; Schwarz, M.; Weitz, J.; Nieberler, M.; Wolff, K.-D.; Fichter, A.M. In-House, Open-Source 3D-Software-Based, CAD/CAM-Planned Mandibular Reconstructions in 20 Consecutive Free Fibula Flap Cases: An Explorative Cross-Sectional Study With Three-Dimensional Performance Analysis. Front. Oncol. 2021, 11, 731336. [Google Scholar] [CrossRef]
- Vollmer, A.; Saravi, B.; Breitenbuecher, N.; Mueller-Richter, U.; Straub, A.; Šimić, L.; Kübler, A.; Vollmer, M.; Gubik, S.; Volland, J.; et al. Realizing in-house algorithm-driven free fibula flap set up within 24 hours: A pilot study evaluating accuracy with open-source tools. Front. Surg. 2023, 10, 1321217. [Google Scholar] [CrossRef]
- Maisi, S.; Dominguez, M.; Gilong, P.C.; Kiong, C.T.; Hajam, S.; Badruddin, A.F.A.; Siew, H.F.; Gopalan, S.; Choon, K.T. In-house virtual surgical planning for mandibular reconstruction with fibula free flap: Case series and literature review. Ann. 3D Print. Med. 2023, 10, 100109. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, W.; Tu, P.; Han, J.; Zhang, C.; Liu, J.; Chen, X. Design and implementation of a surgical planning system for robotic assisted mandible reconstruction with fibula free flap. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 2291–2303. [Google Scholar] [CrossRef]
- Nakao, M.; Aso, S.; Imai, Y.; Ueda, N.; Hatanaka, T.; Shiba, M.; Kirita, T.; Matsuda, T. Automated Planning With Multivariate Shape Descriptors for Fibular Transfer in Mandibular Reconstruction. IEEE Trans. Biomed. Eng. 2017, 64, 1772–1785. [Google Scholar] [CrossRef]
- Raith, S.; Rauen, A.; Möhlhenrich, S.C.; Ayoub, N.; Peters, F.; Steiner, T.; Hölzle, F.; Modabber, A. Introduction of an algorithm for planning of autologous fibular transfer in mandibular reconstruction based on individual bone curvatures. Int. J. Med. Robot. 2018, 14, e1894. [Google Scholar] [CrossRef]
- Wang, E.; Durham, J.S.; Anderson, D.W.; Prisman, E. Clinical evaluation of an automated virtual surgical planning platform for mandibular reconstruction. Head Neck 2020, 42, 3506–3514. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.; Weichel, F.; Kühle, R.; Knaup, P.; Freudlsperger, C.; Eisenmann, U. Automated calculation of ontology-based planning proposals: An application in reconstructive oral and maxillofacial surgery. Int. J. Med. Robot. 2023, 19, e2545. [Google Scholar] [CrossRef]
- Jewer, D.D.; Boyd, J.B.; Manktelow, R.T.; Zuker, R.M.; Rosen, I.B.; Gullane, P.J.; Rotstein, L.E.; Freeman, J.E. Orofacial and mandibular reconstruction with the iliac crest free flap: A review of 60 cases and a new method of classification. Plast. Reconstr. Surg. 1989, 84, 391–403; discussion 404–405. [Google Scholar] [CrossRef]
- German Cancer Research Center (DKFZ). The Medical Imaging Interaction Toolkit (MITK). 2023. Available online: https://www.mitk.org/w/index.php?title=The_Medical_Imaging_Interaction_Toolkit_(MITK)&oldid=3687 (accessed on 20 January 2025).
