Anticoagulation Therapy and Severe Traumatic Brain Injury: A Retrospective Cohort Study on Clinical Outcomes Using TriNetX
Abstract
1. Introduction
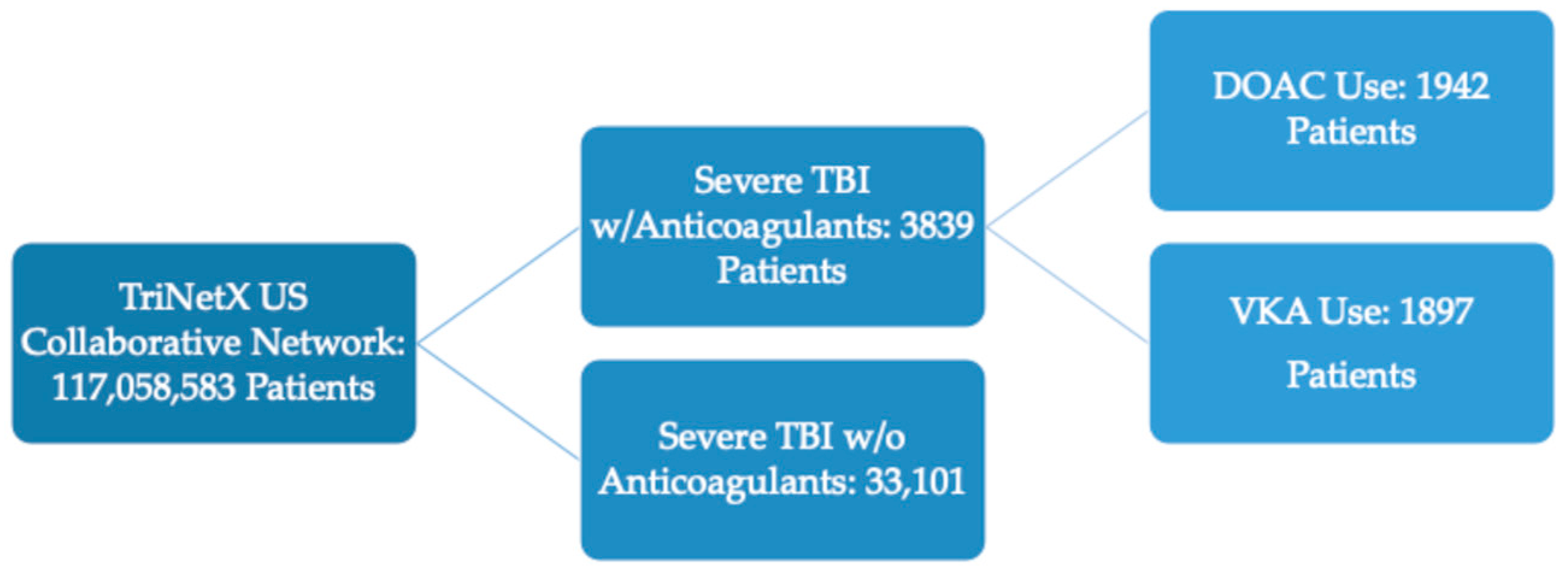
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Primary Outcome
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| DOAC | Direct Oral Anticoagulant |
| DVTs | Deep Vein Thrombosis |
| GCS | Glasgow Coma Scale |
| HCO | Healthcare Organization |
| ICD-10 | International Classification for Disease 10th Edition |
| LOINCs | Logical Observation Identifiers Names and Codes |
| mTBI | Mild Traumatic Brain Injury |
| PE | Pulmonary Embolism |
| sTBI | Severe Traumatic Brain Injury |
| TBI | Traumatic Brain Injury |
| tICH | Traumatic Intracranial Hemorrhage |
| UTI | Urinary Tract Infection |
| VAs | Veterans Affairs |
| VKA | Vitamin K Antagonist (Warfarin) |
References
- McCrea, M.A.; Giacino, J.T.; Barber, J.; Temkin, N.R.; Nelson, L.D.; Levin, H.S.; Dikmen, S.; Stein, M.; Bodien, Y.G.; Boase, K.; et al. Functional Outcomes Over the First Year After Moderate to Severe Traumatic Brain Injury in the Prospective, Longitudinal TRACK-TBI Study. JAMA Neurol. 2021, 78, 982–992. [Google Scholar] [CrossRef]
- Karamian, A.; Seifi, A.; Karamian, A.; Lucke-Wold, B. Incidence of intracranial bleeding in mild traumatic brain injury patients taking oral anticoagulants: A systematic review and meta-analysis. J. Neurol. 2024, 271, 3849–3868. [Google Scholar] [CrossRef]
- Finfer, S.R.; Cohen, J. Severe traumatic brain injury. Resuscitation 2001, 48, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Karamian, A.; Seifi, A.; Lucke-Wold, B. Effects of preinjury oral anticoagulants on the outcomes of traumatic brain injury in elderly patients: A systematic review and meta-analysis. Brain Inj. 2024, 38, 1197–1211. [Google Scholar] [CrossRef]
- Malec, J.F.; Brown, A.W.; Leibson, C.L.; Flaada, J.T.; Mandrekar, J.N.; Diehl, N.N.; Perkins, P.K. The Mayo Classification System for Traumatic Brain Injury Severity. J. Neurotrauma 2007, 24, 1417–1424. [Google Scholar] [CrossRef]
- Ghajar, J.; Hariri, R.J.; Narayan, R.K.; Iacono, L.A.; Firlik, K.; Patterson, R.H. Survey of critical care management of comatose, head-injured patients in the United States. Crit. Care Med. 1995, 23, 560. [Google Scholar] [CrossRef] [PubMed]
