Comparative Analysis of Orbital Morphology Accuracy in 3D Models Based on Cone-Beam and Fan-Beam Computed Tomography Scans for Reconstructive Planning
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martins, C.; Costa e Silva, I.E.; Campero, A.; Yasuda, A.; Aguiar, L.R.; Tatagiba, M.; Rhoton, A. Microsurgical Anatomy of the Orbit: The Rule of Seven. Anat. Res. Int. 2011, 2011, 468727. [Google Scholar] [CrossRef]
- Susarla, S.M.; Duncan, K.; Mahoney, N.R.; Merbs, S.L.; Grant, M.P. Virtual Surgical Planning for Orbital Reconstruction. Middle East Afr. J. Ophthalmol. 2015, 22, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Bielecki-kowalski, B.; Kozakiewicz, M. Assessment of Differences in the Dimensions of Mandible Condyle Models in Fan- versus Cone-beam Computer Tomography Acquisition. Materials 2021, 14, 1388. [Google Scholar] [CrossRef] [PubMed]
- Shumway, C.L.; Motlagh, M.; Wade, M. Anatomy, Head and Neck, Orbit Bones; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Cheon, J.S.; Seo, B.N.; Yang, J.Y.; Son, K.M. Retrobulbar Hematoma in Blow-out Fracture after Open Reduction. Arch. Plast. Surg. 2013, 40, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Bielecki-Kowalski, B.; Bielecka-Kowalska, N.; Jaxa-Kwiatkowski, M.; Osmola, K.; Kozakiewicz, M. Orbital Hematoma Treatment—A Retrospective Study. J. Clin. Med. 2024, 13, 5788. [Google Scholar] [CrossRef]