- Kochanek, D.H.U.; Bartels, R.H. Interpolating splines with local tension, continuity, and bias control. In Proceedings of the 11th Annual Conference on Computer Graphics and Interactive Techniques, Minneapolis, MN, USA, 23-27 July 1984; Association for Computing Machinery: New York, NY, USA, 1984; pp. 33–41. [Google Scholar]
- Brooke, J. SUS: A ‘Quick and Dirty’ Usability Scale. In Usability Evaluation in Industry, 1st ed.; Jordan, P.W., Thomas, B., Weerdmeester, B.A., McClelland, A.L., Eds.; Taylor and Francis: Bristol, PA, USA; London, UK, 1996; pp. 189–195. [Google Scholar]
- German Cancer Research Center (DKFZ). Developer Manual. 2025. Available online: https://docs.mitk.org/nightly/DeveloperManualPortal.html (accessed on 20 January 2025).
- Wu, X.; Xiao, L.; Sun, Y.; Zhang, J.; Ma, T.; He, L. A survey of human-in-the-loop for machine learning. Future Gener. Comput. Syst. 2022, 135, 364–381. [Google Scholar] [CrossRef]
- Bähr, W. Blood supply of small fibula segments: An experimental study on human cadavers. J. Craniomaxillofac. Surg. 1998, 26, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Meade, M.J.; Ng, E.; Weir, T. Digital treatment planning and clear aligner therapy: A retrospective cohort study. J. Orthod. 2023, 50, 361–366. [Google Scholar] [CrossRef]
- Olsson, P.; Nysjö, F.; Rodríguez-Lorenzo, A.; Thor, A.; Hirsch, J.-M.; Carlbom, I.B. Haptics-assisted Virtual Planning of Bone, Soft Tissue, and Vessels in Fibula Osteocutaneous Free Flaps. Plast. Reconstr. Surg. Glob. Open 2015, 3, e479. [Google Scholar] [CrossRef]
- Nakao, M.; Matsuda, T. Sparse Modeling of Mandibular Reconstruction Procedures Using Statistical Geometric Features. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2018, 2018, 3248–3251. [Google Scholar] [CrossRef]
- Liang, Y.; Jiang, C.; Wu, L.; Wang, W.; Liu, Y.; Jian, X. Application of Combined Osteotomy and Reconstruction Pre-Bent Plate Position (CORPPP) Technology to Assist in the Precise Reconstruction of Segmental Mandibular Defects. J. Oral Maxillofac. Surg. 2017, 75, 2026.e1–2026.e10. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; He, J.; Yu, C.; Zhao, W.; Lin, Y.; Wang, H.; Liu, J.; Zhu, H. Utilization of a pre-bent plate-positioning surgical guide system in precise mandibular reconstruction with a free fibula flap. Oral Oncol. 2017, 75, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Miljanovic, D.; Seyedmahmoudian, M.; Horan, B.; Stojcevski, A. Novel and accurate 3D-Printed surgical guide for mandibular reconstruction with integrated dental implants. Comput. Biol. Med. 2022, 151, 106327. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.; Shim, E.; Hwang, B.-Y.; Kim, Y.; Lee, J.-W.; Seo, H. Deep Learning-Based Automatic Segmentation of Mandible and Maxilla in Multi-Center CT Images. Appl. Sci. 2022, 12, 1358. [Google Scholar] [CrossRef]
- Torosdagli, N.; Liberton, D.K.; Verma, P.; Sincan, M.; Lee, J.S.; Bagci, U. Deep Geodesic Learning for Segmentation and Anatomical Landmarking. IEEE Trans. Med. Imaging 2019, 38, 919–931. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeon, K.; Kang, S.-H.; Lee, S.-H. 3D cephalometric landmark detection by multiple stage deep reinforcement learning. Sci. Rep. 2021, 11, 17509. [Google Scholar] [CrossRef]



| HCL Class | Centered Defects | Left-Sided Defect | Right-Sided Defect | Overall |
|---|---|---|---|---|
| H | - | 6 | 4 | 10 |
| HCL | - | 0 | 1 | 1 |
| L | - | 2 | 2 | 4 |
| LC | - | 2 | 2 | 4 |