- Matta, B.; Menon, D. Severe head injury in the United Kingdom and Ireland: A survey of practice and implications for management|. Crit. Care Med. 1996, 24, 1743. [Google Scholar] [CrossRef] [PubMed]
- Santing, J.A.L.; Lee, Y.X.; Van Der Naalt, J.; Van Den Brand, C.L.; Jellema, K. Mild Traumatic Brain Injury in Elderly Patients Receiving Direct Oral Anticoagulants: A Systematic Review and Meta-Analysis. J. Neurotrauma 2022, 39, 458–472. [Google Scholar] [CrossRef]
- Vellek, J.; Tarawneh, O.H.; Kazim, S.F.; Owodunni, O.P.; Arbuiso, S.; Shah, S.; Dicpinigaitis, A.J.; Schmidt, M.H.; McKee, R.G.; Miskimins, R.; et al. Andexanet alfa therapy showed No increased rate of thromboembolic events in spontaneous intracranial hemorrhage patients: A multicenter electronic health record study. World Neurosurg. X 2024, 23, 100367. [Google Scholar] [CrossRef]
- Jung, I.-H.; Yun, J.-H.; Kim, S.J.; Chung, J.; Lee, S.K. Anticoagulation and Antiplatelet Agent Resumption Timing following Traumatic Brain Injury. Korean J. Neurotrauma 2023, 19, 298–306. [Google Scholar] [CrossRef]
- Henry, K.; Naylor, R.; De La Pena, N.; De La Pena, N.; Jarvis, T.; Labott, J.; Van Gompel, J. Timing of restarting antiplatelet and anticoagulation medications after traumatic subdural hematoma—A single institution experience (1699). Neurology 2021, 96, 1699. [Google Scholar] [CrossRef]
- Menditto, V.G.; Rossetti, G.; Sampaolesi, M.; Buzzo, M.; Pomponio, G. Traumatic Brain Injury in Patients under Anticoagulant Therapy: Review of Management in Emergency Department. J. Clin. Med. 2024, 13, 3669. [Google Scholar] [CrossRef]
- TriNetX|Our Real-World Data by Therapeutic Area and World Region. Available online: https://trinetx.com/real-world-data/ (accessed on 7 May 2024).
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on the Review of the Department of Veterans Affairs Examinations for Traumatic Brain Injury. Fact Sheet: Coding Guidance for Traumatic Brain Injury. In Evaluation of the Disability Determination Process for Traumatic Brain Injury in Veterans; National Academies Press (US): Washington, DC, USA, 2019; Available online: https://www.ncbi.nlm.nih.gov/books/NBK542610/ (accessed on 9 September 2024).