- Hwang, K.; Suh, M.S.; Lee, I.; Chung, I.H. Anatomical Studies Zygomaticotemporal Nerve Passage in the Orbit and Temporal Area. J. Craniofacial Surg. 2004, 15, 209–214. [Google Scholar] [CrossRef]
- Loukas, M.; Owens, D.G.; Tubbs, R.S.; Spentzouris, G.; Elochukwu, A.; Jordan, R. Zygomaticofacial, Zygomaticoorbital and Zygomaticotemporal Foramina: Anatomical Study. Anat. Sci. Int. 2008, 83, 77–82. [Google Scholar] [CrossRef]
- Dubron, K.; Verbist, M.; Shaheen, E.; Dormaar, T.J.; Jacobs, R.; Politis, C. Incidence, Aetiology, and Associated Fracture Patterns of Infraorbital Nerve Injuries Following Zygomaticomaxillary Complex Fractures: A Retrospective Analysis of 272 Patients. Craniomaxillofacial Trauma Reconstr. 2022, 15, 139–146. [Google Scholar] [CrossRef]
- Kumar, P.; Godhi, S.; Lall, A.B.; Ram, C.S. Evaluation of Neurosensory Changes in the Infraorbital Nerve Following Zygomatic Fractures. J. Maxillofac. Oral. Surg. 2012, 11, 394–399. [Google Scholar] [CrossRef]
- Shafiq, M.; Khan, T.U.; Qayyum, Z.; Masood, M.; Masooma, S. Frequency of Infraorbital Nerve Injury Associated with Zygomaticomaxillary Complex Fractures in Patients Reporting to Lady Reading Hospital Peshawar. J. Saidu Med. Coll. 2024, 14, 405–410. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Survival and Complications of Zygomatic Implants: An Updated Systematic Review. J. Oral Maxillofac. Surg. 2016, 74, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Newhauser, W.; Jones, T.; Swerdloff, S.; Newhauser, W.; Cilia, M.; Carver, R.; Halloran, A.; Zhang, R. Anonymization of DICOM Electronic Medical Records for Radiation Therapy. Comput. Biol. Med. 2014, 53, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Baillard, C.; Barillot, C. Robust 3D Segmentation of Anatomical Structures with Level Sets. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2000, Proceedings of the 3rd International Conference, Pittsburgh, PA, USA, 11–14 October 2000; Delp, S.L., DiGoia, A.M., Jaramaz, B., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Nilsson, J.; Hindocha, N.; Thor, A. Time Matters—Differences between Computer-Assisted Surgery and Conventional Planning in Cranio-Maxillofacial Surgery: A Systematic Review and Meta-Analysis. J. Cranio Maxillofac. Surg. 2020, 48, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.; Sinko, K.; Dorner, G. Late Reconstruction of the Orbit With Patient-Specific Implants Using Computer-Aided Planning and Navigation. J. Oral Maxillofac. Surg. 2015, 73, S101–S106. [Google Scholar] [CrossRef]
- Dubois, L.; Schreurs, R.; Jansen, J.; Maal, T.J.J.; Essig, H.; Gooris, P.J.J.; Becking, A.G. Predictability in Orbital Reconstruction: A Human Cadaver Study. Part II: Navigation-Assisted Orbital Reconstruction. J. Cranio Maxillofac. Surg. 2015, 43, 2042–2049. [Google Scholar] [CrossRef]