| LCL | 2 | - | - | 2 |
| Overall | 2 | 10 | 9 | 21 |
| Planning Step | Medical Informatician | Surgeon 1 | Surgeon 2 | Surgeon 3 | Overall |
|---|---|---|---|---|---|
| Registration mandibular reference | 5.4 ± 1.4 | - | - | - | - |
| Load and view registration | - | 1.3 ± 0.4 | 1.2 ± 0.5 | 2.4 ± 1.4 | 1.5 ± 1.2 |
| Tumor resection planning | - | 2.4 ± 1.6 | 1.6 ± 0.3 | 5.0 ± 1.4 | 3.2 ± 1.6 |
| Configuration | - | 0.5 ± 0.4 | 0.5 ± 0.3 | 1.4 ± 2.1 | 1.1 ± 1.2 |
| Calculation of reconstruction proposals | - | 0.2 ± 0.2 | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.1 |
| Appraisal and refinement of the proposals | - | 5.3 ± 3.4 | 4.5 ± 1.6 | 6.3 ± 2.1 | 5.4 ± 2.4 |
| Overall | - | 10.4 ± 5.2 | 9.2 ± 2.4 | 16.1 ± 6.1 | 12.0 ± 5.3 |
| Manual Refinement/Adaption of … | Description/Feedback of the Surgeons |
| Tumor Resection (n = 3) | Reassessment of the tumor resection after appraisal of the reconstruction proposals. |
| Transplant Level (n = 3) | Reassessment of the configured transplant level after appraisal of the reconstruction proposals. |
| Reconstructed Jaw Angle (n = 5) | Regarding the postoperative restoration with dental implants, it is necessary to correct the proposed transplant in the jaw angle (towards the mandible base) to ensure parallel positioning to the occlusal plane. |
| Short Segments (n = 2) | Correction of short transplant segments in the anterior region of the mandibular body to prevent necrosis. |
| Jaw Relation (n = 1) | Correction of the proposed graft in the anterior part of the mandible, which exhibits an asymmetrical alignment with the maxilla. |
| Reconstructed Jaw Joint (n = 1) | To prevent the jaw joint and the fossa mandibularis from tilting, it is necessary to refine the proposed transplant due to an overlap with the skull bone. |
| Orientation (n = 4) | Refinement of the orientation (rotation) for a smooth transition between the transplant and the residual mandible, or to ensure a natural bone surface. |
| Mono segment reconstruction (n = 2) | For short mandibular defects, a mono-segment reconstruction variant should be offered by default, even if the defect extends over prominent structures like the jaw angle. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagen, N.; Freudlsperger, C.; Kühle, R.P.; Bouffleur, F.; Knaup, P.; Hoffmann, J.; Eisenmann, U. A User-Friendly Software for Automated Knowledge-Based Virtual Surgical Planning in Mandibular Reconstruction. J. Clin. Med. 2025, 14, 4508. https://doi.org/10.3390/jcm14134508
Hagen N, Freudlsperger C, Kühle RP, Bouffleur F, Knaup P, Hoffmann J, Eisenmann U. A User-Friendly Software for Automated Knowledge-Based Virtual Surgical Planning in Mandibular Reconstruction. Journal of Clinical Medicine. 2025; 14(13):4508. https://doi.org/10.3390/jcm14134508
Chicago/Turabian StyleHagen, Niclas, Christian Freudlsperger, Reinald Peter Kühle, Frederic Bouffleur, Petra Knaup, Jürgen Hoffmann, and Urs Eisenmann. 2025. "A User-Friendly Software for Automated Knowledge-Based Virtual Surgical Planning in Mandibular Reconstruction" Journal of Clinical Medicine 14, no. 13: 4508. https://doi.org/10.3390/jcm14134508
APA StyleHagen, N., Freudlsperger, C., Kühle, R. P., Bouffleur, F., Knaup, P., Hoffmann, J., & Eisenmann, U. (2025). A User-Friendly Software for Automated Knowledge-Based Virtual Surgical Planning in Mandibular Reconstruction. Journal of Clinical Medicine, 14(13), 4508. https://doi.org/10.3390/jcm14134508