- Hecht, J.P.; LaDuke, Z.J.; Cain-Nielsen, A.H.; Hemmila, M.R.; Wahl, W.L. Effect of Preinjury Oral Anticoagulants on Outcomes Following Traumatic Brain Injury from Falls in Older Adults. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Botros, D.; Gautam, D.; Hamrick, F.A.; Nguyen, S.; Cortez, J.; Young, J.B.; Lombardo, S.; McCrum, M.L.; Menacho, S.T.; Grandhi, R. Impact of premorbid oral anticoagulant use on survival in patients with traumatic intracranial hemorrhage. Neurosurg. Focus 2023, 55, E2. [Google Scholar] [CrossRef]
- Pan, L.; Hu, J. Effect of prior anticoagulation therapy on outcomes of traumatic brain injury: A systematic review and meta-analysis. Exp. Ther. Med. 2024, 27, 160. [Google Scholar] [CrossRef]
- Lim, X.T.; Ang, E.; Lee, Z.X.; Hajibandeh, S.; Hajibandeh, S. Prognostic significance of preinjury anticoagulation in patients with traumatic brain injury: A systematic review and meta-analysis. J. Trauma Acute Care Surg. 2021, 90, 191. [Google Scholar] [CrossRef] [PubMed]
- Posti, J.P.; Ruuskanen, J.O.; Sipilä, J.O.T.; Luoto, T.M.; Rautava, P.; Kytö, V. Impact of Oral Anticoagulation and Adenosine Diphosphate Inhibitor Therapies on Short-term Outcome of Traumatic Brain Injury. Neurology 2022, 99, e1122–e1130. [Google Scholar] [CrossRef]
- Pedro, K.M.; Guberman, G.; Scotti, P.; Lafleur, L.; Hua, M.; Troquet, J.-M.; Saluja, R.S.; Marcoux, J. Outcomes of Elderly Patients on Direct Oral Anticoagulants (DOACs) Versus Warfarin After Traumatic Brain Injury. Can. J. Neurol. Sci. 2024, Online ahead of print, 1–7. [Google Scholar] [CrossRef]
- Scotti, P.; Séguin, C.; Lo, B.W.Y.; de Guise, E.; Troquet, J.-M.; Marcoux, J. Antithrombotic agents and traumatic brain injury in the elderly population: Hemorrhage patterns and outcomes. J. Neurosurg. 2019, 133, 486–495. [Google Scholar] [CrossRef]
- Rønning, P.; Helseth, E.; Skaansar, O.; Tverdal, C.; Andelic, N.; Bhatnagar, R.; Melberg, M.; Skaga, N.O.; Aarhus, M.; Halvorsen, S.; et al. Impact of Preinjury Antithrombotic Therapy on 30–Day Mortality in Older Patients Hospitalized with Traumatic Brain Injury (TBI). Front. Neurol. 2021, 12, 650695. [Google Scholar] [CrossRef]
- Nederpelt, C.J.; van der Aalst, S.J.M.; Rosenthal, M.G.; Krijnen, P.; Huisman, M.V.; Peul, W.C.; Schipper, I.B. Consequences of pre-injury utilization of direct oral anticoagulants in patients with traumatic brain injury: A systematic review and meta-analysis. J. Trauma Acute Care Surg. 2020, 88, 186. [Google Scholar] [CrossRef]
- Kobayashi, L.; Barmparas, G.; Bosarge, P.; Brown, C.V.; Bukur, M.; Carrick, M.M.; Catalano, R.D.; Holly-Nicolas, J.; Inaba, K.; Kaminski, S.; et al. Novel oral anticoagulants and trauma: The results of a prospective American Association for the Surgery of Trauma Multi-Institutional Trial. J. Trauma Acute Care Surg. 2017, 82, 827. [Google Scholar] [CrossRef]