- Chepurnyi, Y.; Chernogorskyi, D.; Kopchak, A.; Petrenko, O. Clinical Efficacy of Peek Patient-Specific Implants in Orbital Reconstruction. J. Oral Biol. Craniofacial Res. 2020, 10, 49–53. [Google Scholar] [CrossRef]
- Cheraghi, F.; Shokri, A.; Tapak, L.; Niaee, M.S. A Comparative Investigation of Three-Dimensional Printing Models (Rapid Prototyping) Made by Imaging Systems Including Multidetector Computed Tomography and Cone-Beam Computed Tomography Systems. Saudi J. Oral Sci. 2024, 11, 91–101. [Google Scholar] [CrossRef]
- Molen, A.D. Considerations in the Use of Cone-Beam Computed Tomography for Buccal Bone Measurements. Am. J. Orthod. Dentofac. Orthop. 2010, 137, S130–S135. [Google Scholar] [CrossRef]
- Patcas, R.; Markic, G.; MüLler, L.; Ullrich, O.; Peltomäki, T.; Kellenberger, C.J.; Karlo, C.A. Accuracy of Linear Intraoral Measurements Using Cone Beam CT and Multidetector CT: A Tale of Two CTs. Dentomaxillofacial Radiol. 2012, 41, 637–644. [Google Scholar] [CrossRef]
- Tanimoto, H.; Arai, Y. The Effect of Voxel Size on Image Reconstruction in Cone-Beam Computed Tomography. Oral Radiol. 2009, 25, 149–153. [Google Scholar] [CrossRef]
- Bielecki-Kowalski, B.; Kozakiewicz, M. Clinico-Anatomical Classification of the Processus Condylaris Mandibulae for Traumatological Purposes. Ann. Anat. 2021, 234, 151616. [Google Scholar] [CrossRef]
- Lee, H.T.; Kim, Y.H.; Kim, T.G.; Lee, J. Sensory Impairment in Infraorbital Nerve Following Mid-Facial Fractures. Arch. Plast. Surg. 2011, 38, 43–47. [Google Scholar]
- Nkenke, E.; Hahn, M.; Lell, M.; Wiltfang, J.; Schultze-Mosgau, S.; Stech, B.; Radespiel-Tröger, M.; Neukam, F.W. Anatomic Site Evaluation of the Zygomatic Bone for Dental Implant Placement. Clin. Oral Impl. Res. 2003, 14, 72–79. [Google Scholar] [CrossRef]
- Kontio, R.; Wilkman, T.; Mesimäki, K.; Chepurnyi, Y.; Asikainen, A.; Haapanen, A.; Poutala, A.; Mikkonen, M.; Slobodianiuk, A.; Kopchak, A. Automated 3-D Computer-Aided Measurement of the Bony Orbit: Evaluation of Correlations among Volume, Depth, and Surface Area. J. Pers. Med. 2024, 14, 508. [Google Scholar] [CrossRef]
- Bielecki-Kowalski, B.; Kozakiewicz, M. Choice of Screws for Fixation of Mandibular Condyle Fractures Guided by Anthropometric Data. Appl. Sci. 2021, 11, 3371. [Google Scholar] [CrossRef]

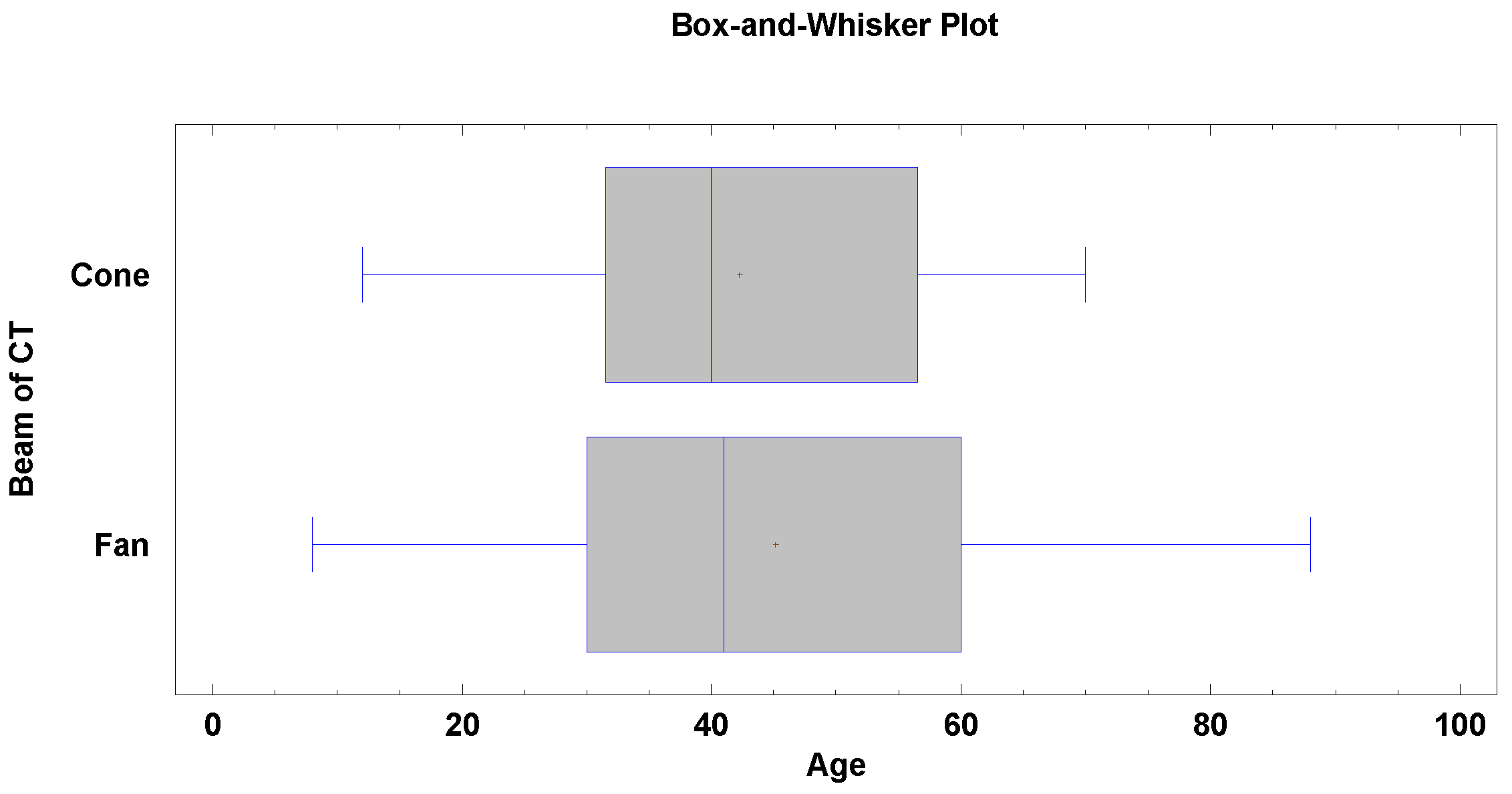
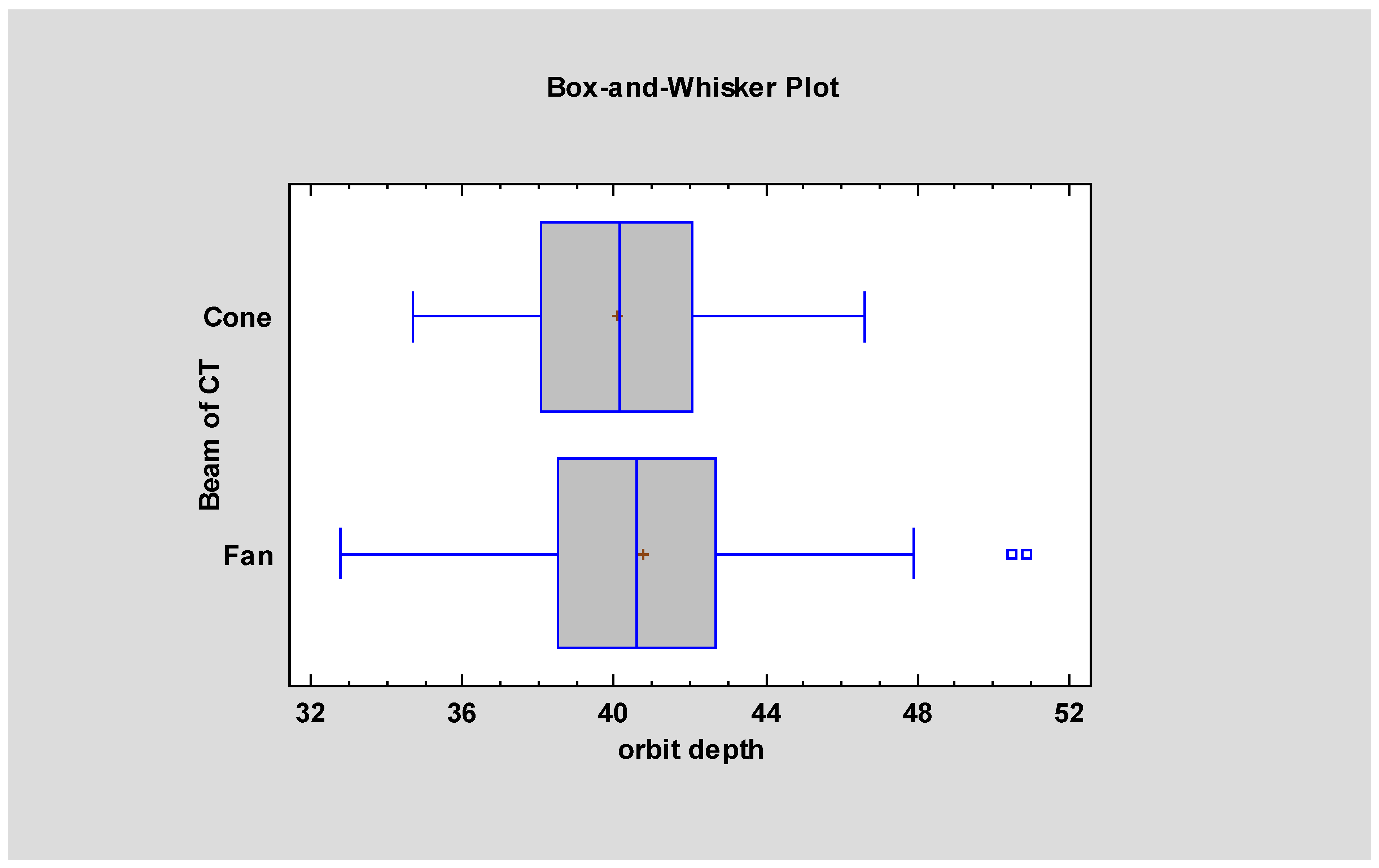
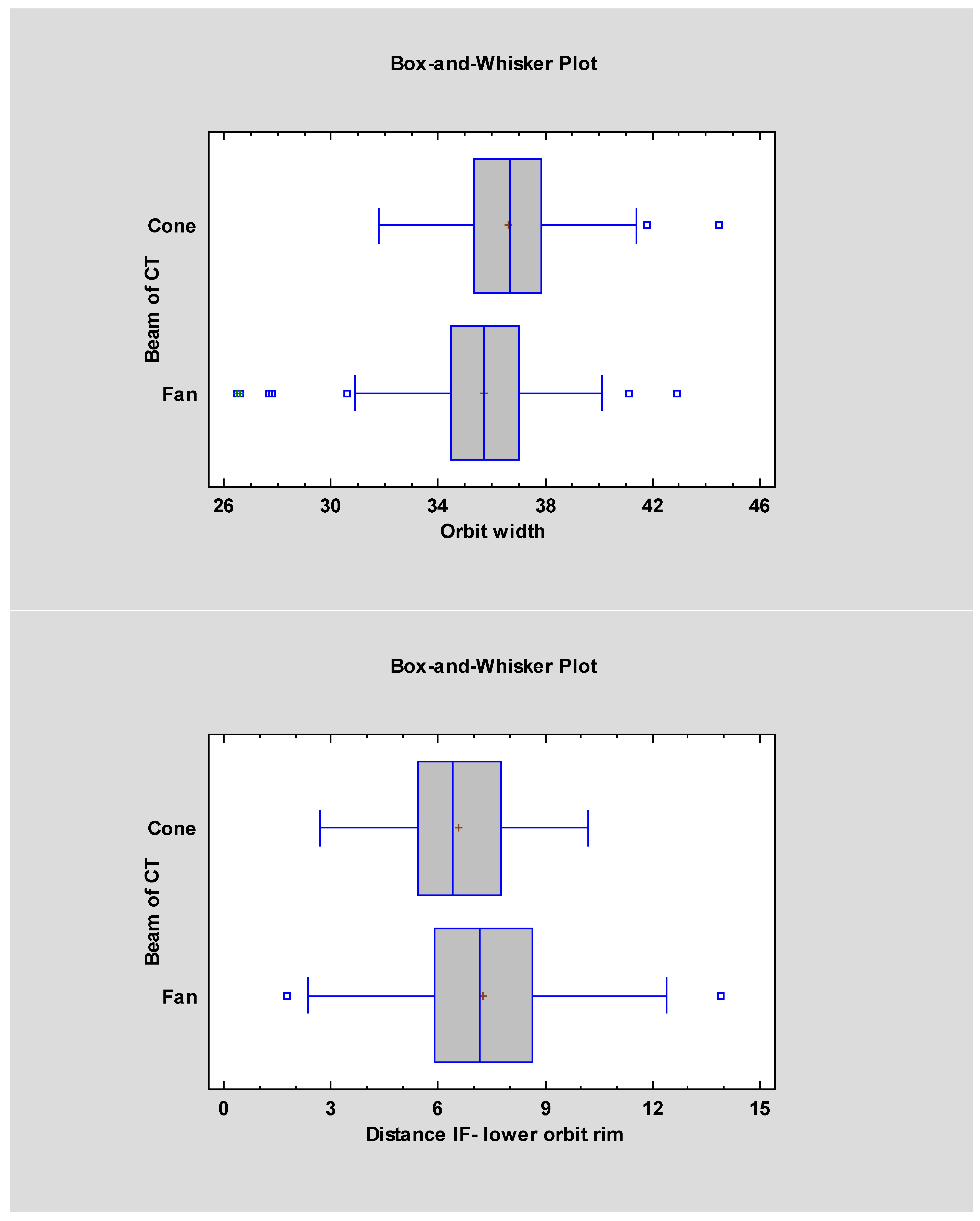
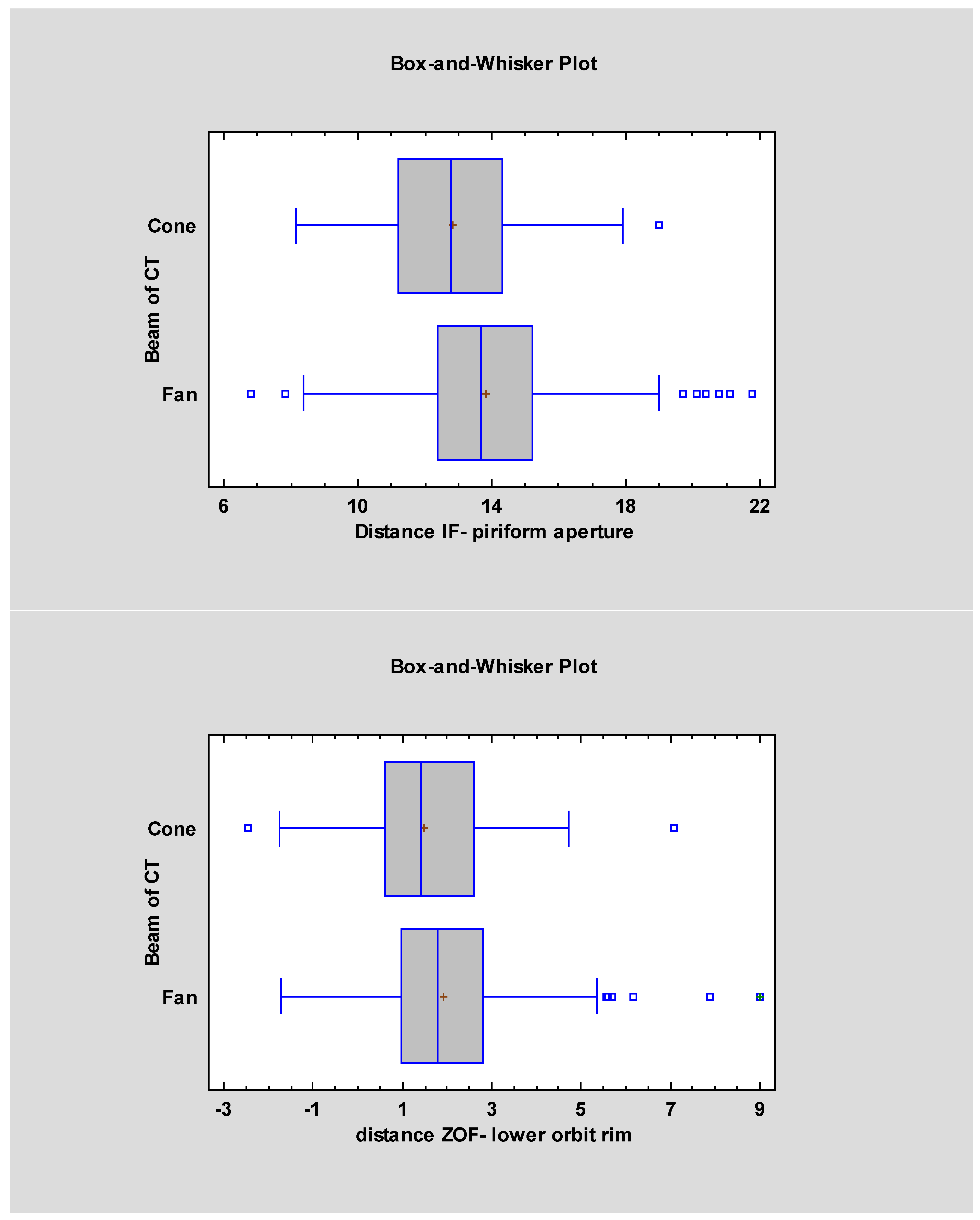
| Measurement Names | FBCT Mean ± SD | FBCT Median | CBCT Mean ± SD | CBCT Median | Statistical Significancy |
|---|---|---|---|---|---|
| Orbital width | 36.61 ± 2.01 | 35.7 | 35.74 ± 2.05 | 36.7 | p < 0.05 |