- Hanger, H.C.; Geddes, J.A.A.; Wilkinson, T.J.; Lee, M.; Baker, A.E. Warfarin-related intracerebral haemorrhage: Better outcomes when reversal includes prothrombin complex concentrates. Intern. Med. J. 2013, 43, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Goldin, M.; Tsaftaridis, N.; Jnani, J.; Spyropoulos, A.C. Reversal of Direct Oral Anticoagulants (DOACs) for Critical Bleeding or Urgent Procedures. J. Clin. Med. 2025, 14, 1013. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Singh, A.; Chaudhary, R.; Bashline, M.; Houghton, D.E.; Rabinstein, A.; Adamski, J.; Arndt, R.; Ou, N.N.; Rudis, M.I.; et al. Evaluation of Direct Oral Anticoagulant Reversal Agents in Intracranial Hemorrhage: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2240145. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jing, J.; Chen, S.; Liu, X.; Wang, J.; Pan, C.; Tang, Z. Reversal and resumption of anticoagulants in patients with anticoagulant-associated intracerebral hemorrhage. Eur. J. Med. Res. 2024, 29, 252. [Google Scholar] [CrossRef]
- Tallroth, M.; Östlundh, L.; Büki, A.; Cao, Y.; von Euler, M.; Ström, J.O. Reversal treatment and clinical outcomes in acute intracranial haemorrhage associated with oral anticoagulant use: Protocol of a planned systematic review and meta-analysis. BMJ Open 2025, 15, e090357. [Google Scholar] [CrossRef]
| Anticoagulant Use Cohort Demographics | ||||
|---|---|---|---|---|
| Characteristics | Propensity-Score-Matched Sample No. (%) | |||
| DOAC (n = 1942) | p-Value a | VKA (n = 1897) | p-Value b | |
| Age at Index (years) | 70 ± 16 | 69 ± 17 | ||
| Demographics | ||||
| Male | 1073 (55.25%) | 0.4021 | 1056 (55.67%) | 0.7688 |
| Female | 811 (41.76%) | 0.3466 | 787 (41.49%) | 0.7173 |
| White | 1219 (62.77%) | 0.9471 | 1216 (64.10%) | 0.9191 |
| Black or African American | 358 (18.44%) | 0.8688 | 347 (18.29%) | 0.8332 |
| Asian | 60 (3.09%) | 0.5684 | 48 (2.53%) | 0.8345 |
| American Indian or Alaska Native | 23 (1.18%) | 0.5349 | 35 (1.85%) | 0.8067 |
| Native Hawaiian or Other Pacific Islander | 10 (0.52%) | 0.8268 | 10 (0.53%) | 1.0000 |
| Other Race | 64 (3.30%) | 0.8584 | 58 (3.06%) | 0.8516 |
| Chronic Medical Conditions | ||||
| Diseases of the circulatory system | 1890 (97.32%) | 1.0000 | 1860 (98.05%) | 1.0000 |
| Essential (primary) hypertension | 1576 (81.15%) | 0.3836 | 1606 (84.66%) | 0.7200 |
| Diseases of the respiratory system | 1608 (82.80%) | 0.2414 | 1585 (83.55%) | 0.9651 |
| Atrial fibrillation and flutter | 1205 (62.05%) | 0.9210 | 1160 (61.15%) | 0.9204 |
| Acute kidney failure and chronic kidney disease | 1119 (57.62%) | 0.5814 | 1149 (60.57%) | 0.8682 |
| Ischemic heart diseases | 1102 (56.75%) | 0.8970 | 1113 (58.67%) | 0.4895 |
| Heart failure | 974 (50.15%) | 0.4224 | 1054 (55.56%) | 0.8703 |
| Diabetes mellitus | 894 (46.04%) | 0.4986 | 897 (47.29%) | 0.6491 |
| Overweight, obesity and other hyperalimentation | 708 (36.46%) | 0.5473 | 666 (35.11%) | 0.8917 |
| BMI | ||||
| 18.5–24.9 kg/m2 | 1028 (52.94%) | 1.0000 | 979 (51.61%) | 0.9224 |
| 25–29.9 kg/m2 | 1264 (65.09%) | 0.9463 | 1227 (64.68%) | 0.4532 |
| 30–70 kg/m2 | 1141 (58.75%) | 0.6960 | 1144 (60.31%) | 0.5948 |
| DOAC-Associated Mortality Risk | |||
|---|---|---|---|
| Follow-Up Interval | Risk Ratio | 95% CI | p-Value |
| 1-Month | 1.03 | (0.94–1.13) | 0.5017 |
| 3-Months | 1.04 | (0.96–1.13) | 0.2906 |
| 6-Months | 1.00 | (0.93–1.08) | 0.9348 |
| 12-Months | 1.01 | (0.94–1.08) | 0.8538 |
| VKA-Associated Mortality Risk | |||
|---|---|---|---|
| Follow-Up Interval | Risk Ratio | 95% CI | p-Value |
| 1-Month | 0.98 | (0.90–1.06) | 0.5675 |
| 3-Months | 0.94 | (0.87–1.02) | 0.1194 |
| 6-Months | 0.94 | (0.88–1.01) | 0.1149 |