| Orbital height | 33.65 ± 2.23 | 33.4 | 34.02 ± 2.17 | 34.05 | |
| Orbital depth | 40.75 ± 3.05 | 40.75 | 40.07 ± 2.66 | 40.15 | p < 0.05 |
| Bone thickness prior to nasolacrimal duct | 3.69 ± 1.07 | 3.5 | 3.45 ± 1.01 | 3.38 | |
| The distance from the infraorbital rim to the infraorbital foramen | 7.26 ± 1.99 | 7.17 | 6.57 ± 1.7 | 6.42 | p < 0.05 |
| The distance from the piriform aperture and the infraorbital foramen | 13.82 ± 2.36 | 13.7 | 12.82 ± 2.21 | 12.8 | p < 0.05 |
| Distance from the zygomatico-orbital foramen to the infraorbital rim | 1.93 ± 1.53 | 1.8 | 1.49 ± 1.56 | 1.43 | p < 0.05 |
| Distance from supraorbital foramen to the supraorbital rim | 2.01 ± 1.43 | 1.43 | 1.54 ± 1.52 | 1.52 |
| Bone | Orbital Location | Thin Bone? | Clinical Remarks |
|---|---|---|---|
| Ethmoid bone (lamina papyracea) | Medial wall | ✅ Yes | Thinnest structure (<0.2 mm) |
| Maxillary bone (orbital floor) | Inferior wall | ✅ Yes | Particularly thin medially |
| Lacrimal bone | Anterior part of medial wall | ✅ Yes | Small and fragile |
| Palatine bone (orbital process) | Posteromedial part of orbital floor | ✅ Yes | Thin and difficult to visualize |
| Frontal bone | Superior wall | ❌ No | Thick and structurally stable |
| Zygomatic bone | Lateral wall and inferior orbital rim | ❌ No | Robust, supports patient-specific implants (PSI) |