| 12-Months | 0.95 | (0.89–1.02) | 0.1709 |
| DOAC-Associated Post-Injury Outcomes | |||
|---|---|---|---|
| Follow-Up Interval | Risk Ratio | 95% CI | p-Value |
| 30-day Outcomes | |||
| UTI | 1.01 | (0.72–1.41) | 0.9597 |
| DVT | 1.13 | (0.70–1.85) | 0.6111 |
| Pneumonia | 0.98 | (0.77–1.25) | 0.8661 |
| Stroke | 1.17 | (0.86–1.59) | 0.3148 |
| Myocardial Infarction | 1.03 | (0.68–1.55) | 0.8970 |
| GI Bleed | 0.99 | (0.53–1.83) | 0.9705 |
| Anticoagulation Reversal Agent | 0.85 | (0.54–1.33) | 0.4695 |
| Nontraumatic Subdural Hemorrhage | 1.00 | (0.80–1.26) | 0.9748 |
| Nontraumatic Subarachnoid Hemorrhage | 1.09 | (0.84–1.41) | 0.5359 |
| Surgical Site Infection | 0.99 | (0.41–2.38) | 0.9876 |
| Infection Following Procedure | 0.99 | (0.41–2.38) | 0.9900 |
| Hospital Stay | 0.91 | (0.41–2.06) | 0.8296 |
| Tracheostomy | 1.13 | (0.84–1.53) | 0.4223 |
| PEG Tube | 1.05 | (0.79–1.39) | 0.7220 |
| Craniectomy or Craniotomy Procedure | 0.92 | (0.62–1.36) | 0.6697 |
| Seizure | 1.08 | (0.80–1.45) | 0.6281 |
| PE | 0.99 | (0.59–1.66) | 0.9689 |
| VKA-Associated Post-Injury Outcomes | |||
|---|---|---|---|
| Follow-Up Interval | Risk Ratio | 95% CI | p-Value |
| 30-day Outcomes | |||
| UTI | 0.98 | (0.71–1.36) | 0.9107 |
| DVT | 0.95 | (0.61–1.47) | 0.8194 |
| Pneumonia | 1.01 | (0.80–1.29) | 0.9046 |
| Stroke | 0.91 | (0.70–1.20) | 0.5190 |
| Myocardial Infarction | 1.03 | (0.70–1.49) | 0.8929 |
| GI Bleed | 0.87 | (0.50–1.52) | 0.6231 |
| Anticoagulation Reversal Agent | 0.77 | (0.63–0.93) | 0.0069 |
| Nontraumatic Subdural Hemorrhage | 0.87 | (0.72–1.05) | 0.1390 |
| Nontraumatic Subarachnoid Hemorrhage | 0.93 | (0.73–1.17) | 0.5224 |
| Surgical Site Infection | 1.00 | (0.42–2.39) | 0.9933 |
| Infection Following Procedure | 0.99 | (0.41–2.37) | 0.9830 |
| Hospital Stay | 1.03 | (0.43–2.47) | 0.9421 |
| Tracheostomy | 0.93 | (0.69–1.24) | 0.6141 |
| PEG Tube | 0.99 | (0.75–1.31) | 0.9473 |
| Craniectomy or Craniotomy Procedure | 0.84 | (0.62–1.15) | 0.2880 |
| Seizure | 0.92 | (0.71–1.20) | 0.5380 |
| PE | 0.71 | (0.41–1.22) | 0.2083 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmussen, S.; Shaik, K.; Rawson, C.; Saloum, A.; Rahme, R.; Karsy, M. Anticoagulation Therapy and Severe Traumatic Brain Injury: A Retrospective Cohort Study on Clinical Outcomes Using TriNetX. J. Clin. Med. 2025, 14, 4510. https://doi.org/10.3390/jcm14134510
Rasmussen S, Shaik K, Rawson C, Saloum A, Rahme R, Karsy M. Anticoagulation Therapy and Severe Traumatic Brain Injury: A Retrospective Cohort Study on Clinical Outcomes Using TriNetX. Journal of Clinical Medicine. 2025; 14(13):4510. https://doi.org/10.3390/jcm14134510
Chicago/Turabian StyleRasmussen, Spencer, Kamal Shaik, Clayton Rawson, Ammar Saloum, Rudy Rahme, and Michael Karsy. 2025. "Anticoagulation Therapy and Severe Traumatic Brain Injury: A Retrospective Cohort Study on Clinical Outcomes Using TriNetX" Journal of Clinical Medicine 14, no. 13: 4510. https://doi.org/10.3390/jcm14134510
APA StyleRasmussen, S., Shaik, K., Rawson, C., Saloum, A., Rahme, R., & Karsy, M. (2025). Anticoagulation Therapy and Severe Traumatic Brain Injury: A Retrospective Cohort Study on Clinical Outcomes Using TriNetX. Journal of Clinical Medicine, 14(13), 4510. https://doi.org/10.3390/jcm14134510