| Sphenoid bone (wings) | Superior and lateral walls | ❌ No | Dense, central bone of the skull base |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielecka-Kowalska, N.; Bielecki-Kowalski, B.; Kozakiewicz, M. Comparative Analysis of Orbital Morphology Accuracy in 3D Models Based on Cone-Beam and Fan-Beam Computed Tomography Scans for Reconstructive Planning. J. Clin. Med. 2025, 14, 5541. https://doi.org/10.3390/jcm14155541
Bielecka-Kowalska N, Bielecki-Kowalski B, Kozakiewicz M. Comparative Analysis of Orbital Morphology Accuracy in 3D Models Based on Cone-Beam and Fan-Beam Computed Tomography Scans for Reconstructive Planning. Journal of Clinical Medicine. 2025; 14(15):5541. https://doi.org/10.3390/jcm14155541
Chicago/Turabian StyleBielecka-Kowalska, Natalia, Bartosz Bielecki-Kowalski, and Marcin Kozakiewicz. 2025. "Comparative Analysis of Orbital Morphology Accuracy in 3D Models Based on Cone-Beam and Fan-Beam Computed Tomography Scans for Reconstructive Planning" Journal of Clinical Medicine 14, no. 15: 5541. https://doi.org/10.3390/jcm14155541
APA StyleBielecka-Kowalska, N., Bielecki-Kowalski, B., & Kozakiewicz, M. (2025). Comparative Analysis of Orbital Morphology Accuracy in 3D Models Based on Cone-Beam and Fan-Beam Computed Tomography Scans for Reconstructive Planning. Journal of Clinical Medicine, 14(15), 5541. https://doi.org/10.3390/jcm14155541







